Grain Refinement Efficiency in Commercial-Purity Aluminum Influenced by the Addition of Al-4Ti Master Alloys with Varying TiAl3 Particles
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
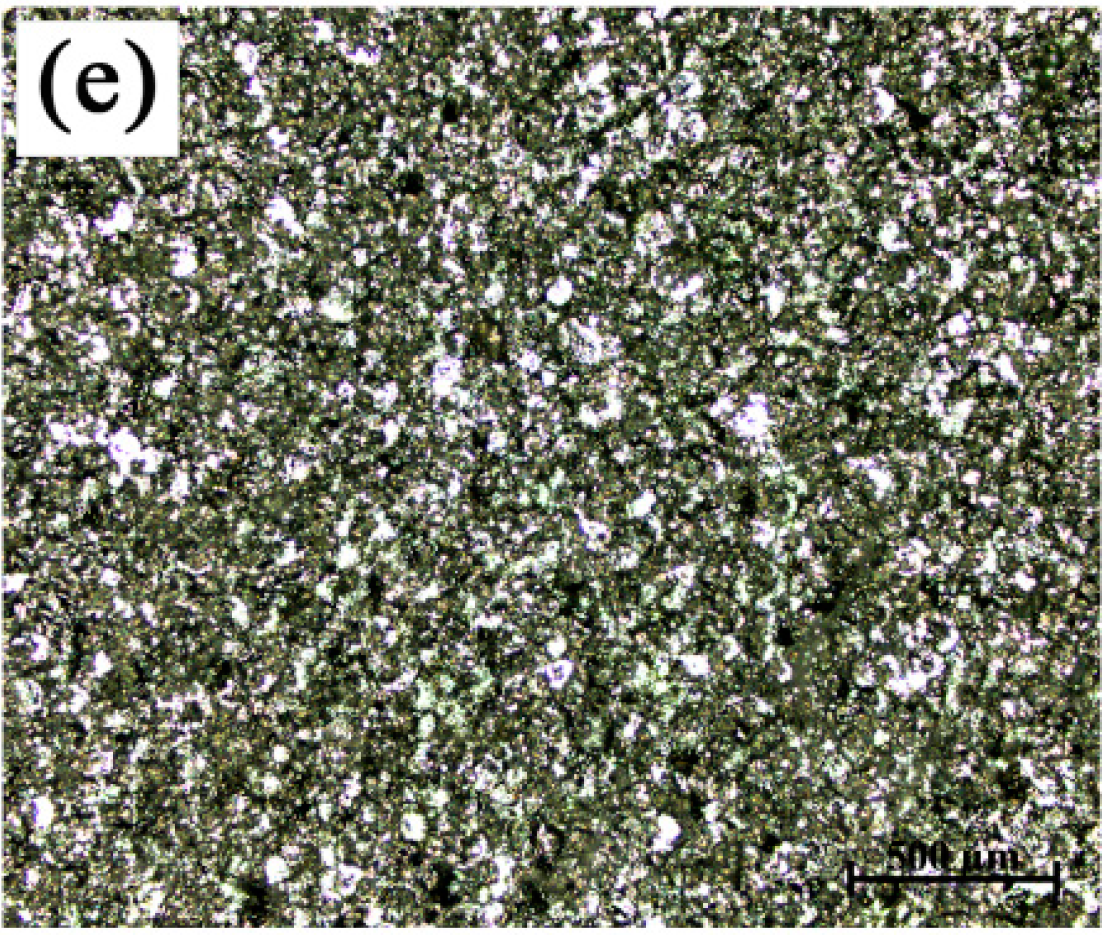
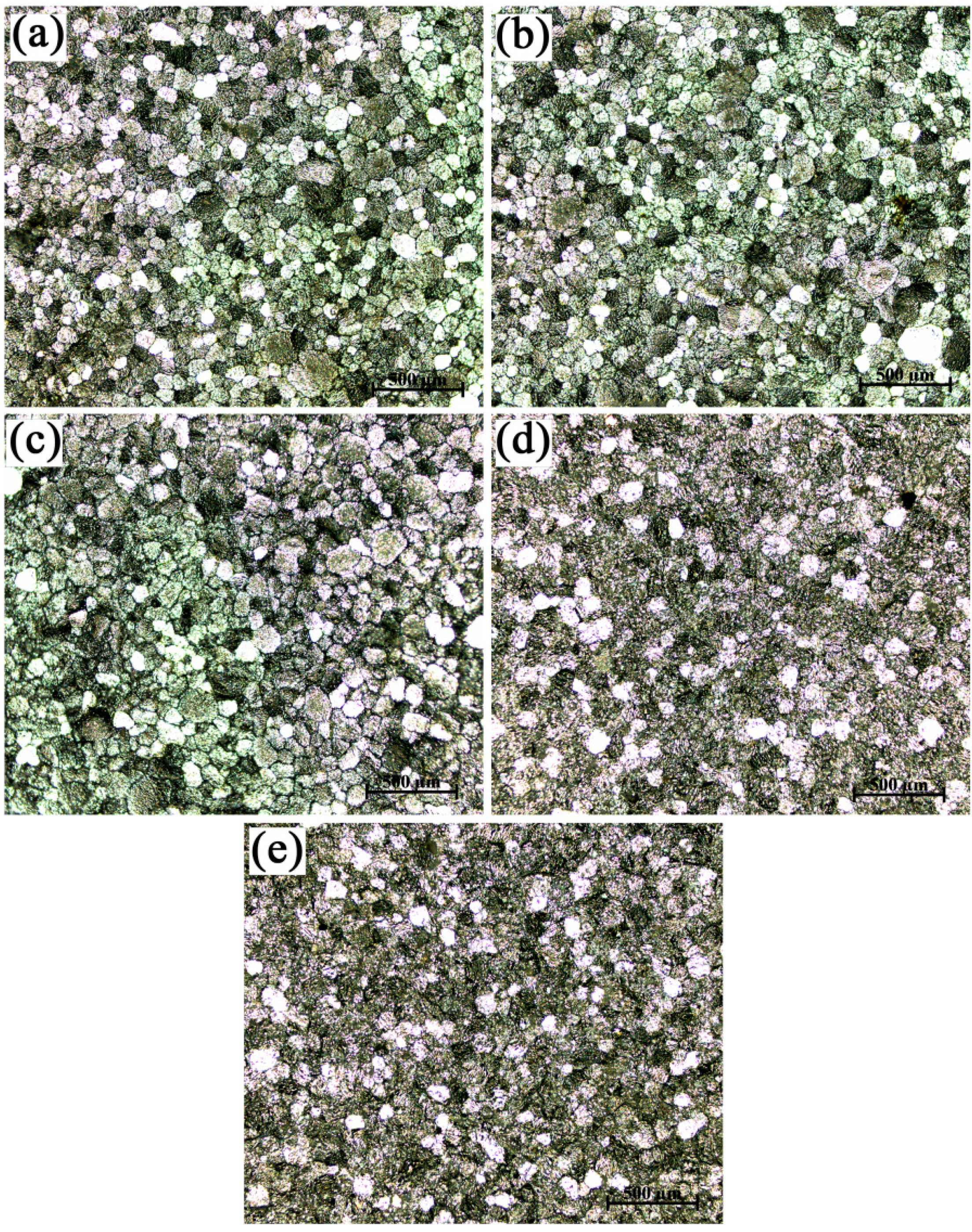
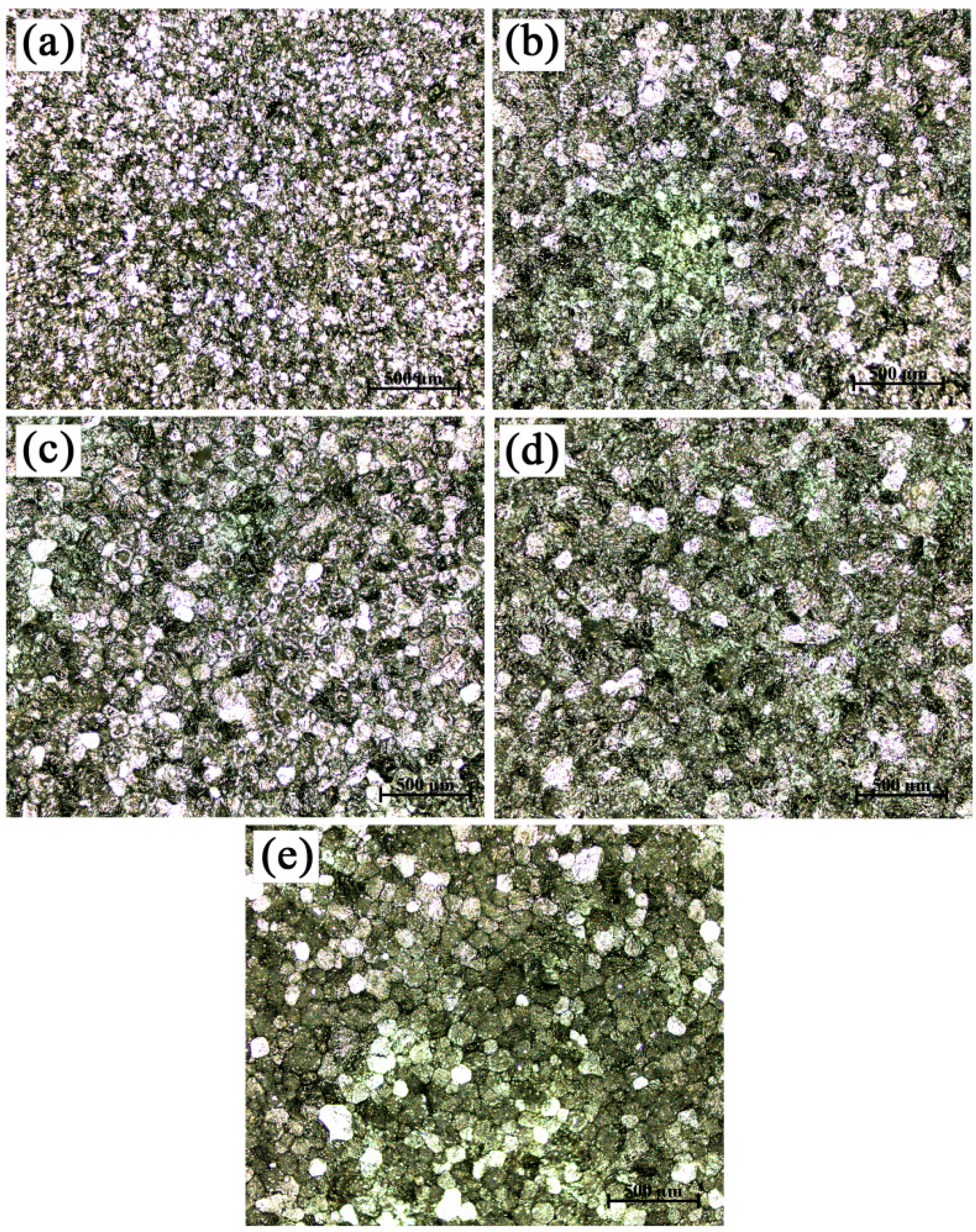
3.1. Microstructures and Morphologies
3.2. Refinement Performance of Al-4Ti Master Alloys
4. Conclusions
- (i)
- There were three different types of TiAl3 particles in Al-4Ti master alloys: petal-like structures, blocky structures, and flaky structures. The petal-like TiAl3 particles were only formed according to the growth mechanism of repeated twins when the aluminum melt augmented with K2TiF6 was casted into the graphite mold. Whether the K2TiF6 or sponge titanium was added into the aluminum melt which was cast into the sand mold, the flaky TiAl3 particles were generated. In addition, the blocky TiAl3 particles were found after the remaining three different processes.
- (ii)
- The grains in commercial-purity aluminum after grain refinement of Al-4Ti master alloys showed a hereditary effect on grain size. The larger the average grain sizes of the original TiAl3 particles were, the larger that of the refined commercial-purity aluminum was.
- (iii)
- With the increase in the hold time, the grain size of the commercial-purity aluminum refined by the Al-4Ti master alloy with petal-like TiAl3 particles increased, at first, and then decreased rapidly. The grain refinement efficiency of the Al-4Ti master alloy with blocky TiAl3 particles was the best and did not fade away with the change of the hold time. The Al-4Ti master alloy with flaky TiAl3 particles could instantly achieve a fine grain refinement under a hold time of five minutes; however, the fine refinement efficiency went down as the hold time increased.
