A Study on a Novel Phase Change Material Panel Based on Tetradecanol/Lauric Acid/Expanded Perlite/Aluminium Powder for Building Heat Storage
Abstract
:1. Introduction
2. Experimental
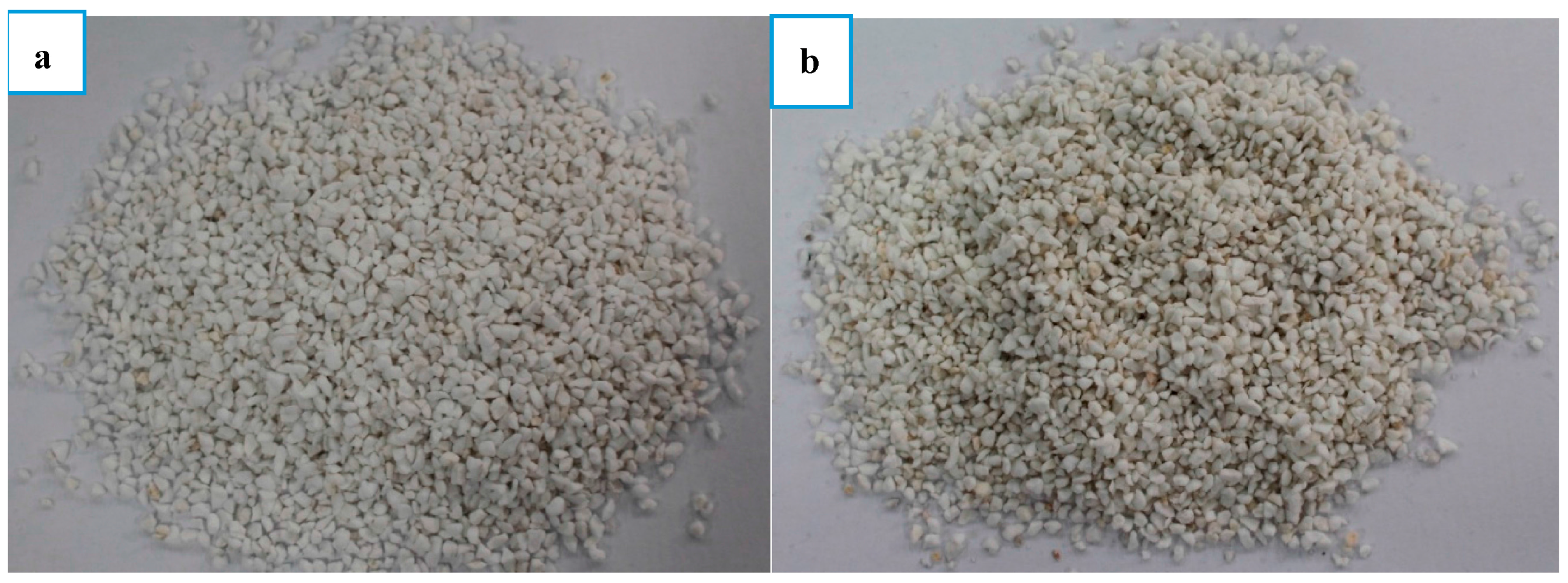
2.1. Materials
2.2. Preparation of Novel Composite PCM
2.2.1. Binary Eutectic Mixture
2.2.2. Novel PCMP
- (1)
- TD and LA with a mass proportion of 53.60%:46.40% were mixed, melted and stirred in a vessel that was heated by the water bath for 30 min under 60 °C, and then the melting TD-LA mixture was poured into a feeding funnel in the top of a filtering flask, the inlet valve of which remained closed.
- (2)
- EP was placed into the filtering flask, in which the internal space was maintained to be vacuum state and the inside air of EP’s porous structure was evacuated by a circulating water vacuum pump.
- (3)
- After the inlet valve opened, the liquid of TD-LA flowed into the feeding funnel and mixed with EP through magnetic stirring. Because of the strong capillary action under vacuum state, the liquid TD-LA can be absorbed into the micropores of EP. The mass fraction ratio of TD-LA vs. EP was 50%:50%.
- (4)
- The vacuum process was continued for 2 h at vacuum pressure of 65 kPa, and the mixture of TD-LA and EP in the filtering flask was heated by water bath, so as to maintain TD-LA melting in the vacuum process. Due to achieving the full penetration of TD-LA into EP, the air was allowed into the filtering flask to force the liquid PCM to further penetrate into the micropore structures of EP, when finishing the vacuum process.
- (5)
- The low thermal conductivity of TD, LA and EP possibly leads to melting-solidifying circulation incompletion [35]. In order to enhance the thermal conduction of TD-LA/EP, AP was added and uniformly mixed with TD-LA/EP through stirrer (Figure 2b). Then, the styrene-acrylic emulsion was also put into the stirrer to form the film attached on the TD-LA/EP-AP surface.
