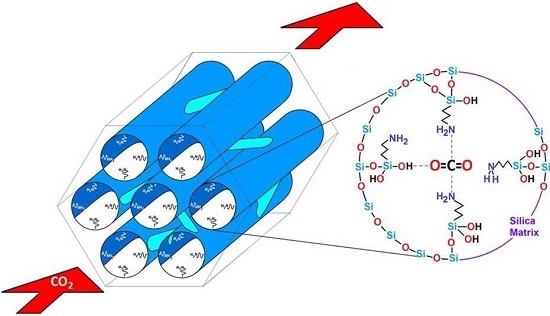
Optimal Surface Amino-Functionalization Following Thermo-Alkaline Treatment of Nanostructured Silica Adsorbents for Enhanced CO2 Adsorption
Abstract
:1. Introduction
1.1. The Problem
1.2. Carbon Capture and Storage (CCS) Alternatives
- -
- Absorption processes, which involve the bubbling of the adsorptive in solutions containing species such as amines, carbonates, and ions. In this case, the solvent becomes very important, since ion order to achieve a good capture of CO2, solvents of a low polarity and relatively low vapor pressure are very much preferred. Nevertheless, absorption processes involve a great disadvantage that is concerned with the short lifetimes that the trapped species can attain as these molecules usually interact (react) with the solvent species.
- -
1.3. CO2 Chemisorption and Physisorption
2. Stages Followed Concerning the Choice and Implementation of Proficient Silica Adsorbents for Enhanced CO2 Capture
2.1. Adsorbent Choice
2.2. Habilitation of the SiO2 Surface to Attain an Efficient and Sufficient Amine-Functionalization Process
2.3. Supplementary Aspects in Relation to the Choice of Santa Barbara Amorphous Silica No. 15 (SBA-15) for Enhanced CO2 Trapping
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Precursor SBA-15 Solids
3.3. Soft Thermo-Alkaline Treatment Performed over SBA-15 Precursor Materials
3.4. Chemical Anchoring of 3-Aminopropyltriethoxysilane (APTES) on the Surface of SiO2 Mesoporous Solids
3.5. CO2 Sorption Experiments at Different Temperatures
4. Characterization Techniques
- -
- FTIR: This technique will be helpful for realizing the presence of silanol and siloxane species on the surface of the adsorbents. FTIR signals were obtained from a Perkin Elmer Paragon 1000 (FT-IR) instrument equipped with an Attenuated Total Reflectance (ATR) tool.
- -
- TGA: This analysis was performed on a Perkin Elmer Diamond instrument on a N2 flow of 50 mL·min−1, subjected to at temperature ramp of 5 °C·min−1 along a temperature interval of 323–823 K.
- -
- Raman: These spectra are useful for providing an overall view of the structural consolidation of the adsorbents prepared in this work; in addition to the structure of the substrate itself, in particular, of silica bonds as well as free or vicinal silanol groups that lie on the silica surface. Through this technique it could be possible, in principle, to realize the arrangement of adsorbate molecules throughout the adsorbent surface. The instrument employed was a Raman Microscope (ThermoFisher Scientific, Waltham, MA, USA) with a 636 nm laser delivering a maximum power of 10 mW.
- -
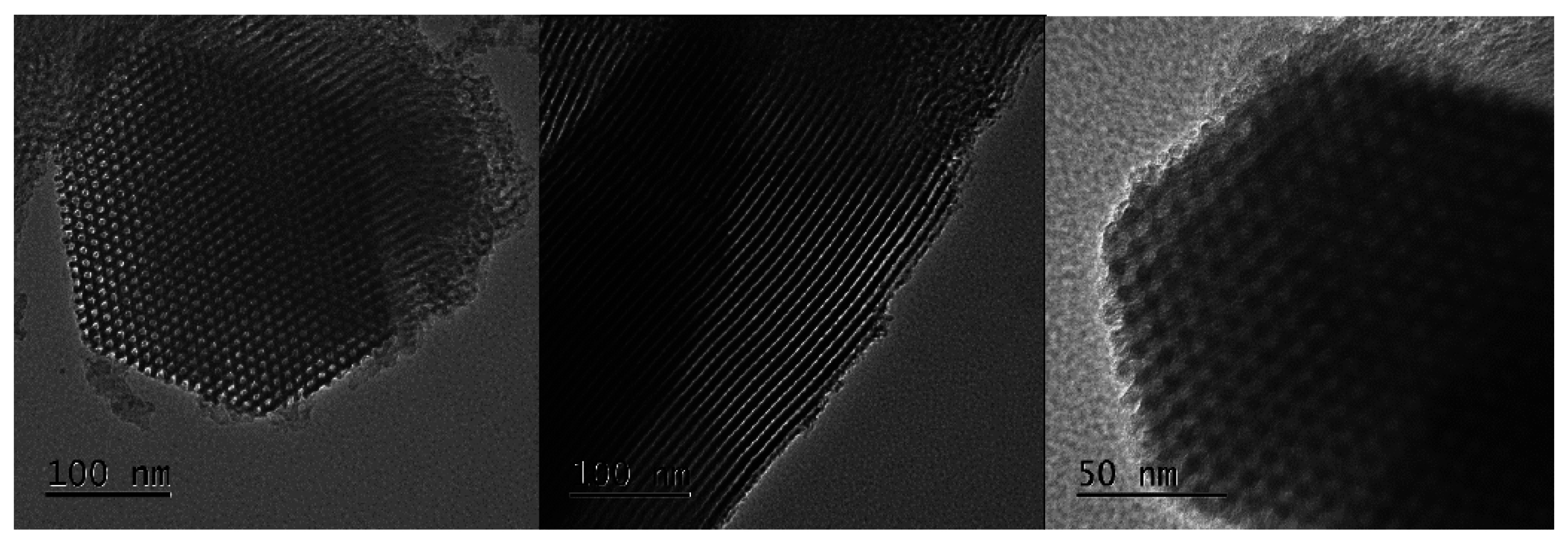
- TEM: Images were obtained from a HRTEM Jeol 2100F microscope (JEOL Ltd., Tokyo, Japan) operating at an acceleration voltage of 200 keV. This instrument was employed to observe the structural arrangement of the samples and to realize if the preparation procedure had affected (or not) the shape and structure of the SiO2 substrates.
- -
- XRD: It is not only useful for checking the ordered hexagonal arrangement of the nanotubes making the SBA-15 hexagonal arrangement but also for determining the pore width and the thickness of their pore walls. The XRD parameters of the network were measured from a Bruker D8 Advance instrument (Bruker AXS, Madison, WI, USA) employing a Cu-Kα radiation wavelength of 1.54 Å in the low region (0.6° to 5.0 ° in the 2θ scale).
- -
- N2 Sorption: The textural properties of the silica adsorbents were determined from an ASAP 2020 automatic instrument (Micromeritics Instrument Corp., Norcross, GA, USA), employing N2 at its boiling point (76 K at Mexico City’s altitude). The materials were previously outgassed at 373 K for 12 h. under a turbomolecular vacuum of 10−6 mbar. The surface area was determined from either the BET or t-methods, while the pore size distribution was obtained through the Non-Localized Functional Theory (NLDFT) approach. The kernel employed for this calculation was that corresponding to the filling of cylindrical pores along the boundary N2 adsorption curve at 77 K.
- -
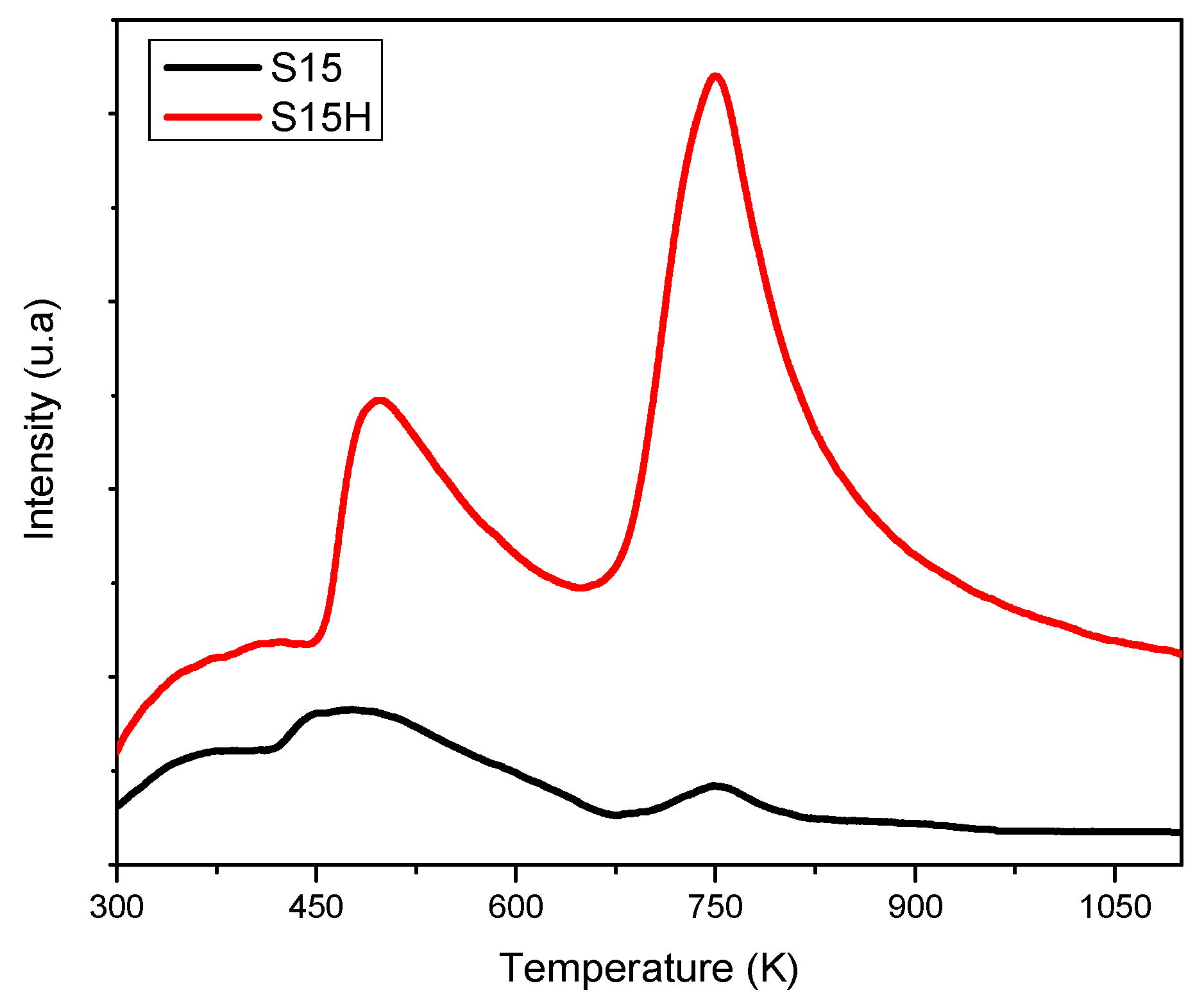
- NH3 TPD: The total acidity (Brönsted + Lewis) was qualitatively realized from a Micromeritics TPD/TPR 2900 NH3 programmed desorption (TPD) device (Micromeritics Instrument Corp.) provided with a TCD detector. The experimental procedure consisted in outgassing the sample under a mixture of N2 and air flowing at individual rates of 60 cm3·min−1 before being mixed from room temperature up to 773 K; the system was then kept at this temperature for 15 min. This was followed by the concurrence of N2 and NH3 flows of 60 cm3·min−1 each, up to a final temperature of 1073 K. Subsequently, the system was left to cool down to 303 K for about one hour under the same NH3 and N2 flows. Subsequently, an additional cleaning of the surface at 303 K was performed with a He flow of 60 cm3·min−1 during one hour. The definitive TPD experiment was carried out under a N2 flow of 60 cm3·min−1 used as a carrier gas and following to a heating ramp of 10 °C·min−1 up to 1073 K; while registering the amount of NH3 desorbed at each temperature.