- (iv)
- The grain refinement efficiency of Al-4Ti master alloys with the same morphology, size, and distribution of TiAl3 particles prepared through different processes was almost identical.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminium and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification processing. Metall. Trans. 1974, 5, 2121–2134. [Google Scholar] [CrossRef]
- Hassanbeygi, V.; Shafyei, A. Investigation on microstructure and grain refining performance of a new type of Al-3Ti-1C master alloy. OJMetal 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Birol, Y. Effect of the salt addition practice on the grain refining efficiency of Al-Ti-B master alloys. J. Alloys Compd. 2006, 420, 207–212. [Google Scholar] [CrossRef]
- Birol, Y. An improved practice to manufacture Al-Ti-B master alloys by reacting halide salts with molten aluminium. J. Alloys Compd. 2006, 420, 71–76. [Google Scholar] [CrossRef]
- Auradi, V.; Kori, S.A. Effect of processing temperature on the microstructure of Al-7Ti master alloy and on refinement of α-Al dendrites in Al-7Si alloys. Adv. Mater. Lett. 2015, 6, 252–259. [Google Scholar] [CrossRef]
- Kashyap, K.T.; Chandrashekar, T. Effects and mechanisms of grain refinement in aluminium alloys. Bull. Mater. Sci. 2001, 24, 345–353. [Google Scholar] [CrossRef]