- (6)
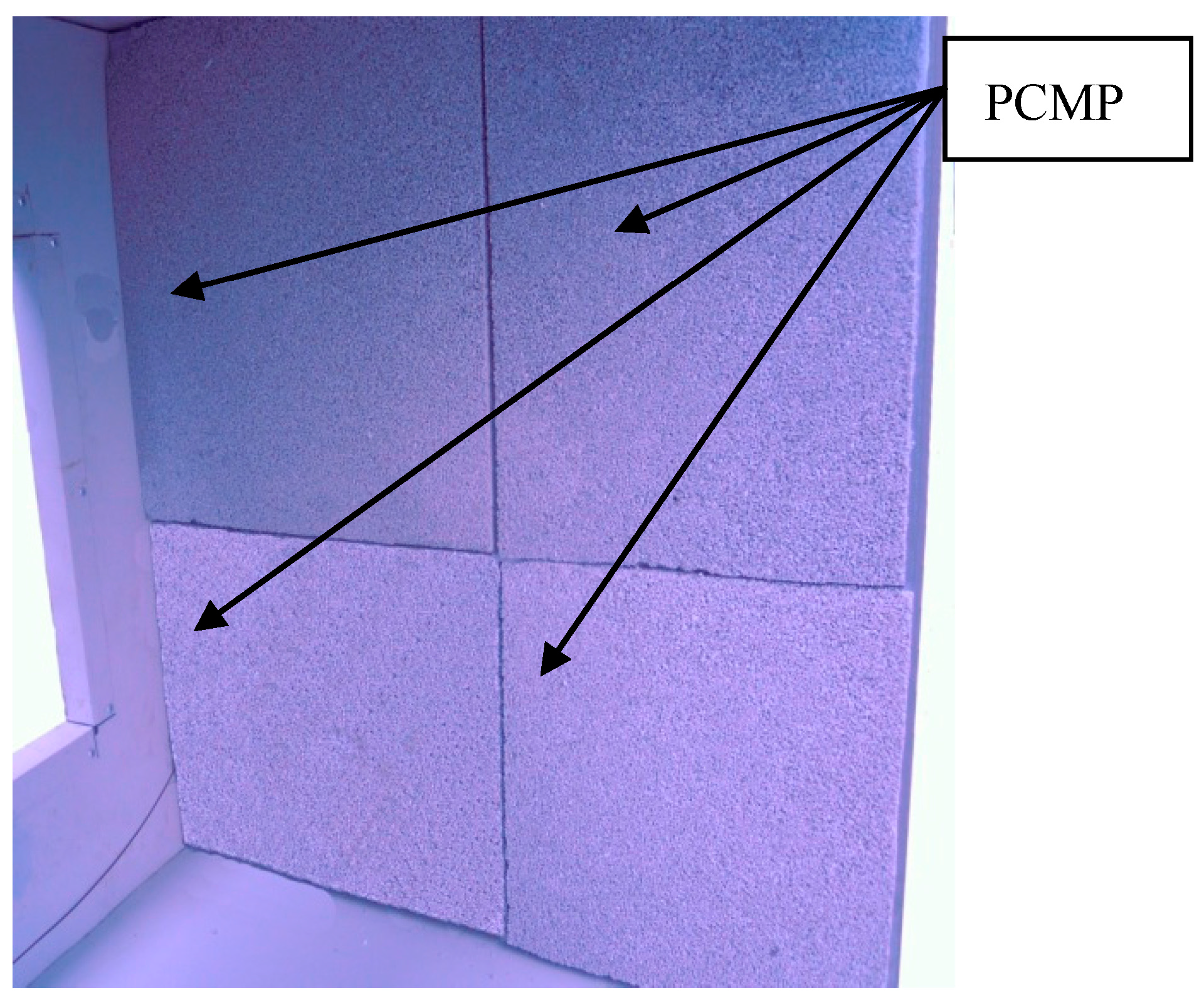
- The styrene-acrylic emulsion was also the binding element for the PCMP. When finishing the stirring process, the composite TD-LA/EP-AP with styrene-acrylic emulsion was compacted to the novel PCMP through the square-shape mould, as shown in Figure 2c. When the novel PCMP was completely dry, the mould was removed. Finally, the novel PCMP has been prepared well, as shown in Figure 3.