- -
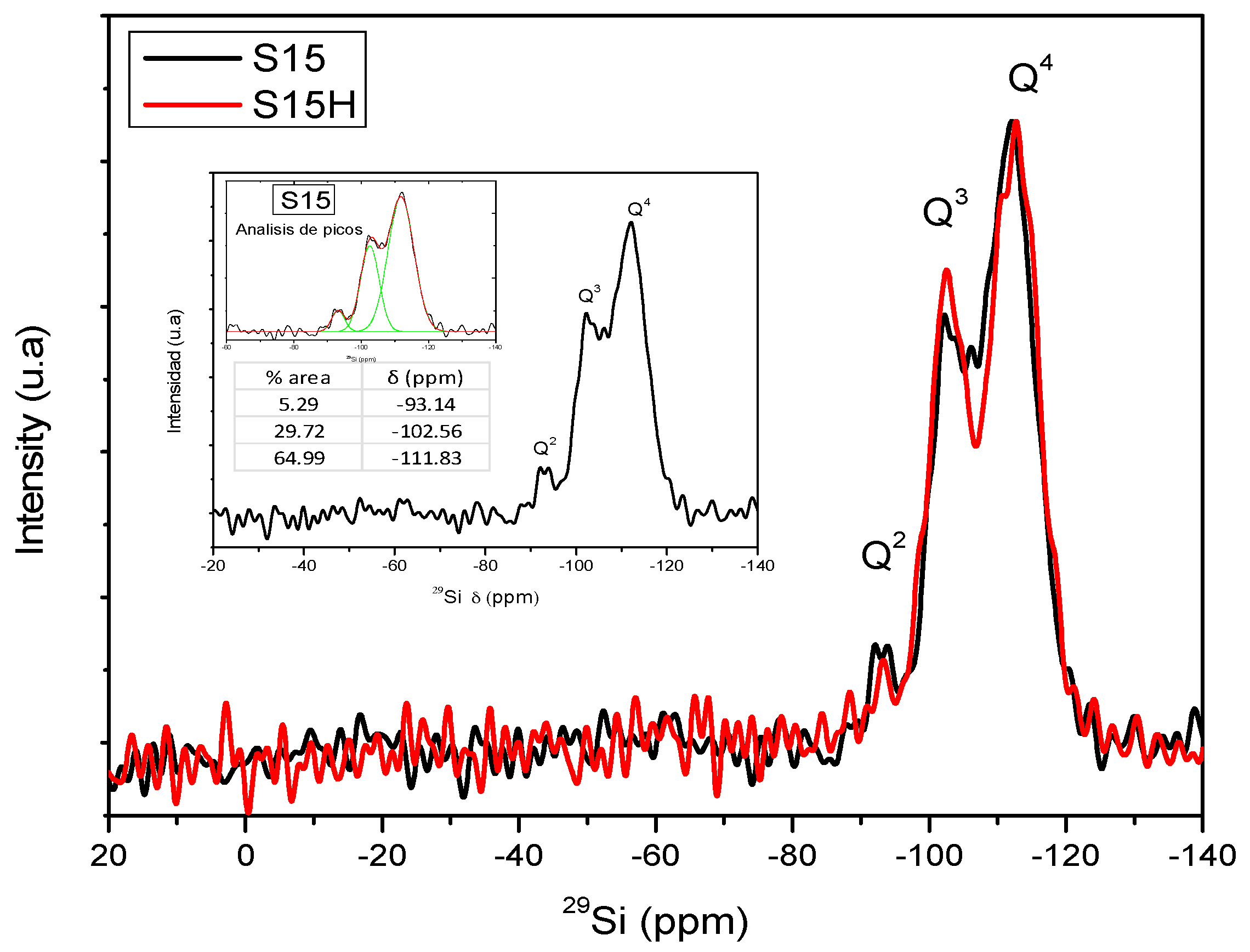
- Solid NMR: Solid State Nuclear Magnetic Resonance experiments were run on a Bruker Advance II300 spectrometer (Bruker BioSpin, The Woodlands, TX, USA), operating at 59.62 MHz for 29Si. The magic angle spinning technique was altogether employed with the NMR results as a reference of tetramethylsilane (TMS). This technique is very important in order to ascertain the number of bonds established between the surface silanol groups and the ethoxy groups of the APTES functionalizing species.
- -
- CO2 and CH4 adsorption and selectivity: Finally, CO2 and CH4 adsorption studies at different temperatures were performed on a Quantachrome Autosorb-1LC instrument; for this task, the samples were previously outgassed at 373 K for 6 h. CO2 adsorption isotherms were obtained from 263 to 303 K in order to obtain the CO2 enthalpy of adsorption at pressures from 0.001 to 1 bar.
5. Results and Discussion
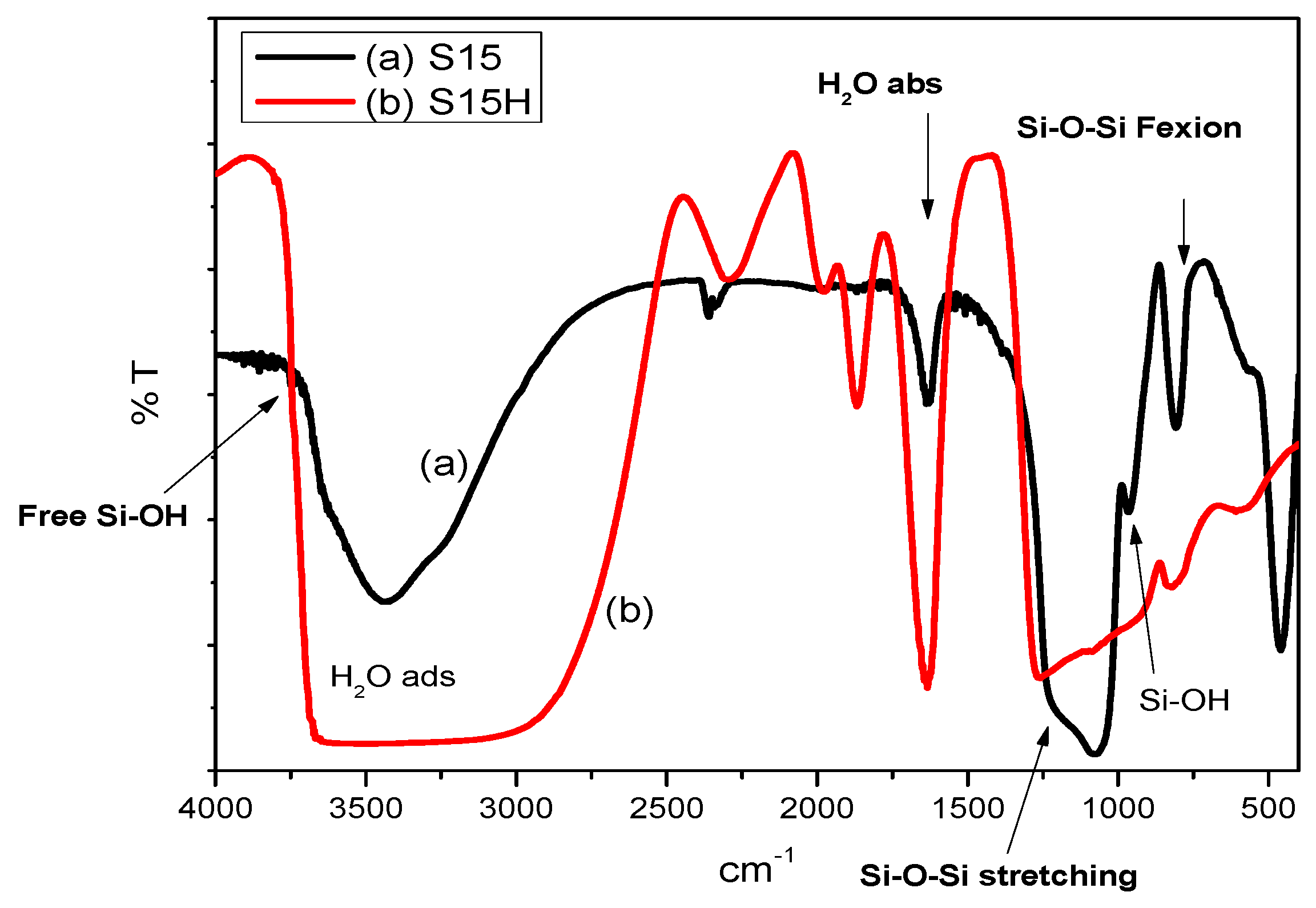
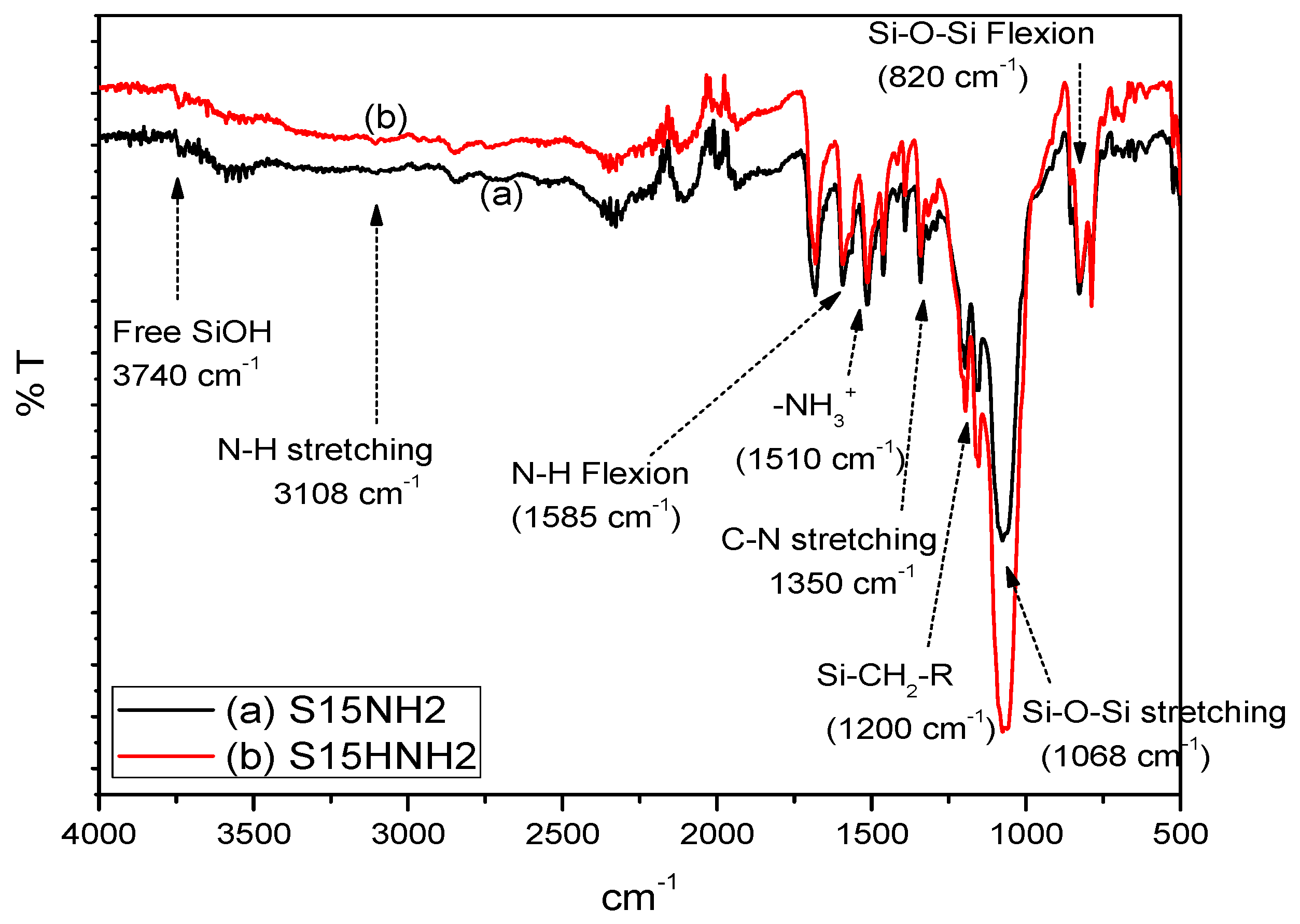
5.1. Middle-Infrared Spectroscopy Studies
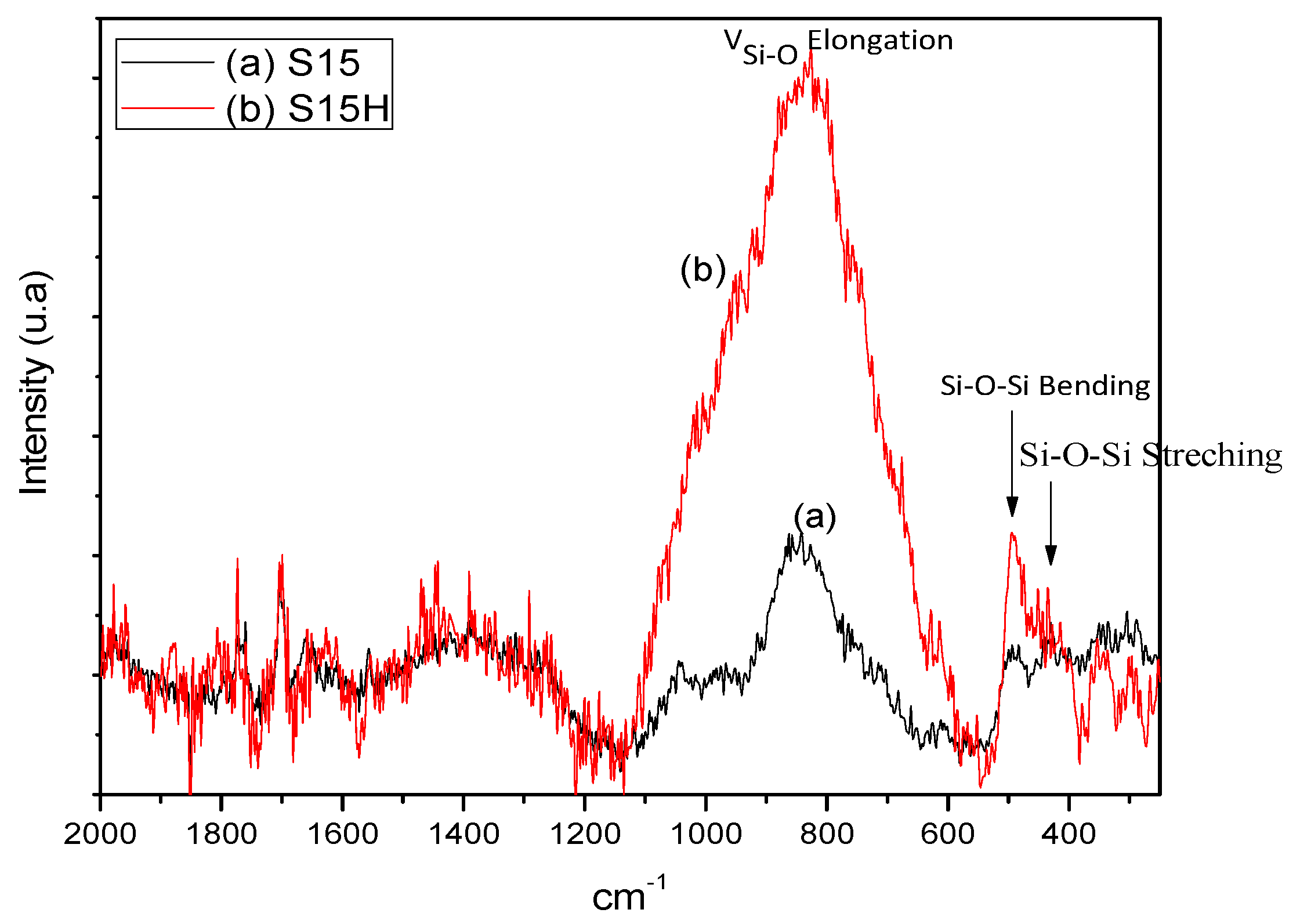
5.2. Raman Spectra of Precursor and Thermo Alkaline Treated Silica Samples
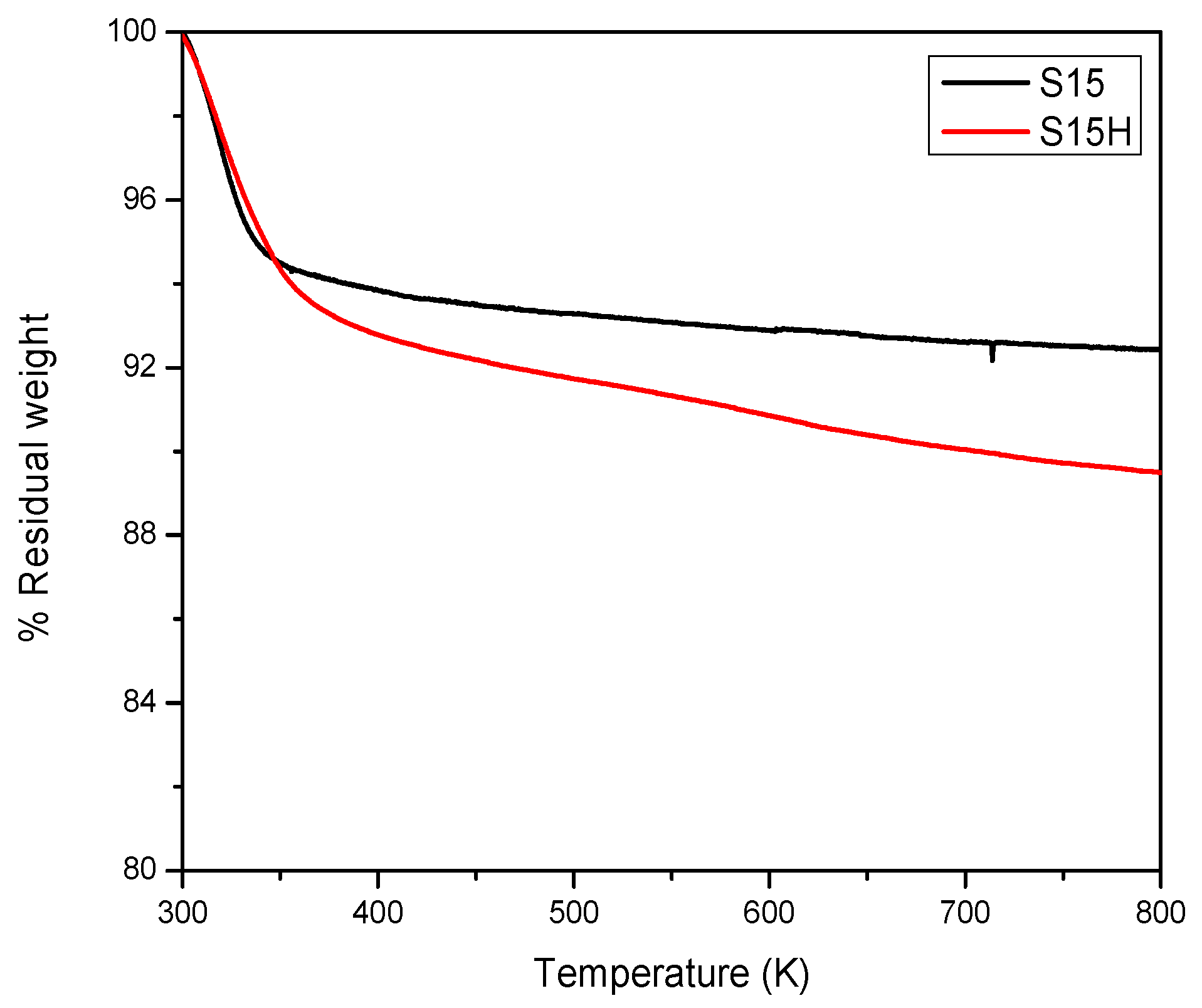
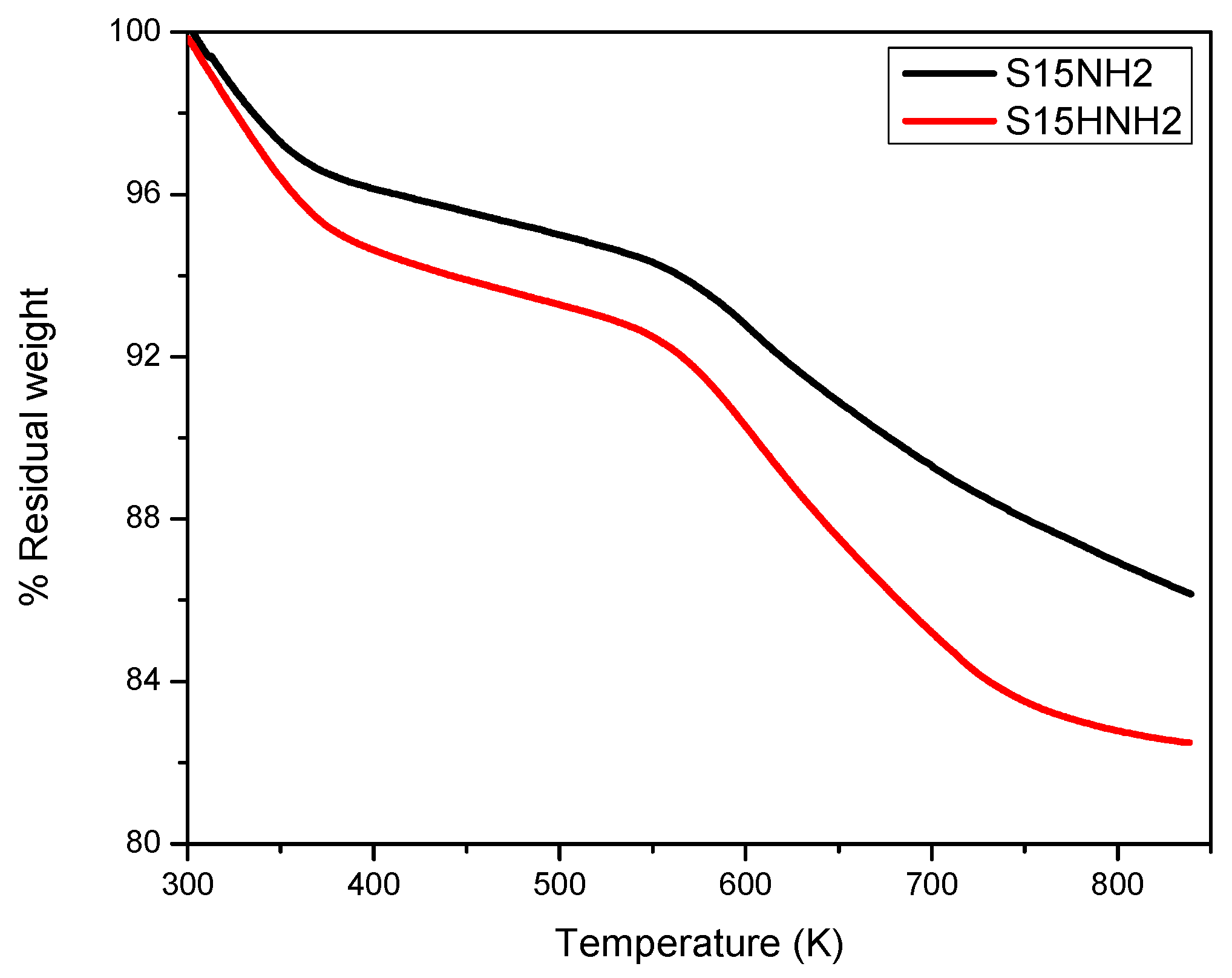
5.3. Thermogravimetric Analysis (TGA) Analysis of Precursor and Functionalized Silica Samples
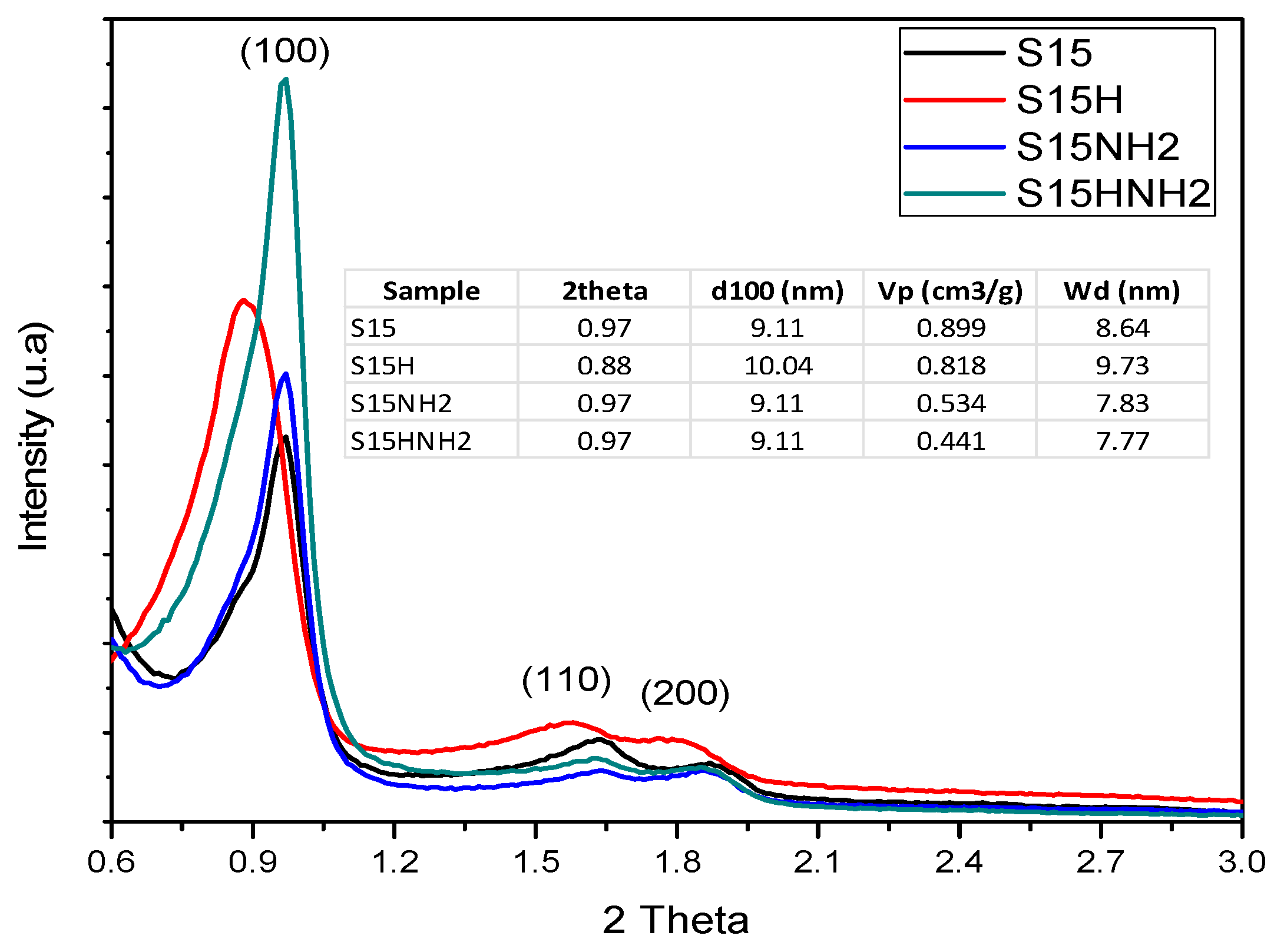
5.4. X-ray Diffraction (XRD) Analysis at Low Angle
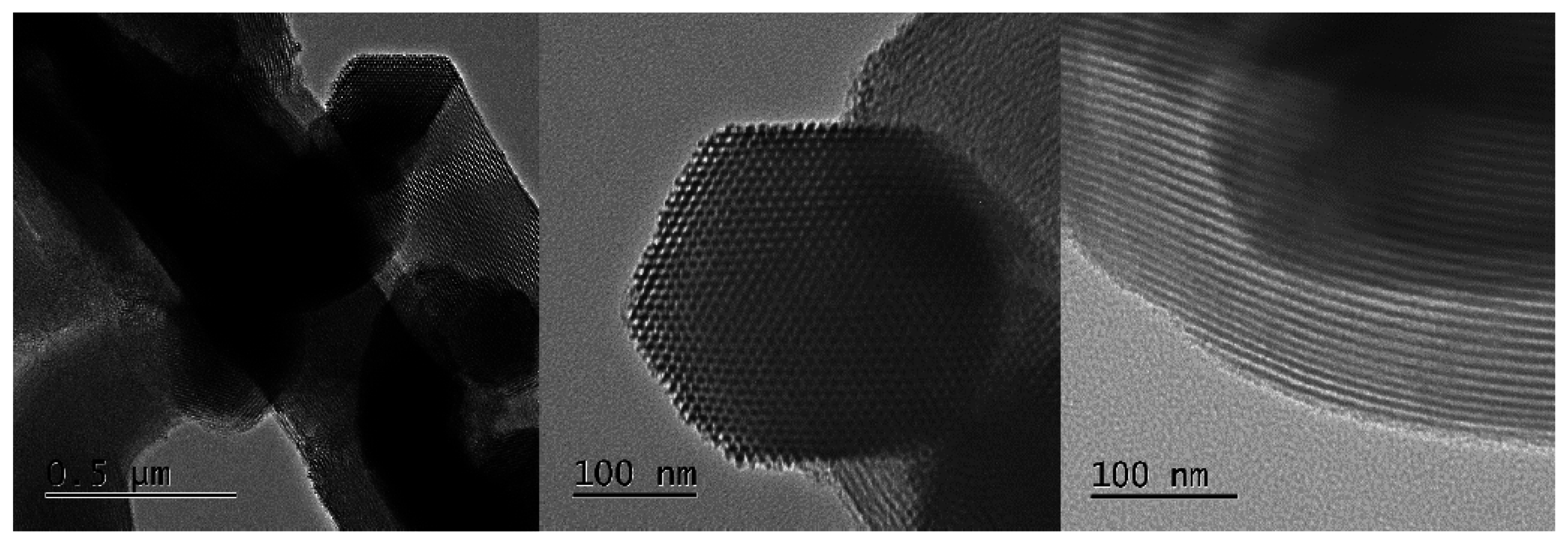
5.5. Transmission Electron Microscopy (TEM)
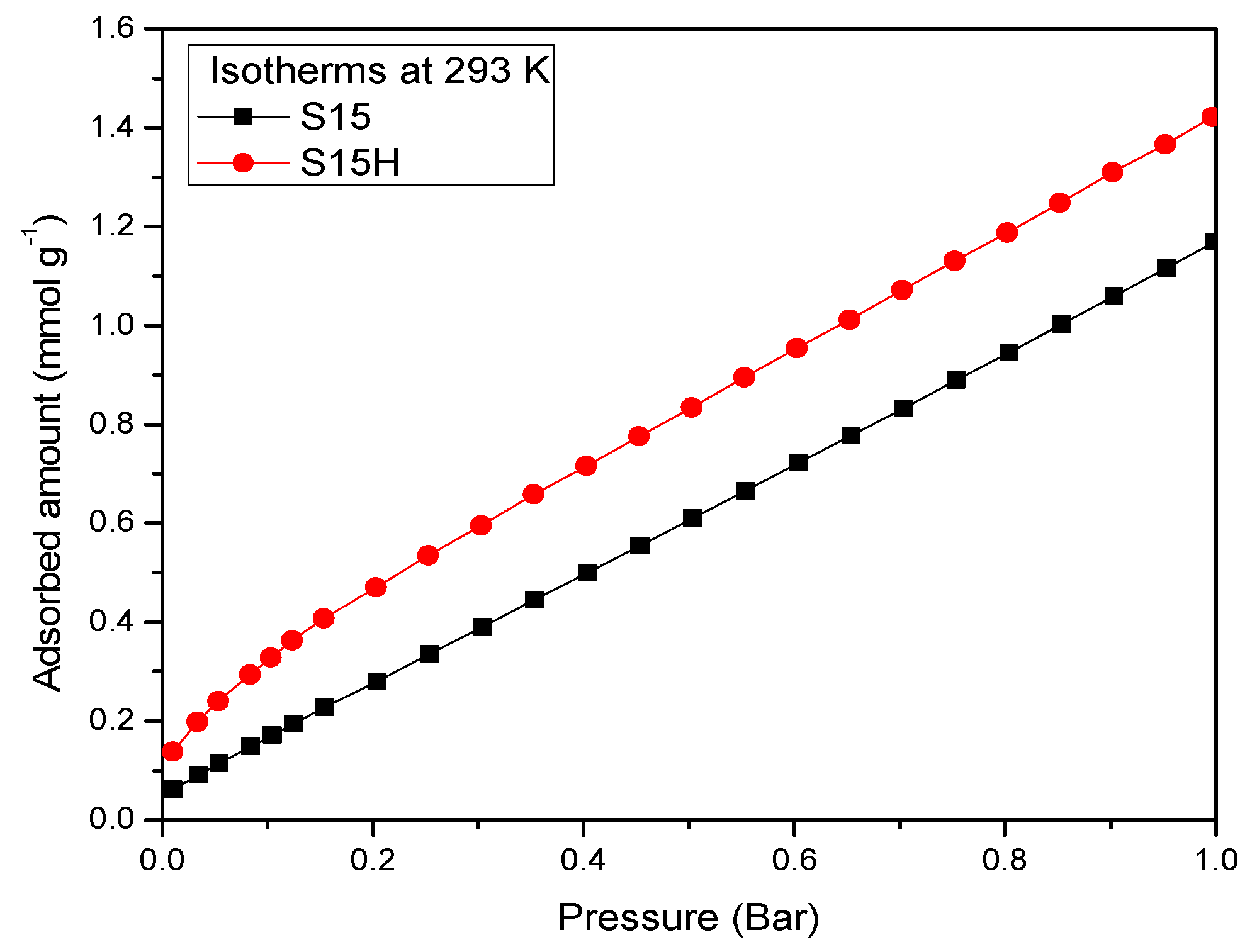
5.6. N2 Adsorption at 76 K
- (1)
- Compared to the precursor unhydroxylated substrate (S15), the presence of APTES has caused a decrease of the mean pore size in the S15HNH2 sample by 10.1%. Likewise, a decrease of 40.6% of the total pore volume. In turn, the S15H adsorbent depicted a pore size diminution of 13.2% and a reduction of 44.1% in the total pore volume. These facts suggest that more APTES molecules have been anchored on the hydroxylated specimen with respect to the unhydroxylated specimen.