- Jones, G.P.; Pearson, J. Factors affecting the grain-refinement of aluminum using titanium and boron additives. Metall. Trans. B 1976, 7, 223–234. [Google Scholar] [CrossRef]
- Birol, Y. AlB3 master alloy to grain refine AlSi10Mg and AlSi12Cu aluminium foundry alloys. J. Alloys Compd. 2012, 513, 150–153. [Google Scholar] [CrossRef]
- Savas, O.; Kayikci, R.A. Taguchi optimisation for production of Al-B master alloys using boron oxide. J. Alloys Compd. 2013, 580, 232–238. [Google Scholar] [CrossRef]
- Easton, M.A.; StJohn, D.H. A model of grain refinement incorporating alloy constitution and potency of heterogeneous nucleant particles. Acta Mater. 2001, 49, 1867–1878. [Google Scholar] [CrossRef]
- Guzowski, M.M.; Sigworth, G.K.; Sentner, D.A. The role of boron in the grain-refinement of aluminum with titanium. Metall. Trans. A Phys. Metall. Mater. Sci. 1987, 18, 603–619. [Google Scholar] [CrossRef]
- Li, P.; Kandalova, E.G.; Nikitin, V.I. Grain refining performance of Al–Ti master alloys with different microstructures. Mater. Lett. 2005, 59, 723–727. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Li, H.; Sritharan, T.; Lam, Y.M.; Leng, N.Y. Effects of processing parameters on the performance of Al grain refinement master alloys Al Ti and Al B in small ingots. J. Mater. Process. Technol. 1997, 66, 253–257. [Google Scholar] [CrossRef]