2.3. Property Test Methods
2.4. Thermal Performance Test
3. Results and Discussion
3.1. Thermal Property Analysis
3.2. Construction Characterization and Mechanical Property Analysis
3.3. Thermal Conductivity Improvement Analysis
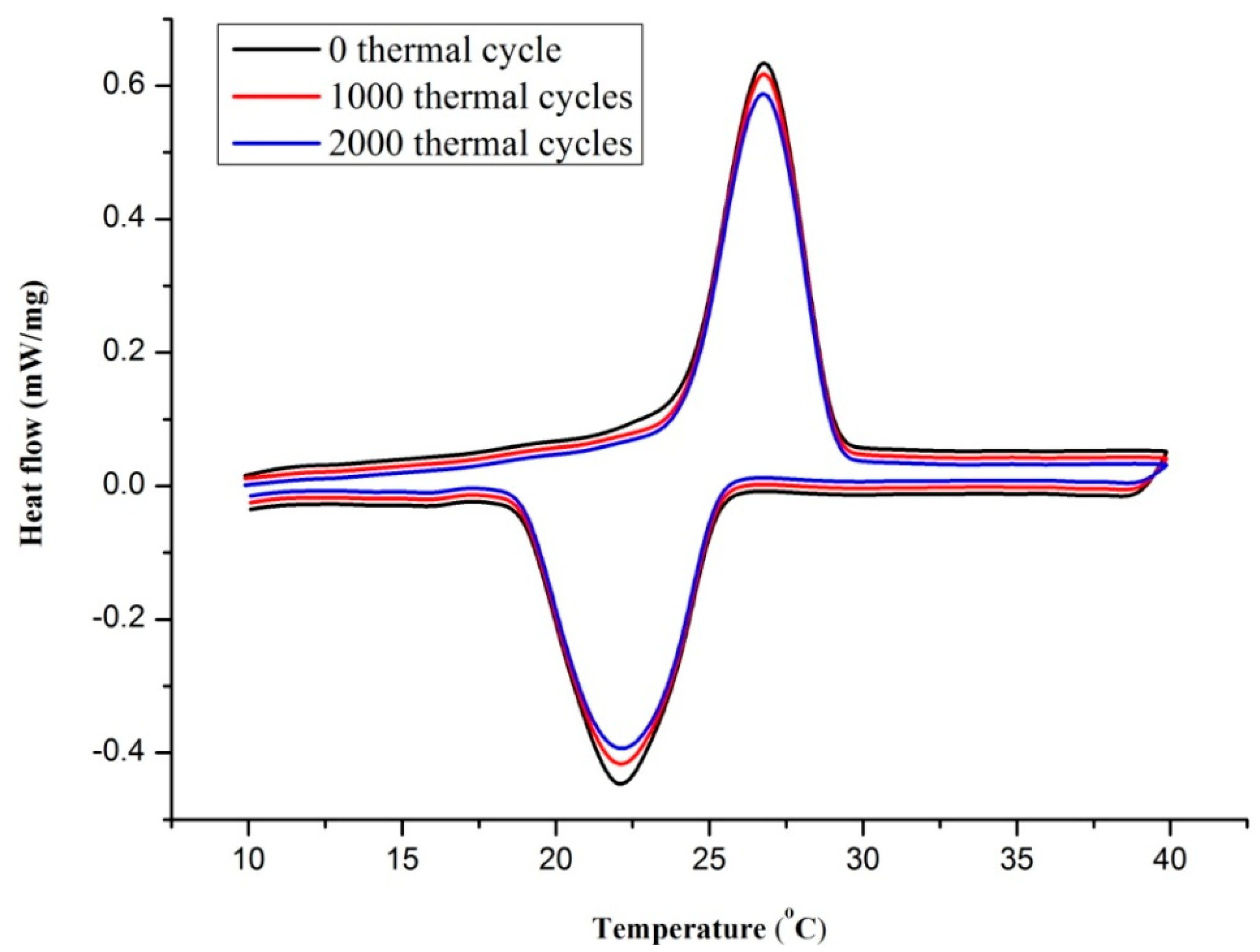
3.4. Thermal Reliability Analysis
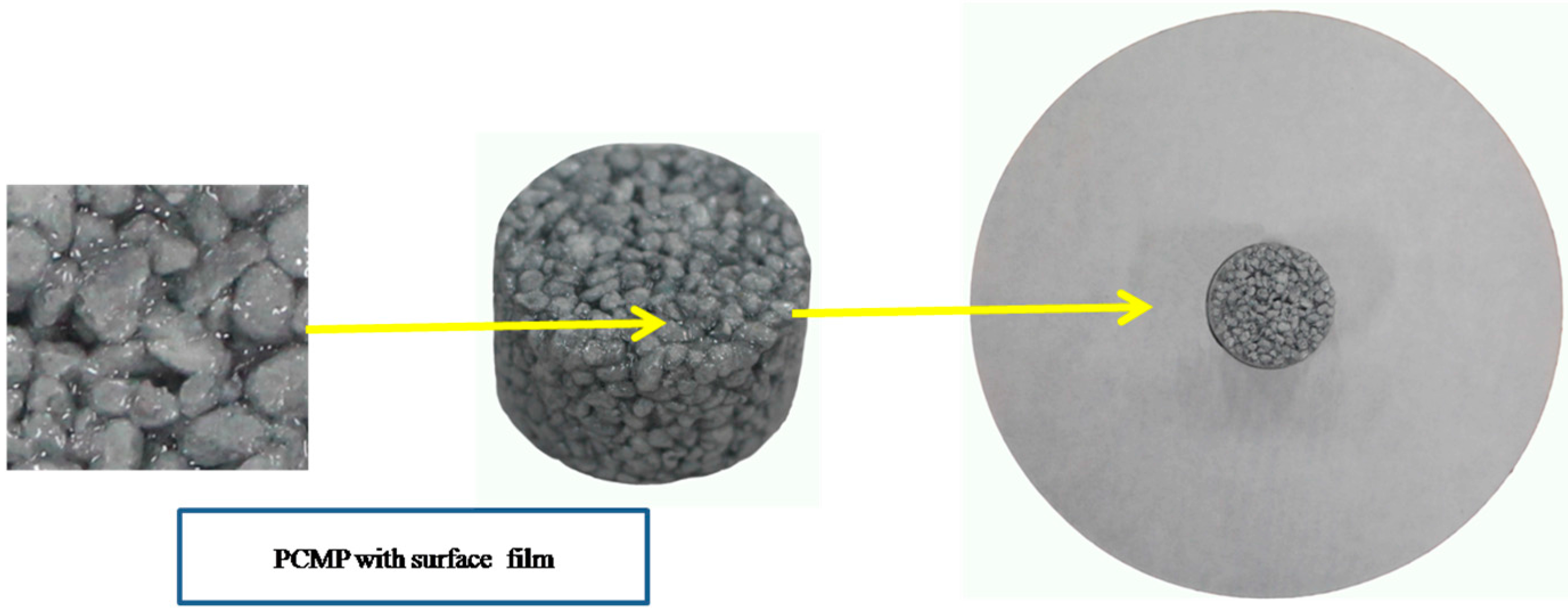
3.5. Leakage Analysis
3.6. Thermal Performance Analysis
- (1)
- In day time, when the indoor temperature is higher than the melting temperature of TD-LA/EP-5 wt % AP, PCMP started to absorb the heat from inside cell to restrain the indoor temperature rise. The peak temperature and mean inside temperatures of PCM cell were 6.96 °C and 4.10 °C lower than that of reference room in daytime (6:00–18:00), respectively. On the first day, a relative smooth temperature section emerged at noon. Temperatures in this smooth section were much higher than the melting point of PCMP, indicating the latent heat storage of PCMP was possibly finished, and the sensible heat storage of PCMP played a leading role in this section. Similarly, the peak temperature decreasing may also mainly result from the sensible heat storage of PCMP. Therefore, the heat storage of PCMP included sensible heat storage and latent heat storage, and they simultaneously effected in the inside temperature decreasing. The ratio of latent/sensible heat storage will be studied in detail in the future.
- (2)
- For night time (18:00–6:00), because of ambient temperature decreasing and without solar radiation, the indoor temperature was reduced, and PCMP began to release the heat stored during daytime if the indoor temperature was lower than the freezing point of PCM. Therefore, mean inside temperature of PCM cell was 2.43 °C higher than that of reference cell. In other words, the heat absorbing and releasing by prepared PCMP can effectively reduce the indoor temperature fluctuation.