- (2)
- Additionally, the surface area decreased by 10.4% with respect to its original magnitude (S15). As the textural analysis shows, the S15H material depicts no micropores, thus suggesting that practically all the APTES molecules reside in the interior of mesopores and that they are uniformly dispersed on the surface. However, the disappearance of micropores from the S15 solid indicates that the access to these voids is somewhat precluded by the APTES species, perhaps anchored at pore entrances.
5.7. NH3 Thermal Programmed Desorption from Silica Specimens (TPD-NH3)
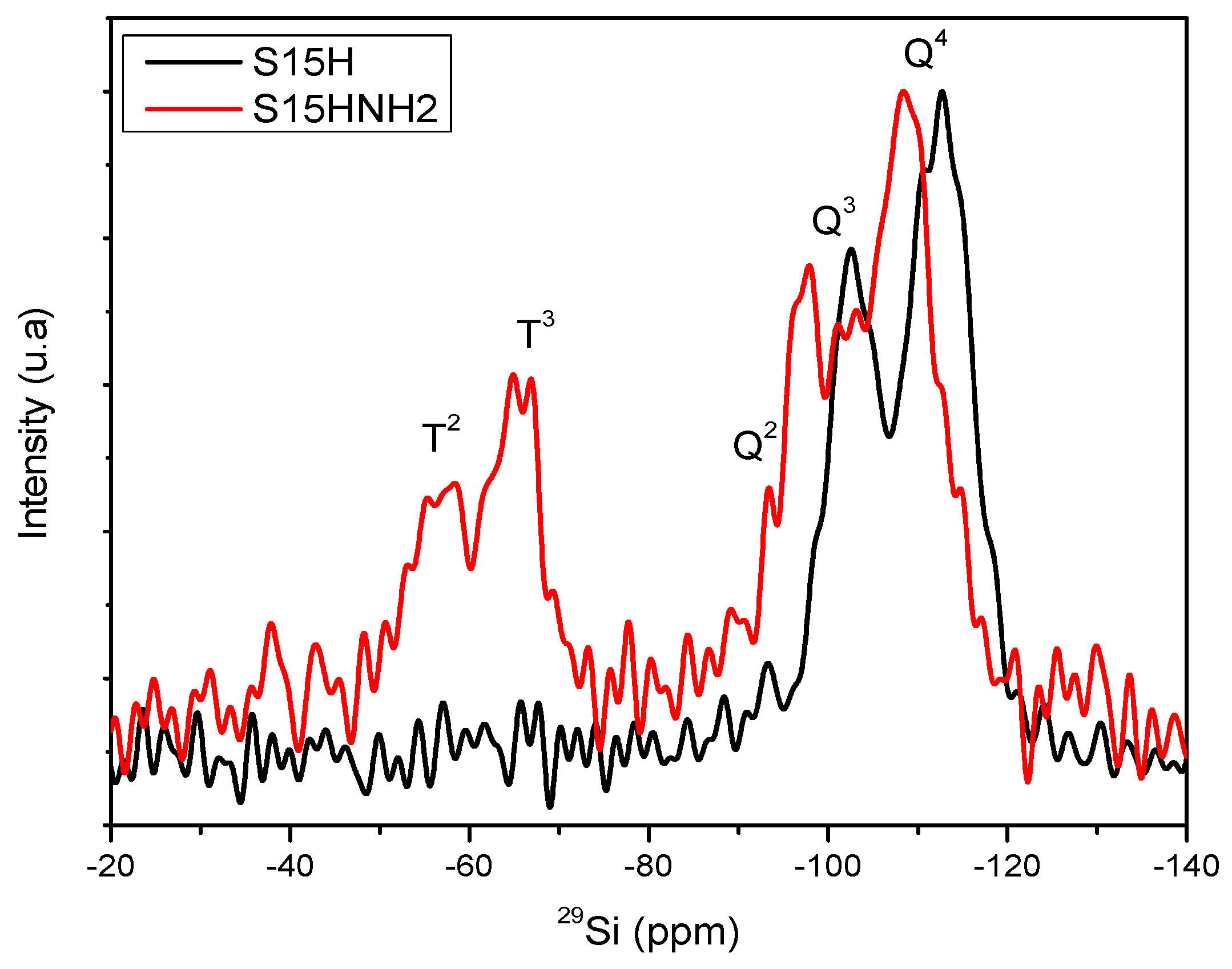
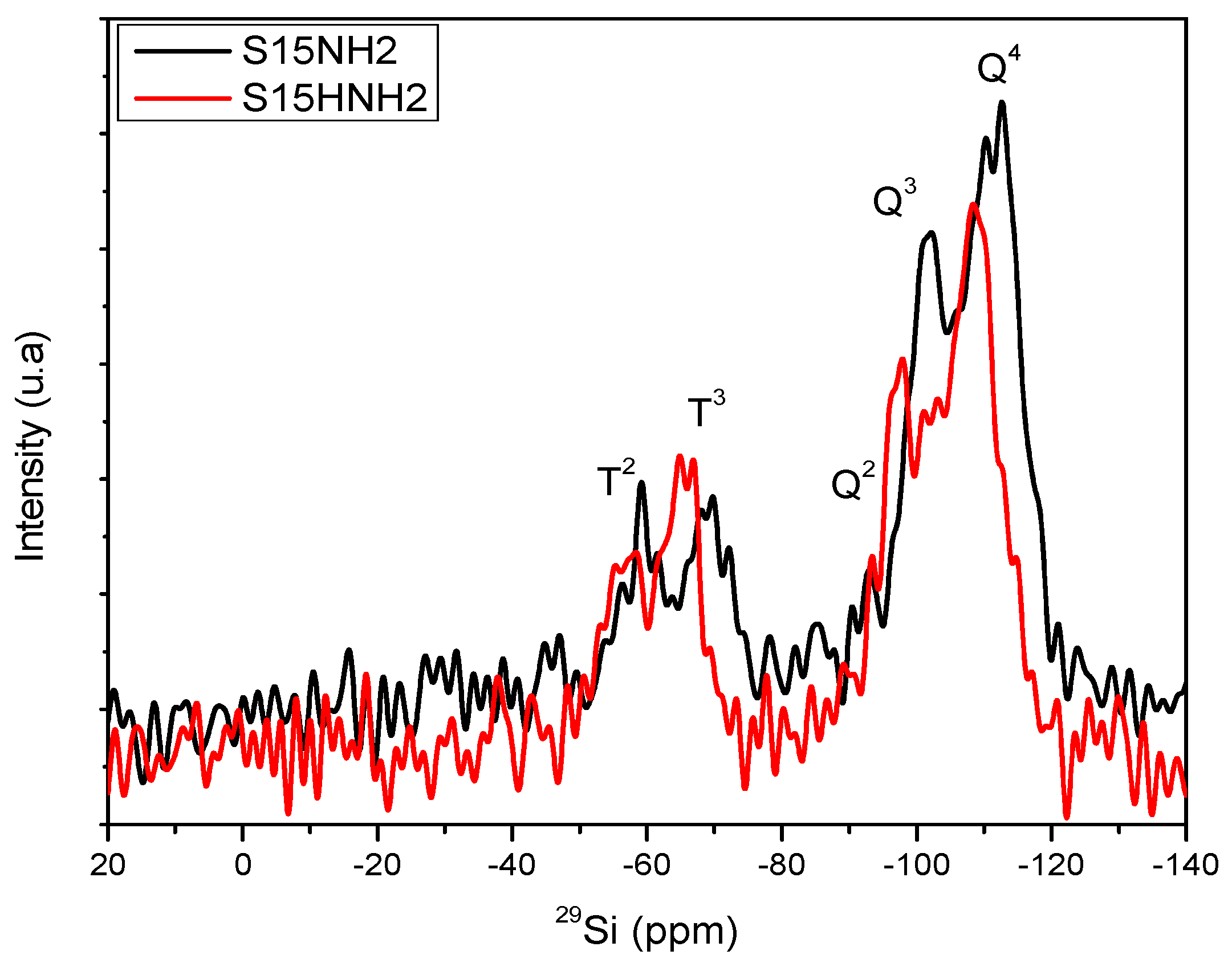
5.8. Nuclear Magnetic Resonance (NMR) Studies
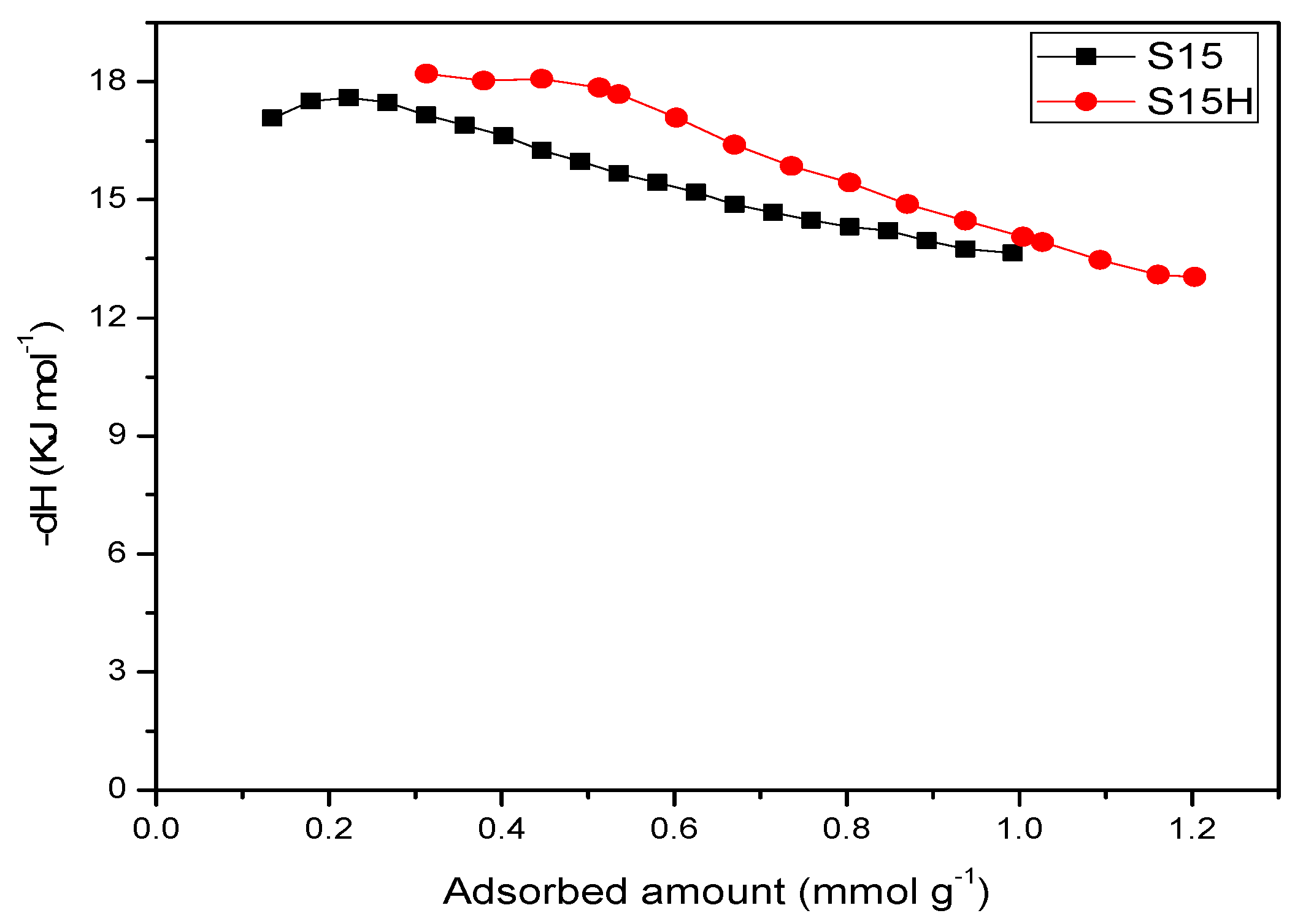
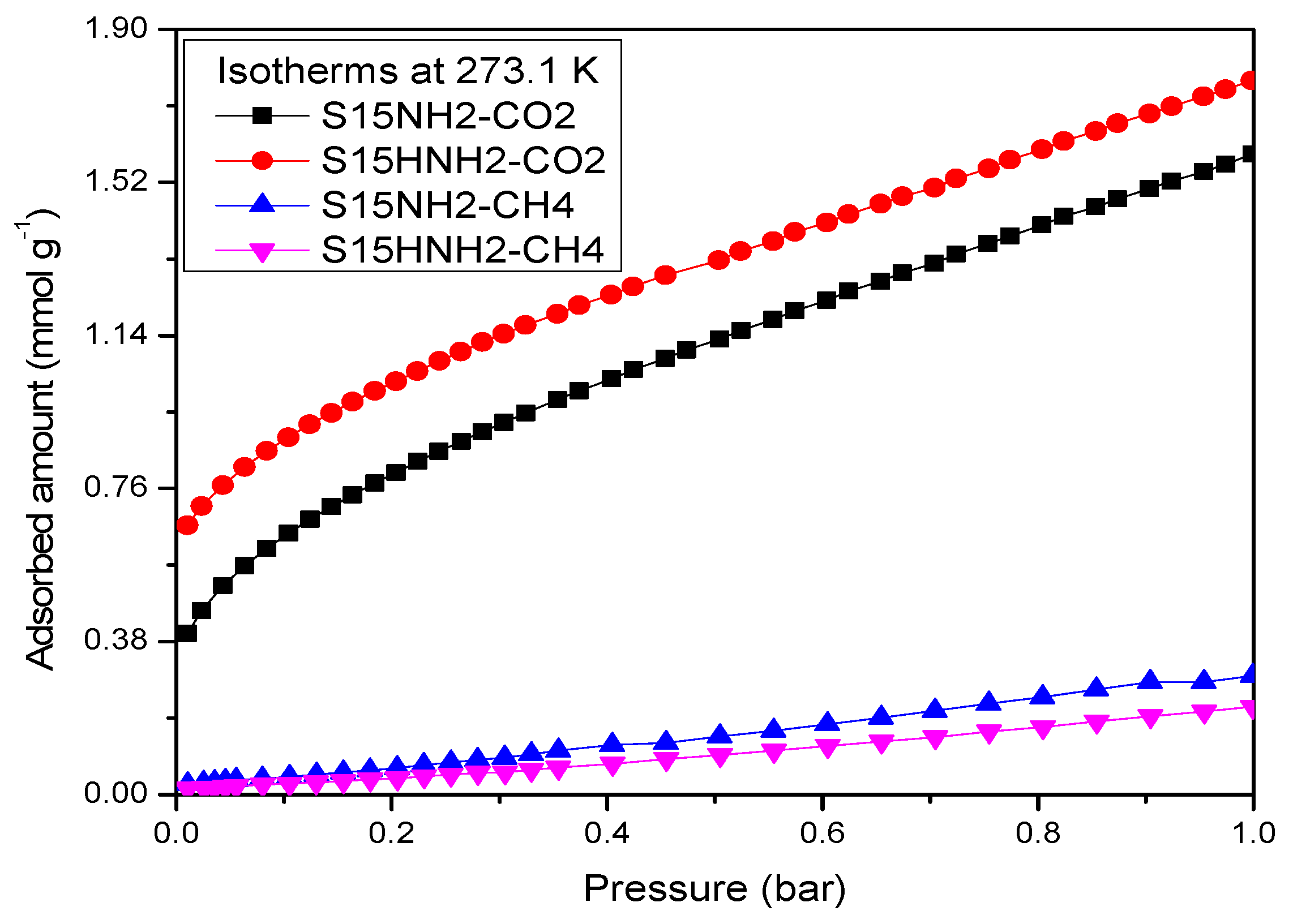
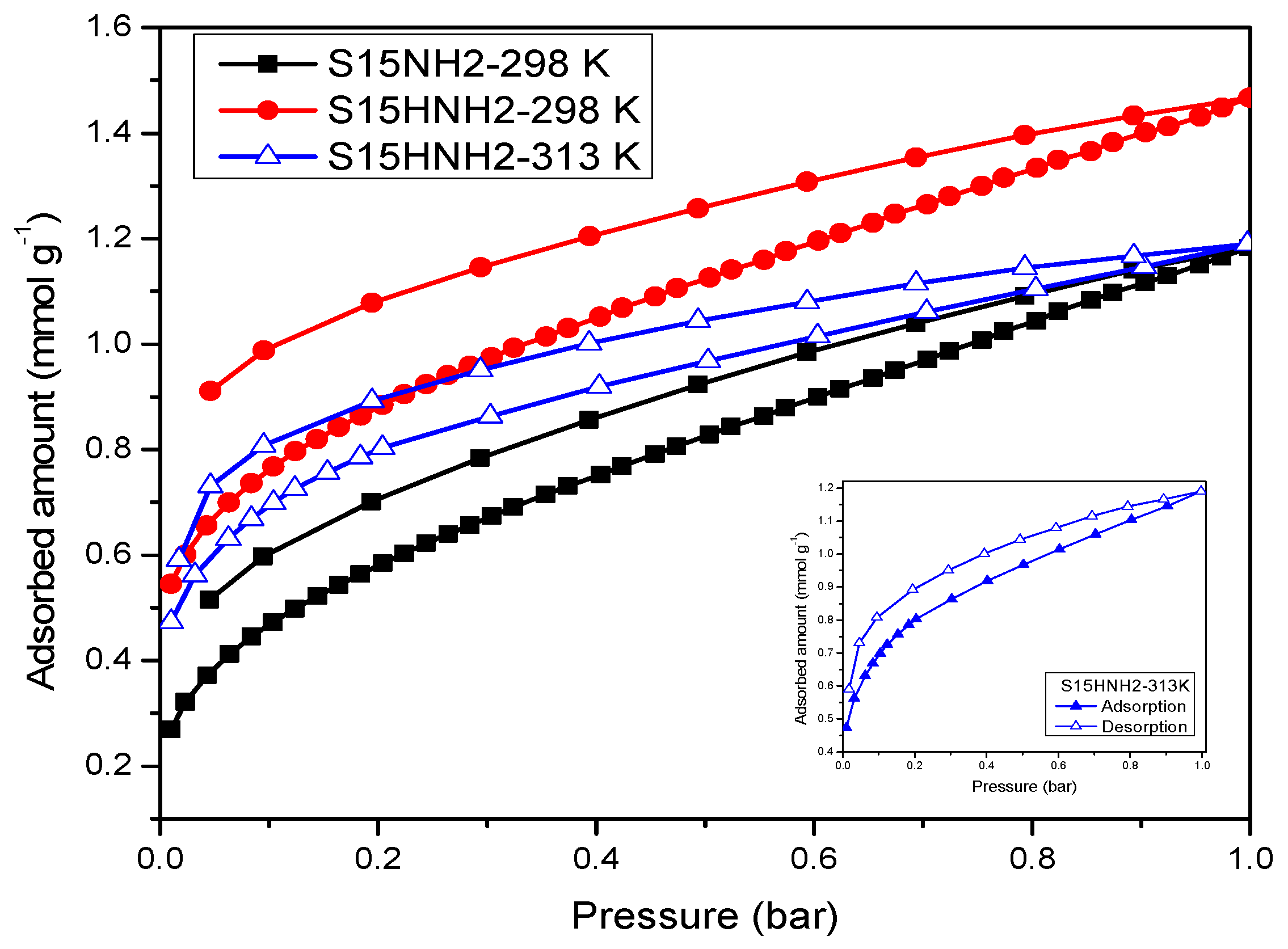
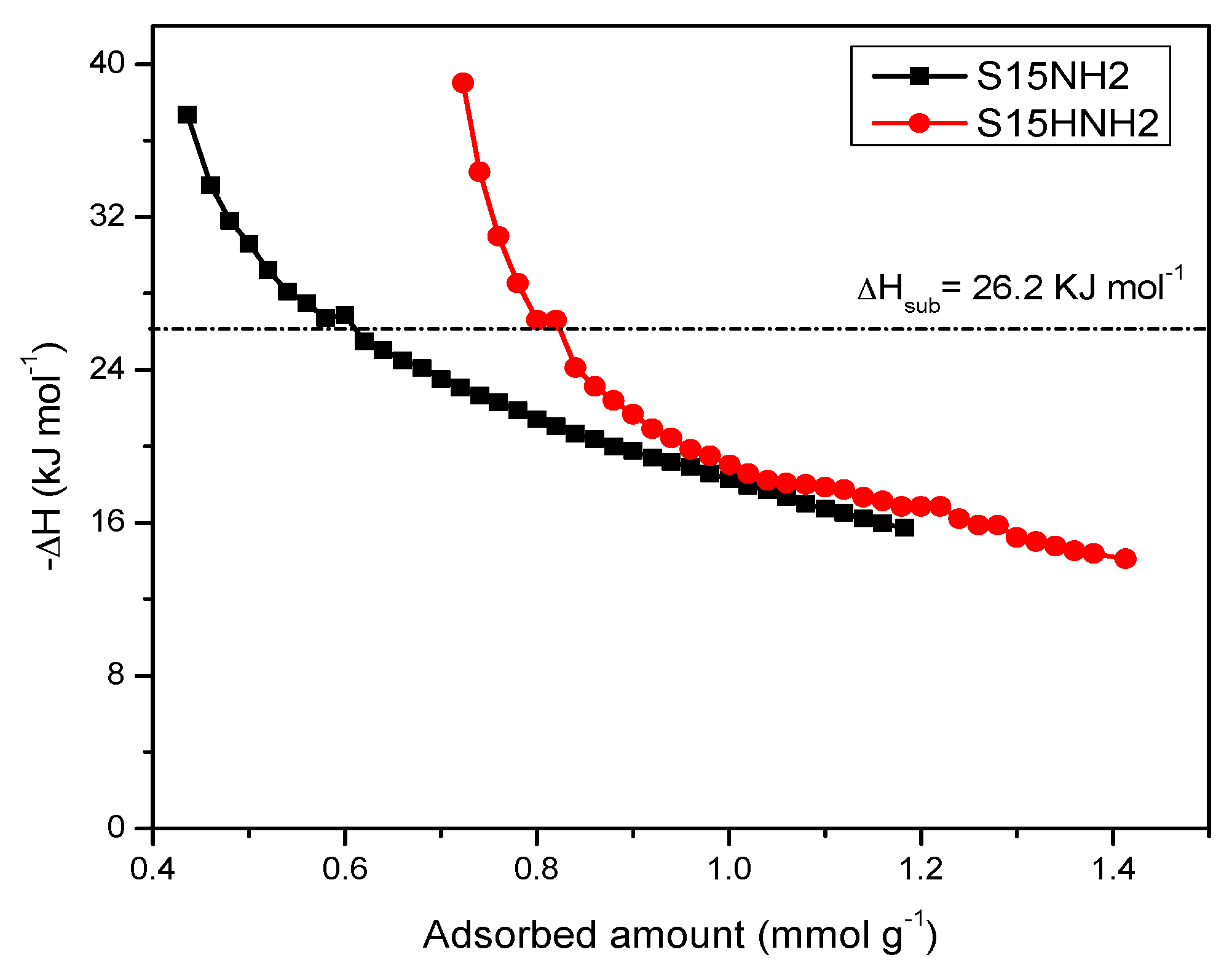
5.9. CO2 Adsorption Studies and Determination of the Isosteric Enthalpy of Adsorption
- (1)
- (2)
- (3)
- (4)
- The quantity of amine compound employed for the surface functionalization [35].
- (5)
- The functionalization method (e.g., impregnation, chemical anchorage or a combination of both). Olea et al. [56] have taken into account most of the above factors, including the exploration of different surface functionalization approaches of SBA-15 materials.
- (6)
- The experimental conditions selected for CO2 capture (e.g., temperature, pressure, CO2 flow rate, etc.).
- (a)
- The basic property of amino functionalized silica acts as a Lewis base that provides an electron pair to the CO2 molecule, i.e., a coordination link is formed. The adsorbed CO2 molecule then establishes additional coordination links with the hydrogen or oxygen atoms proceeding from adjacent APTES or with the hydroxyl groups of the silica substrate.
- (b)