- Li, P.T.; Ma, X.G.; Li, Y.G.; Nie, J.F.; Liu, X.F. Effects of trace C addition on the microstructure and refining efficiency of Al–Ti–B master alloy. J. Alloys Compd. 2010, 503, 286–290. [Google Scholar] [CrossRef]
- Birol, Y. The effect of holding conditions in the conventional halide salt process on the performance of Al–Ti–B grain refiner alloys. J. Alloys Compd. 2007, 427, 142–147. [Google Scholar] [CrossRef]
- Majumdar, A.; Muddle, B.C. Microstructure in rapidly solidified Al-Ti alloys. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 1993, 169, 135–147. [Google Scholar] [CrossRef]
- Birol, Y. Al–Ti–B grain refiners via powder metallurgy processing of Al/K2TiF6/KBF4 powder blends. J. Alloys Compd. 2009, 480, 311–314. [Google Scholar] [CrossRef]
- Zhao, Z.; Guan, R.; Guan, X.; Zhang, J.; Sun, X.; Liu, H. Effects of electromagnetic stirring, shearing, and extrusion on TiB2 and TiAl3 Particles in Al–5Ti–1B(wt. %) Alloy. Mater. Manuf. Process. 2015, 30, 1223–1228. [Google Scholar] [CrossRef]
- Arnberg, L.; Backerud, L.; Klang, H. Grain refinement of aluminum by addition of master alloys of the Al-Ti-B type. In The Czech Aluminum Symposium, Fourth, Extended Abstracts; Mid-County Press: London, UK, 1980; pp. 17–26. [Google Scholar]
- Liu, X.F.; Bian, X.F.; Yang, Y. The formation law of TiAl3 morphologies in AlTi5B master alloy. Spec. Cast. Nonferrous Alloys 1997, 5, 4–6. [Google Scholar]