- (3)
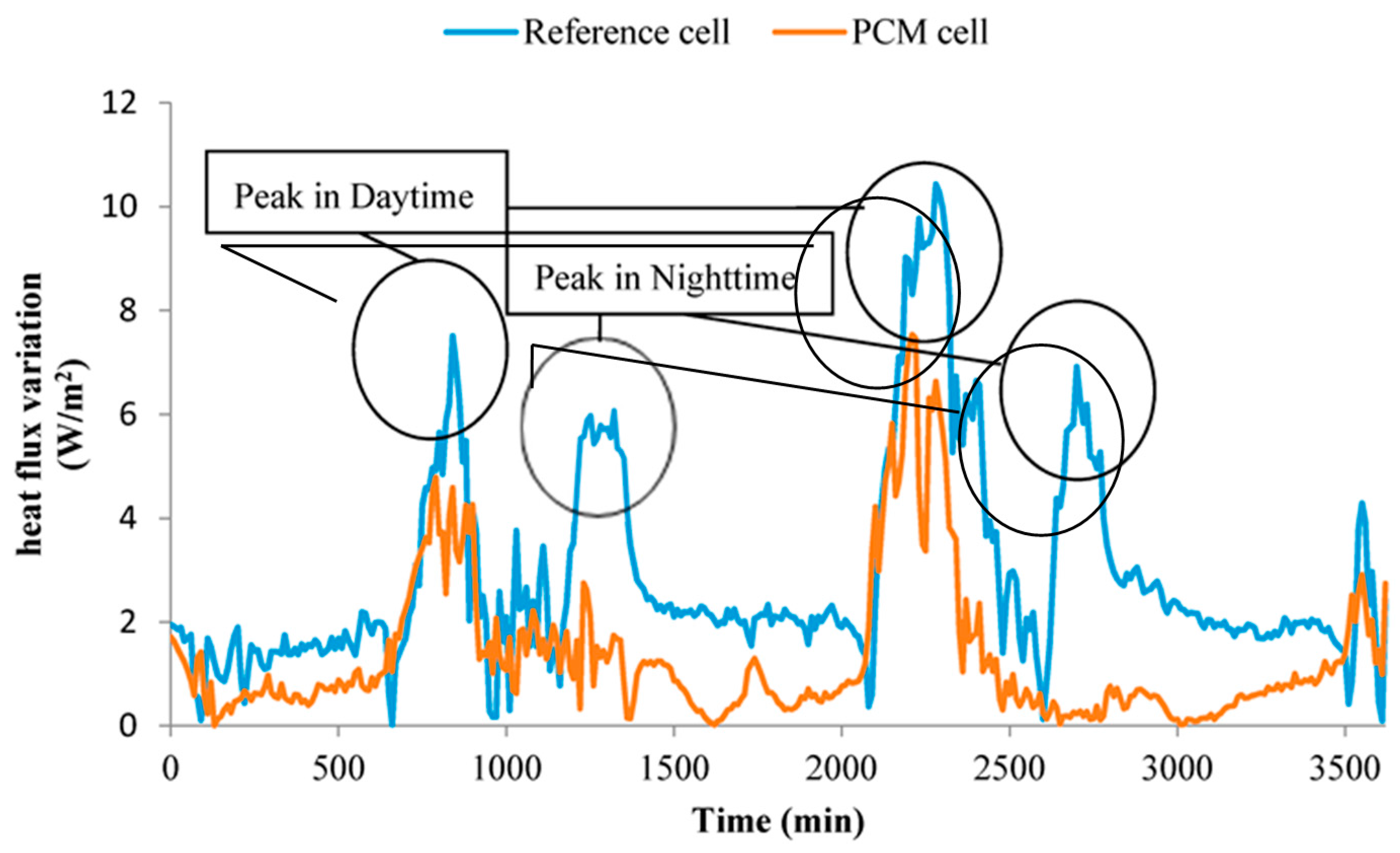
- The heat flux variations of PCM cell wall and reference cell wall are shown in Figure 15, in which the heat flux value indicated the heat exchange capacity between cells and external environment. As listed in Table 9, the peak and mean heat fluxes of PCM cell were 2.81 W/m2 and 1.64 W/m2 smaller than that of reference cell, respectively. Besides, the fluctuation of heat flux values of PCM cell were smaller than that of reference cell during all the experiment period. The small heat flux value and range have showed that the prepared PCMP can reduce the influence of external disturbance on the inside thermal environment.
4. Conclusions
- (1)
- TD-LA/EP has melting/freezing temperature and latent heat of 24.9 °C/25.2 °C and 78.2 J/g/81.3 J/g, respectively, which are suitable for building heat storage.
- (2)
- With the mass ratio of thermal conductivity promoter of AP increasing, the thermal conductivity value has been enhanced linearly, but the latent heat was linearly decreased. It seems that an AP mass ratio range of 5–15 wt % was reasonable to obtain a high thermal conductivity and while maintain a large latent heat.
- (3)
- The leakage test using diffusion–effusion circle method indicated that the film attached on the surface of composite PCM particles was an effective solution for the leaking issue of composite PCMs.
- (4)
- The results of mechanical test and thermal cycling test demonstrated that the fabricated PCMP have good mechanical property and thermal reliability for building application.
- (5)
- The thermal performance test has been conducted through two cells, PCM cell and reference cell, with lightweight envelope (CSSIB) under the same outdoor climatic condition during 2.5 days. Compared with the result of reference cell in thermal performance test, the cell with PCMP was on average 4.10 °C cooler during day time and 2.43 °C warmer during night time, and had lower heat flux value. It also was found that both the latent and sensible heat storage capacities of PCMP functioned in indoor temperature decreasing. Because was PCMP installed in PCM cell, the two cells had different thermal transmittance and thermal mass. Thus, a qualitative thermal performance of PCMP was obtained at present stage, and an in-depth contrastive study for PCMP performance under the same thermal transmittance and thermal mass in building envelopes will be carried out in the next stage.