- When the surface population of Si–OH species is increased via the hydroxylation treatment, the APTES molecules exist over larger domains of the pore structure, therefore a larger amount of CO2 molecules gets captured. This last process could be carried out by the zwitterionic reaction that renders ammonium carbonate after the reaction of amine and CO2 species [57]. The capture of CO2 by neighboring (i.e., more concentrated) amine species results to be more stable than if only an individual, isolated amine species reacts with CO2. A proton of one of the neighboring amines is transferred to the adjacent one; the former NH2 group attracts the carbon dioxide to it due to its refurbished electron density. This is a reflection of the assertion, since therein a considerable enthalpy of adsorption is attained by the amine-functionalized and pre-hydroxylated SBA-15 species (Figure 18).
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Post-combustion carbon dioxide capture: Evolution towards utilization of nanomaterials. Renew. Sustain. Energy Rev. 2012, 16, 2599–2609. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported Absorption of CO2 by Tetrabutylphosphonium Amino Acid Ionic Liquids. Chem. Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, D.; Mimeault, M.; Hausler, R. Determination of the Structural Features of Distinct Amines Important for the Absorption of CO2 and Regeneration in Aqueous Solution. Ind. Eng. Chem. Res. 2003, 42, 3179–3184. [Google Scholar] [CrossRef]
- Hart, A.; Gnanendran, N. Cryogenic CO2 Capture in Natural Gas. Energy Procedia 2008, 1, 697–706. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P. Adsorption of CO2 on Zeolites at Moderate Temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Kim, S.; Ida, J.; Guliants, V.V.; Lin, J.Y.S. Tailoring Pore Properties of MCM-48 Silica for Selective Adsorption of CO2. J. Phys. Chem. B 2005, 109, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Lu, C.; Kuo, S.C.; Zeng, W. Adsorption of CO2 on Amine-Functionalized Y-Type Zeolites. Energy Fuels 2010, 24, 1441–1448. [Google Scholar] [CrossRef]