- Arnberg, L.; Bäckerud, L.; Klang, H. Production and properties of master alloys of Al–Ti–B type and their ability to grain refine aluminium. Met. Technol. 1982, 9, 1–6. [Google Scholar] [CrossRef]
- Ding, W.W.; Zhang, X.Y.; Zhao, W.J.; Xia, T.D.; Zhang, H.X. Microstructure evolution and grain refining performance of new green kind of Al-5Ti-C master alloy. Int. J. Cast Met. Res. 2015, 28, 89–96. [Google Scholar] [CrossRef]
- Wang, T.; Gao, T.; Nie, J.; Li, P.; Liu, X. Influence of carbon source on the microstructure of Al–Ti–C master alloy and its grain refining efficiency. Mater. Charact. 2013, 83, 13–20. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Liu, X.F.; Liu, Y.H.; Zhang, J.Y.; Yu, L.N.; Bian, X.F. Structural heredity of TiC and its influences on refinement behaviors of AlTiC master alloy. Trans. Nonferrous Met. Soc. China 2003, 13, 790–793. [Google Scholar]
- Arnberg, L.; Backerud, L.; Klang, H. Intermetallic particles in Al-Ti-B-type master alloys for grain-refinement of aluminum. Met. Technol. 1982, 9, 7–13. [Google Scholar] [CrossRef]
- Lee, M.S.; Terry, B.S. Effects of processing parameters on aluminide morphology in aluminium grain refining master alloys. Mater. Sci. Technol. 1991, 7, 608–612. [Google Scholar] [CrossRef]
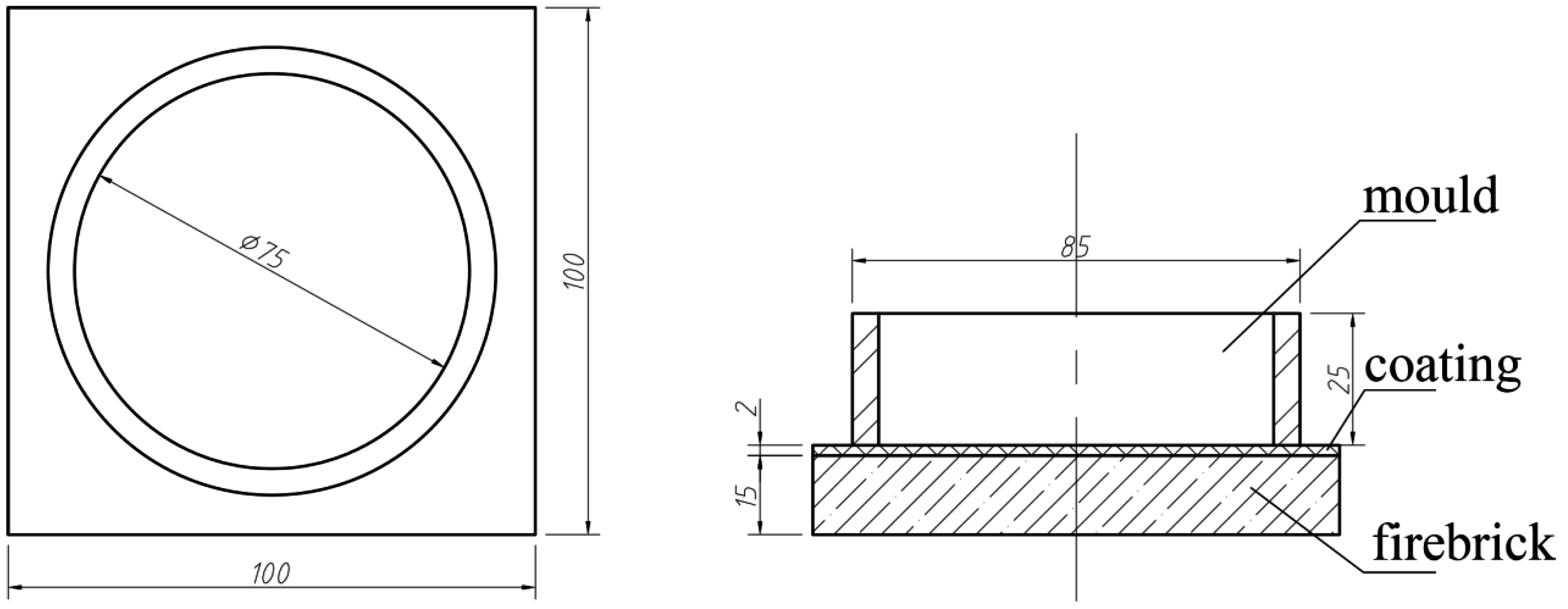
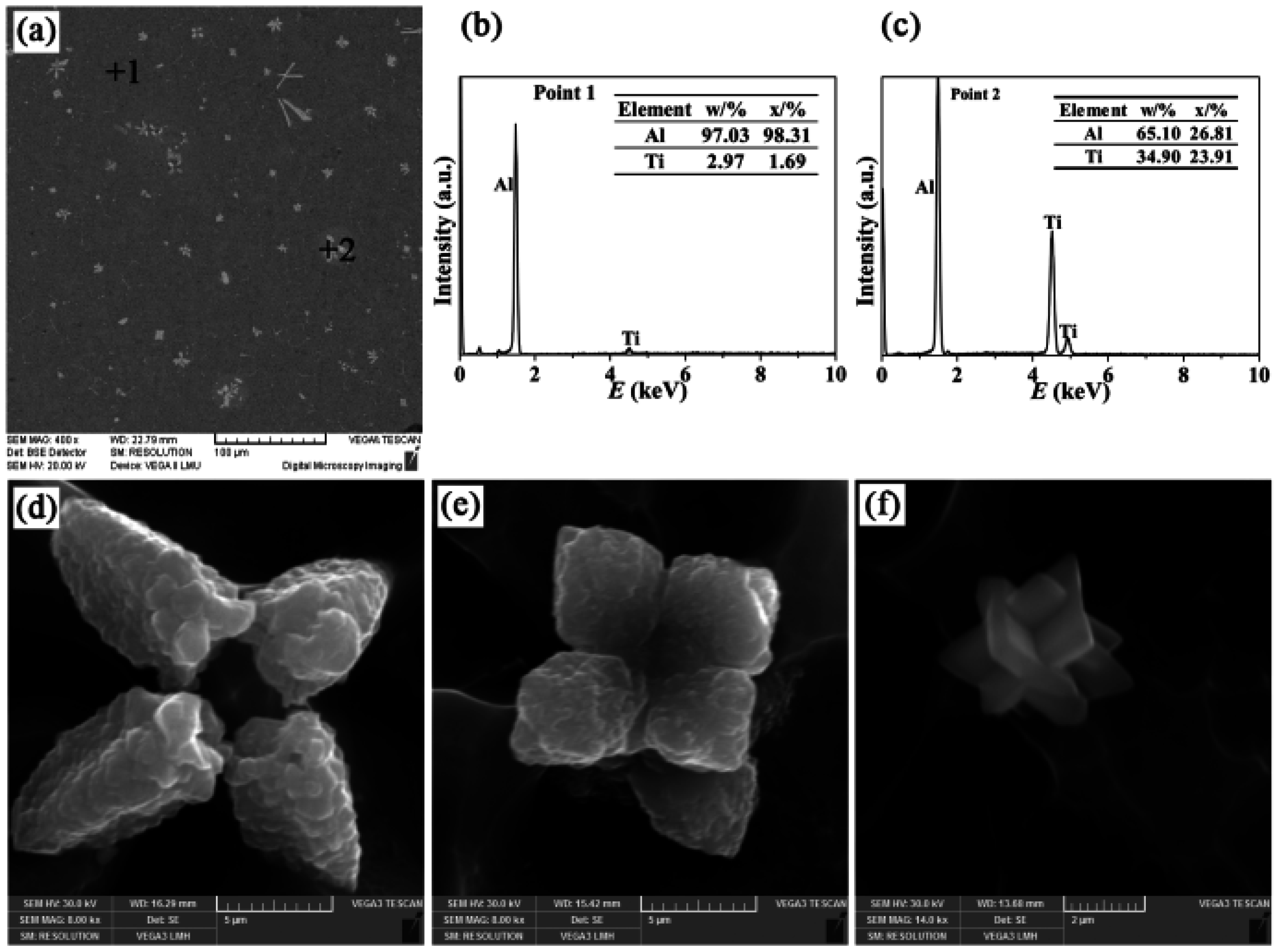
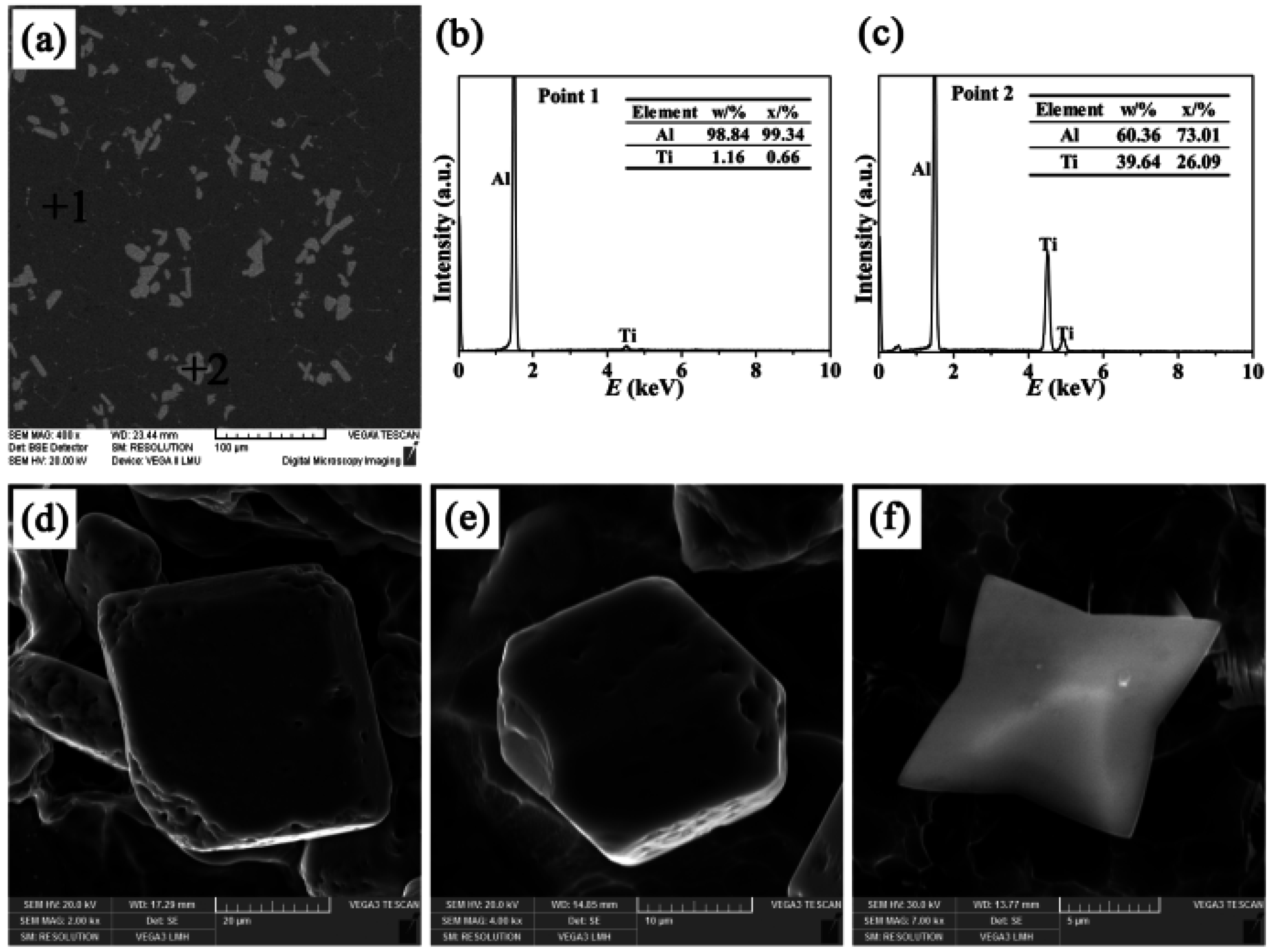