- (6)
- Based on all results, it can also be concluded that the developed novel PCMP can be used as building heat storage panel in the wall inside to diminish the building energy consumption and adjust the indoor comfort.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsinghua University. Annual Report on China Building Energy Efficiency; China Architecture & Building Press: Beijing, China, 2015. [Google Scholar]
- Zhang, Y.; He, C.Q.; Tang, B.J.; Wei, Y.M. China’s energy consumption in the building sector: A life cycle approach. Energy Build. 2015, 94, 240–251. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Li, Y.; Huang, J.; Liu, S. Numerical study on the thermal performance of building wall and roof incorporating phase change material panel for passive cooling application. Energy Build. 2014, 81, 404–415. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellon, C.; Nogues, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Cruceru, M. Novel concept of composite phase change material wall system for year-round thermal energy savings. Energy Build. 2010, 42, 1759–1772. [Google Scholar] [CrossRef]
- Serrano, S.; Barreneche, C.; Navarro, A.; Haurie, L.; Fernandez, A.I.; Cabeza, L.F. Use of multi-layered PCM gypsums to improve fire response. Physical, thermal and mechanical characterization. Energy Build. 2016, 127, 1–9. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Huang, J.; Cai, Z.; Wei, S. Experimental research on the use of phase change materials in perforated brick rooms for cooling storage. Energy Build. 2013, 62, 597–604. [Google Scholar] [CrossRef]
- Bastani, A.; Haghighat, F.; Manzano, C.J. Investigating the Effect of Control Strategy on the Shift of Energy Consumption in a Building Integrated with PCM Wallboard. Energy Proced. 2015, 78, 2280–2285. [Google Scholar] [CrossRef]
- El Omari, K.; Le Guer, Y.; Bruel, P. Analysis of micro-dispersed PCM-composite boards behavior in a building’s wall for different seasons. J. Build. Eng. 2016, 7, 361–371. [Google Scholar] [CrossRef]
- Chung, M.H.; Park, J.C. Development of PCM cool roof system to control urban heat island considering temperate climatic conditions. Energy Build. 2016, 116, 341–348. [Google Scholar] [CrossRef]
- Alawadhi, E.M.; Alqallaf, H.J. Building roof with conical holes containing PCM to reduce the cooling load: Numerical study. Energy Conversi. Manag. 2011, 52, 2958–2964. [Google Scholar] [CrossRef]
- Roman, K.K.; O’Brien, T.; Alvey, J.B.; Woo, O. Simulating the effects of cool roof and PCM (phase change materials) based roof to mitigate UHI (urban heat island) in prominent US cities. Energy 2016, 96, 103–117. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Ge, X. Predication of the melting temperature and the fusion heat of (Quasi-) eutectic PCM. J. China Univ. Min. Technol. 1995, 25, 474–478. [Google Scholar]
- Shilei, L.; Neng, Z.; Guohui, F. Impact of phase change wall room on indoor thermal environment in winter. Energy Build. 2006, 38, 18–24. [Google Scholar] [CrossRef]
- Shilei, L.; Neng, Z.; Guohui, F. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar] [CrossRef]
- Shilei, L.; Guohui, F.; Neng, Z.; Li, D. Experimental study and evaluation of latent heat storage in phase change materials wallboards. Energy Build. 2007, 39, 1088–1091. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation, thermal properties and thermal reliability of eutectic mixtures of fatty acids/expanded vermiculite as novel form-stable composites for energy storage. J. Ind. Eng. Chem. 2010, 16, 767–773. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.; Zhang, N.; Du, Y.; Cao, X. Preparation and thermal characterization of capric–myristic–palmitic acid/expanded graphite composite as phase change material for energy storage. Mater. Lett. 2014, 125, 154–157. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation and characterization of fatty acid ester/building material composites for thermal energy storage in buildings. Energy Build. 2011, 43, 1952–1959. [Google Scholar] [CrossRef]