- Hudson, M.R.; Queen, W.L.; Mason, J.A.; Fickel, D.W.; Lobo, R.F.; Brown, C.M. Unconventional, Highly Selective CO2 Adsorption in Zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Li, P.; Yu, J.G.; Cunha, A.F.; Rodrigues, A.E. K-Promoted Hydrotalcites for CO2 Capture in Sorption Enhanced Reactions. Chem. Eng. Technol. 2013, 36, 567–574. [Google Scholar] [CrossRef]
- Rezaei, F.; Lively, R.P.; Labreche, Y.; Chen, G.; Fan, Y.; Koros, W.J.; Jones, C.W. Aminosilane-Grafted Polymer/Silica Hollow Fiber Adsorbents for CO2 Capture from Flue Gas. Appl. Mater. Interfaces 2013, 5, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S.K. A comparative study of CO2 sorption properties for different oxides. Mater. Renew. Sustain. Energy 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Demuth, D.G.; Feng, P.; Gier, T.E.; Sieger, P.; Firouzi, A.; Chmelka, B.F.; Schuth, F.; et al. Organization of organic molecules with inorganic molecular species into nanocomposite biphase arrays. Chem. Mater. 1994, 6, 1176–1191. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Mccarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.; Balbuena, P.B.; Zhou, H. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Cavka, J.H.; Grande, C.A.; Mondino, G.; Blom, R. High Pressure Adsorption of CO2 and CH4 on Zr-MOFs. Ind. Eng. Chem. Res. 2014, 53, 15500–15507. [Google Scholar] [CrossRef]
- Xian, S.; Peng, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. Highly enhanced and weakened adsorption properties of two MOFs by water vapor for separation of CO2/CH4 and CO2/N2 binary mixtures. Chem. Eng. J. 2015, 270, 385–392. [Google Scholar] [CrossRef]
- Kato, M.; Yoshikawa, S.; Nakagawa, K. Carbon dioxide absorption by lithium orthosilicate in a wide range of temperature and carbon dioxide concentrations. J. Mater. Sci. Lett. 2002, 21, 485–487. [Google Scholar] [CrossRef]
- Subha, P.V.; Nair, B.N.; Hareesh, P.; Mohamed, A.P.; Yamaguchi, T.; Warrier, K.G.K.; Hareesh, U.S. Enhanced CO2 absorption kinetics in lithium silicate platelets synthesized by a sol-gel approach. J. Mater. Chem. 2014, 2, 12792–12798. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordoñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixes Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Naik, B.; Ghosh, N.N. A Review on Chemical Methodologies for Preparation of Mesoporous Silica and Alumina Based Materials. Recent Pat. Nanotechnol. 2009, 3, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Abelló, S.; Bonilla, A.; Groen, J.C. Tailored Mesoporosity Development in Zeolite Crystals by Partial Detemplation and Desilication. Adv. Funct. Mater. 2009, 19, 164–172. [Google Scholar] [CrossRef]
- Andrade, G.F.; Ferreira-Soares, D.C.; De Sousa Almeida, R.K.; Barros Sousa, E. Mesoporous Silica SBA-16 Functionalized with Alkoxysilane Groups: Preparation, Characterization, and Release Profile Study. J. Nanomater. 2012, 816496, 1–10. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Wovchko, E.A.; Camp, J.C.; Glass, J.A., Jr.; Yates, J.T. Active Sites on SiO2: Role in CH3OH Decomposition. Langmuir 1995, 11, 2592–2599. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol-gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Finch, J.A. Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles: I. Direct silanation. Appl. Surf. Sci. 1997, 120, 269–278. [Google Scholar] [CrossRef]
- Chong, M.A.S.M.; Zhao, X.S. Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Galeener, F.L. Band limits and the vibrational spectra of tetrahedral glasses. Phys. Rev. B 1979, 19, 4292. [Google Scholar] [CrossRef]
- Pasquarello, A.; Car, R. Identification of Raman Defect Lines as Signatures of Ring Structures in Vitreous Silica. Phys. Rev. Lett. 1998, 80, 5145–5147. [Google Scholar] [CrossRef]
- Galeener, F.L.; Barrio, R.A.; Martinez, E.; Elliott, R.J. Vibrational decoupling of rings in amorphous solids. Phys. Rev. Lett. 1984, 53, 2429–2432. [Google Scholar] [CrossRef]
- McMillan, P.; Remmele, R.L., Jr. Hydroxyl sites in SiO2 glass: A note on infrared and Raman spectra. Am. Miner. 1986, 71, 772–778. [Google Scholar]