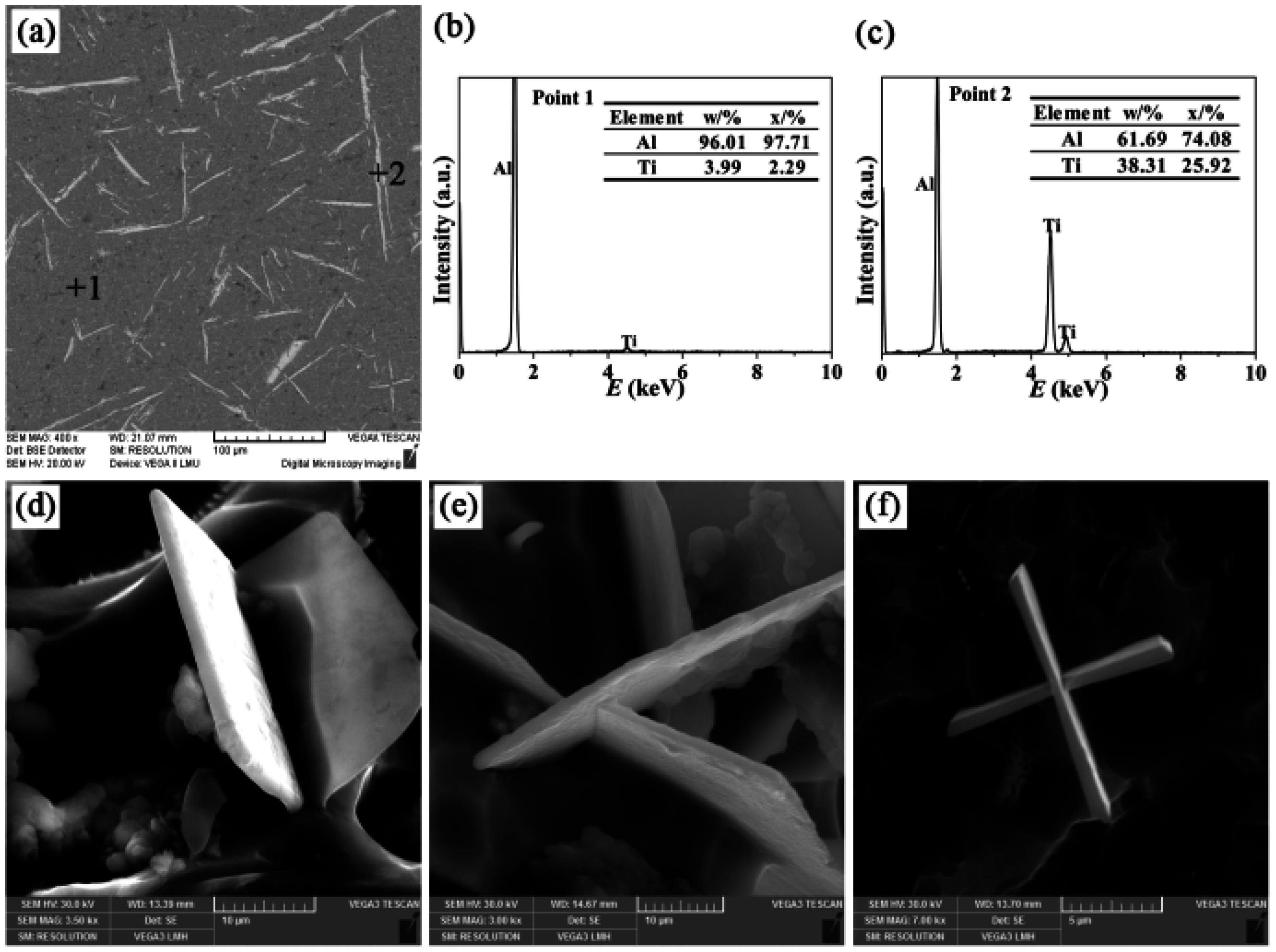


| Cast Material | Addition | Mold Materials | Alloy Designation |
|---|---|---|---|
| Pure aluminum | K2TiF6 | Graphite | I |
| Copper | II | ||
| Sand | III | ||
| Sponge titanium | Graphite | IV | |
| Copper | V | ||
| Sand | VI |
| Alloy | Morphology | Average Length, μm | Quantity, cm2 |
|---|---|---|---|
| I | Petal-like | 11 | 24,340 |
| II | Blocky | 16 | 20,411 |
| III | Needle-like | 90 | 7462 |
| IV | Blocky | 13 | 23,750 |
| V | Blocky | 22 | 17,900 |
| VI | Needle-like | 106 | 6358 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; He, J.; Tang, Q.; Wang, T.; Chen, J. Grain Refinement Efficiency in Commercial-Purity Aluminum Influenced by the Addition of Al-4Ti Master Alloys with Varying TiAl3 Particles. Materials 2016, 9, 869. https://doi.org/10.3390/ma9110869
Zhao J, He J, Tang Q, Wang T, Chen J. Grain Refinement Efficiency in Commercial-Purity Aluminum Influenced by the Addition of Al-4Ti Master Alloys with Varying TiAl3 Particles. Materials. 2016; 9(11):869. https://doi.org/10.3390/ma9110869
Chicago/Turabian StyleZhao, Jianhua, Jiansheng He, Qi Tang, Tao Wang, and Jing Chen. 2016. "Grain Refinement Efficiency in Commercial-Purity Aluminum Influenced by the Addition of Al-4Ti Master Alloys with Varying TiAl3 Particles" Materials 9, no. 11: 869. https://doi.org/10.3390/ma9110869