- Sarı, A. Fabrication and thermal characterization of kaolin-based composite phase change materials for latent heat storage in buildings. Energy Build. 2015, 96, 193–200. [Google Scholar] [CrossRef]
- Jiao, C.; Ji, B.; Fang, D. Preparation and properties of lauric acid–stearic acid/expanded perlite composite as phase change materials for thermal energy storage. Mater. Lett. 2012, 67, 352–354. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Song, X.; Hou, H.; Yang, Z.; Zhu, J. Preparation and properties of gypsum based energy storage materials with capric acid–palmitic acid/expanded perlite composite PCM. Energy Build. 2015, 92, 155–160. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, X.; Sanjayan, J.G. Development of thermal energy storage composites and prevention of PCM leakage. Appl. Energy 2014, 135, 225–233. [Google Scholar] [CrossRef]
- Li, X.; Sanjayan, J.G.; Wilson, J.L. Fabrication and stability of form-stable diatomite/paraffin phase change material composites. Energy Build. 2014, 76, 284–294. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Evola, G.; Marletta, L.; Sicurella, F. A methodology for investigating the effectiveness of PCM wallboards for summer thermal comfort in buildings. Build. Environ. 2013, 59, 517–527. [Google Scholar] [CrossRef]
- Huang, J.; Lu, S.; Kong, X.; Liu, S. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: Preparation and performance analysis. Materials 2013, 6, 4758–4775. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Baran, G.; Sari, A. Phase change and heat transfer characteristics of a eutectic mixture of palmitic and stearic acids as PCM in a latent heat storage system. Energy Convers. Manag. 2003, 44, 3227–3246. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Kim, S.; Drzal, L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z. The study of evaluation methodology on the leakage performance of PCM used in buildings. Insul. Mater. Build. Energy Sav. 2004, 7, 43–46. [Google Scholar]
- Zheng, Y. Energy Consumption Research of Double Skin Facade with Presence of Solar Radiation. Master’s Thesis, Zhejiang University, Hangzhou, China, 9 March 2015. [Google Scholar]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building Energy Storage Panel Based on Paraffin/Expanded Perlite: Preparation and Thermal Performance Study. Materials 2016, 9, 70. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using β-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Sarı, A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials. Energy Convers. Manag. 2016, 117, 132–141. [Google Scholar] [CrossRef]
- Memon, S.A.; Lo, T.Y.; Cui, H.; Barbhuiya, S. Preparation, characterization and thermal properties of dodecanol/cement as novel form-stable composite phase change material. Energy Build. 2013, 66, 697–705. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A.; Kaygusuz, K. Capric acid and stearic acid mixture impregnated with gypsum wallboard for low-temperature latent heat thermal energy storage. Int. J. Energy Res. 2008, 32, 154–160. [Google Scholar] [CrossRef]
- He, H.; Zhao, P.; Yue, Q.; Gao, B.; Yue, D.; Li, Q. A novel polynary fatty acid/sludge ceramsite composite phase change materials and its applications in building energy conservation. Renew. Energy 2015, 76, 45–52. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Development and thermal performance of pumice/organic PCM/gypsum composite plasters for thermal energy storage in buildings. Sol. Energy Mater. Sol. Cells 2016, 149, 19–28. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A. Preparation and thermal energy storage properties of building material-based composites as novel form-stable PCMs. Energy Build. 2012, 51, 73–83. [Google Scholar] [CrossRef]
- Zhu, Y.X. Built Environment; China Architecture and Building Press: Beijing, China, 2005. [Google Scholar]









| Constituent | SiO2 | Al2O3 | K2O | Na2O | MgO | CaO |
|---|---|---|---|---|---|---|
| Mass percent (wt %) | 73 | 18 | 4.3 | 4.1 | 0.4 | 0.2 |
| PCM | Melting Point (°C) | Mass Percentage in Binary Eutectic Mixture (%) | |
|---|---|---|---|
| Measured Value | Predicted Value | ||
| TD | 37.00 | – | 53.60 |