- McMillan, P. Structural studies of silicate glasses and melts-applications and limitations of Raman Spectroscopy. Am. Miner. 1984, 69, 622–644. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica Zhuravlev model. Colloid Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Cao, L.; Man, T.; Kruk, M. Synthesis of Ultra-Large-Pore SBA-15 Silica with Two Dimensional Hexagonal Structure Using Triisopropylbenzene as Micelle Expander. Chem. Mater. 2009, 21, 1144–1153. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moacou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Nassa, E.J.; Messaddeq, Y.; Ribeiro, S.J.L. Base and acid catalysis in the preparation of functionalized silica obtain by sol-gel method. Quim. Nova 2002, 25, 27–31. [Google Scholar]
- Chong, A.S.M.; Zhao, X.S.; Kustedjo, A.T.; Qiao, S.Z. Functionalization of large-pore mesoporous silicas with organosilanes by direct synthesis. Microporous Mesoporous Mater. 2004, 72, 33–42. [Google Scholar] [CrossRef]
- Hernández-Huesca, R.; Aguilar-Armenta, G.; Domínguez, G. Isosteric Heats of Adsorption of N2O and NO on Natural Zeolites. J. Mex. Chem. Soc. 2010, 54, 111–116. [Google Scholar]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 Uptake and Adsorption Kinetics of Pore-Expanded SBA-15 Double-Functionalized with Amino Groups. Energy Fuels 2013, 27, 7637–7644. [Google Scholar] [CrossRef]
- Brunauer, S. The Adsorption of Gases and Vapors; Princeton University Press: Princeton, NJ, USA, 1943; Volume 1, pp. 397–408. [Google Scholar]
- Ojeda-López, R.; Pérez-Hermosillo, I.J.; Esparza-Schulz, J.M.; Cervantes-Uribe, A.; Domínguez-Ortiz, A. SBA-15 materials: Calcination temperature influence on textural properties and total silanol ratio. Adsorption 2015, 21, 659–669. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing Selective CO2 Adsorption on Amine-Grafted SBA-15 by Increasing Silanol Density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Yokoi, T.; Yoshitakeb, H.; Tatsumi, T. Synthesis of amino-functionalized MCM-41 via direct co-condensation and post-synthesis grafting methods using mono-, di- and tri-amino organoalkoxysilanes. J. Mater. Chem. 2004, 14, 951–957. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Yoo, J.; Pruski, M.; Lin, V.S.Y. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. J. Phys. Chem. C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Olea, A.; Sanz-Pérez, E.S.; Arencibia, A.; Sanz, R.; Calleja, G. Amino-functionalized pore-expanded SBA-15 for CO2 adsorption. Adsorption 2013, 19, 589–600. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashim, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
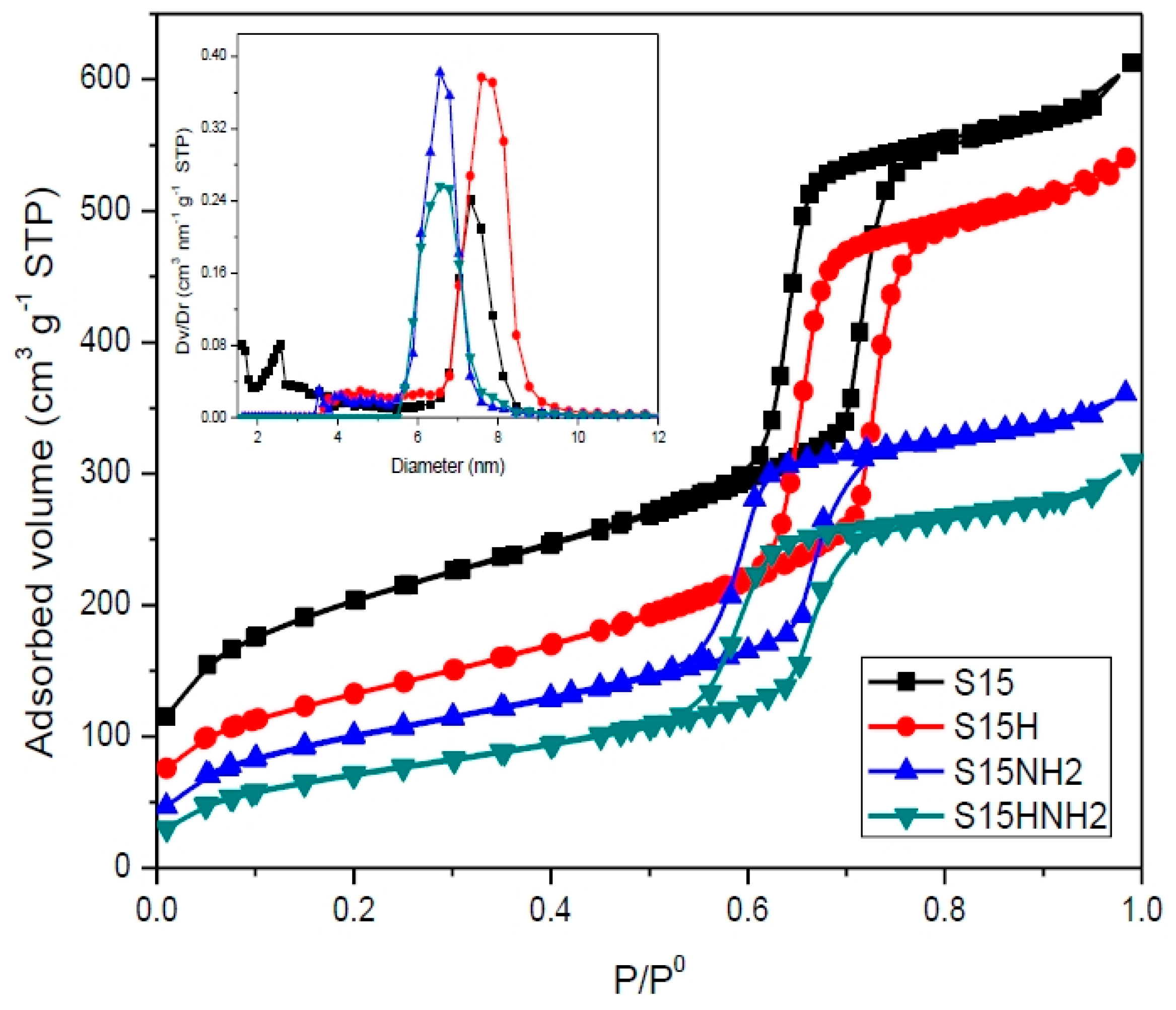
| Sample | ABET (m2·g−1) | Vt (cm3·g−1) | Aext (m2·g−1) | Amic (m2·g−1) | Dmode (nm) |
|---|---|---|---|---|---|
| S15 | 696.4 | 0.899 | 456.4 | 240.0 | 7.30 |
| S15H | 466.9 | 0.818 | 439.7 | 27.2 | 7.59 |
| S15NH2 | 360.9 | 0.534 | 335.8 | 25.1 | 6.56 |
| S15HNH2 | 260.9 | 0.441 | 260.9 | 0.0 | 6.56 |
| Material | % Q2 Area | % Q3 Area | % Q4 Area | % T2 Area | % T3 Area |
|---|---|---|---|---|---|
| S15 | 5.29 | 29.72 | 64.99 | - | - |
| S15H | 2.87 | 35.28 | 61.85 | - | - |
| S15NH2 | 0.16 | 32.84 | 48.75 | 7.91 | 10.34 |
| S15HNH2 | 5.30 | 21.25 | 45.00 | 13.54 | 14.90 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Juárez, O.; García-Sánchez, M.Á.; Arellano-Sánchez, U.; Kornhauser-Straus, I.; Rojas-González, F. Optimal Surface Amino-Functionalization Following Thermo-Alkaline Treatment of Nanostructured Silica Adsorbents for Enhanced CO2 Adsorption. Materials 2016, 9, 898. https://doi.org/10.3390/ma9110898
Medina-Juárez O, García-Sánchez MÁ, Arellano-Sánchez U, Kornhauser-Straus I, Rojas-González F. Optimal Surface Amino-Functionalization Following Thermo-Alkaline Treatment of Nanostructured Silica Adsorbents for Enhanced CO2 Adsorption. Materials. 2016; 9(11):898. https://doi.org/10.3390/ma9110898
Chicago/Turabian StyleMedina-Juárez, Obdulia, Miguel Ángel García-Sánchez, Ulises Arellano-Sánchez, Isaac Kornhauser-Straus, and Fernando Rojas-González. 2016. "Optimal Surface Amino-Functionalization Following Thermo-Alkaline Treatment of Nanostructured Silica Adsorbents for Enhanced CO2 Adsorption" Materials 9, no. 11: 898. https://doi.org/10.3390/ma9110898