| LA | 43.20 | – | 46.40 |
| TD-LA | 24.2 | 25.8 | 100 |
| Item | Reference Cell | PCMP Cell | ||||||
|---|---|---|---|---|---|---|---|---|
| Wall | Ceiling | Floor | Window | Wall | Ceiling | Floor | Window | |
| Structure | CSSIB | CSSIB | CSSIB | Single glazing Aluminium alloy | CSSIB + PCMP | CSSIB + PCMP | CSSIB | Single glazing Aluminium alloy |
| Heat transfer coefficient (W/m2·K) | 0.763 | 0.763 | 0.763 | 6.4 | 0.645 | 0.645 | 0.763 | 6.4 |
| Thickness (mm) | 50 | 50 | 50 | 6 | 0.645 | 0.645 | 50 | 6 |
| Reflectivity (%) | – | – | – | 89 | – | – | – | 89 |
| Transmittance (%) | – | – | – | 8 | – | – | – | 8 |
| PCM | Phase Change Point (°C) | Latent Heat (J/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Melting | Freezing | Melting | Freezing | Calculating | |||||
| Onset | Peak | End | Onset | Peak | End | ||||
| TD-LA | 24.2 | 26.5 | 28.8 | 24.8 | 22 | 19.9 | 162.7 | 165.3 | – |
| TD-LA/EP | 24.9 | 26.9 | 29 | 25.2 | 22.3 | 20.1 | 78.2 | 81.3 | 81.4 |
| CHR (%) | 2.89% | 1.51% | 0.69% | 1.61% | 1.36% | 1.01% | – | – | – |
| Item | Curing Time (Days) | Compressive Strength (N/m2) | Reference |
|---|---|---|---|
| Paraffin/expanded perlite/cement mortar | 7 | 4.04 | [29] |
| 28 | 7.53 | ||
| PCM powder/cement mortar | 7 | 3.62 | [27] |
| 28 | 7.16 | ||
| Paraffin/expanded perlite | – | 4.61 | [39] |
| TD-LA/EP-AP | 3 | 2.09 | This study |
| 7 | 4.21 | ||
| 15 | 4.23 |
| Item | Melting Point (°C) | Freezing Point (°C) | Latent Heat of Melting (J/g) | Latent Heat of Freezing (J/g) | Reference |
|---|---|---|---|---|---|
| Bentonite/dodecanol/5 wt % EG | 22.16 | 21.05 | 57.84 | 55.45 | [42] |
| Bentonite/heptadecane/5 wt % EG | 22.09 | 21.53 | 34.05 | 32.43 | [42] |
| Dodecanol/cement | 21.6 | – | 18.39 | – | [43] |
| Capric–stearic acid/gypsum | 23.8 | 23.9 | 49 | – | [44] |
| Stearic–palmitic–myristic–lauric acid/sludge ceramsite | 26.6 | – | 47.1 | – | [45] |
| Pumice/capric–palmitic acid | 23.13 | 21.65 | 56.45 | 55.4 | [46] |
| Pumice/heptadecane | 22.18 | 21.14 | 72.38 | 70.24 | [46] |
| Pumice/dodecanol | 23.27 | 20.98 | 67.32 | 66.48 | [46] |
| TD-LA/EP-5 wt % AP | 24.5 | 25.4 | 77.78 | 79.02 | This study |
| TD-LA/EP-10 wt % AP | 24.7 | 25.6 | 74.22 | 73.89 | This study |
| TD-LA/EP-15 wt % AP | 24.3 | 24.9 | 69.15 | 69.75 | This study |
| PCMP Samples | Sample 1# | Sample 2# | Sample 3# | Sample 4# | Sample 5# | Sample 6# | Sample 7# | Sample 8# | Sample 9# |
|---|---|---|---|---|---|---|---|---|---|
| AP percentage (%) | 0 | 2.5 | 5 | 7.5 | 10 | 12.5 | 15 | 17.5 | 20 |
| Heat transfer coefficient (W/m2·K) | 2.38 | 2.42 | 2.59 | 2.68 | 2.84 | 2.98 | 3.05 | 3.11 | 3.24 |
| PCM | Number of the Cycle | Melting Onset Point (°C) | Freezing Onset Point (°C) | Melting Latent Heat (J/g) | Freezing Latent Heat (J/g) |
|---|---|---|---|---|---|
| TD-LA/EP-5 wt % AP | 0 cycle | 24.5 | 25.4 | 77.78 | 79.02 |
| 1000 cycles | 24.9 | 25 | 75.63 | 77.89 | |
| 2000 cycles | 25.1 | 24.7 | 74.39 | 75.92 |
| Item | PCM Cell | Reference Cell |
|---|---|---|
| Peak temperature (°C) | 36.85 | 43.81 |
| Mean temperature of day time (°C) | 28.11 | 32.21 |
| Mean temperature of night time (°C) | 16.3 | 13.87 |
| Peak heat flux (W/m2) | 6.16 | 8.97 |
| Mean heat flux (W/m2) | 1.44 | 3.08 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Kong, X.; Rong, X.; Yao, C.; Yang, H.; Qi, C. A Study on a Novel Phase Change Material Panel Based on Tetradecanol/Lauric Acid/Expanded Perlite/Aluminium Powder for Building Heat Storage. Materials 2016, 9, 896. https://doi.org/10.3390/ma9110896
Wang E, Kong X, Rong X, Yao C, Yang H, Qi C. A Study on a Novel Phase Change Material Panel Based on Tetradecanol/Lauric Acid/Expanded Perlite/Aluminium Powder for Building Heat Storage. Materials. 2016; 9(11):896. https://doi.org/10.3390/ma9110896
Chicago/Turabian StyleWang, Enyu, Xiangfei Kong, Xian Rong, Chengqiang Yao, Hua Yang, and Chengying Qi. 2016. "A Study on a Novel Phase Change Material Panel Based on Tetradecanol/Lauric Acid/Expanded Perlite/Aluminium Powder for Building Heat Storage" Materials 9, no. 11: 896. https://doi.org/10.3390/ma9110896






