Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering
Abstract
:1. Introduction
2. Experimental
2.1. Materials
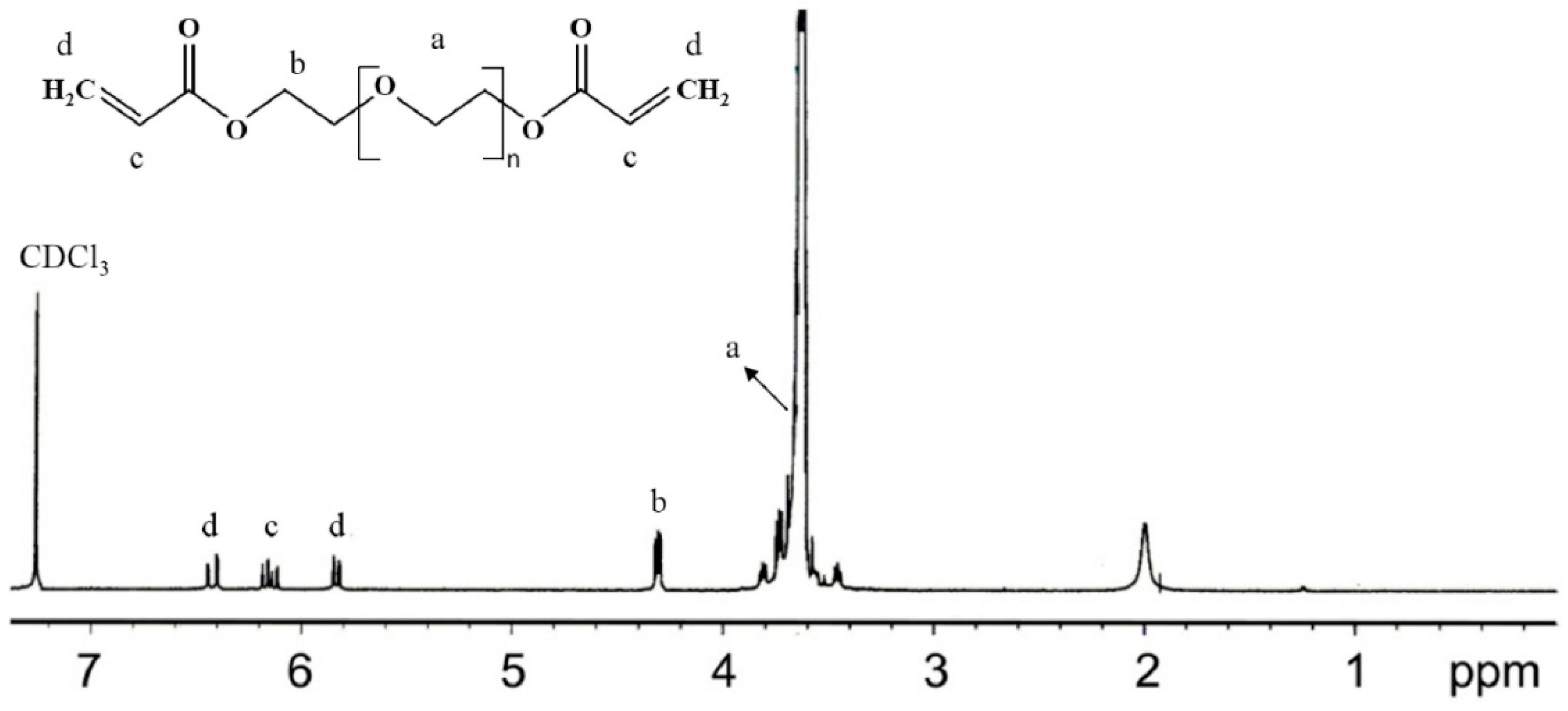
2.2. Synthesis of PEGDA with Different Molecular Weights
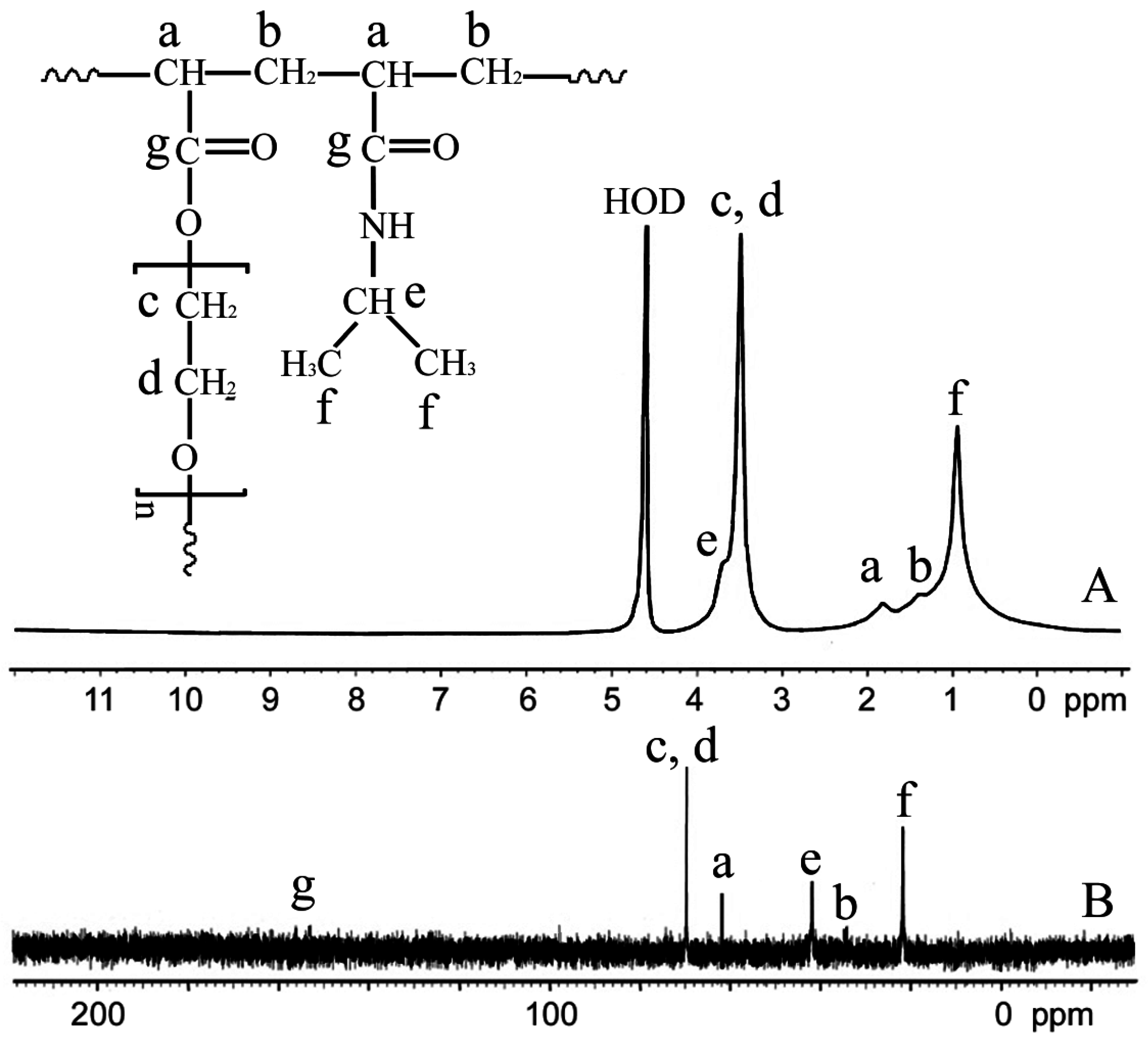
2.3. Synthesis of the p(NIPAm-co-PEGDA) Hydrogels
2.4. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
2.5. Microstructure Analysis
2.6. Swelling Ratio Measurement
2.7. Kinetics of Deswelling and Reswelling
2.8. LCST Measurements
2.9. Application of the Prepared Hydrogels as 2D Monolayer Cell Culture Substrates for Cell Sheet Engineering
2.10. Characterization of the Cell Sheet
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the p(NIPAm-co-PEGDA) Hydrogels
3.2. Microstructure Analysis
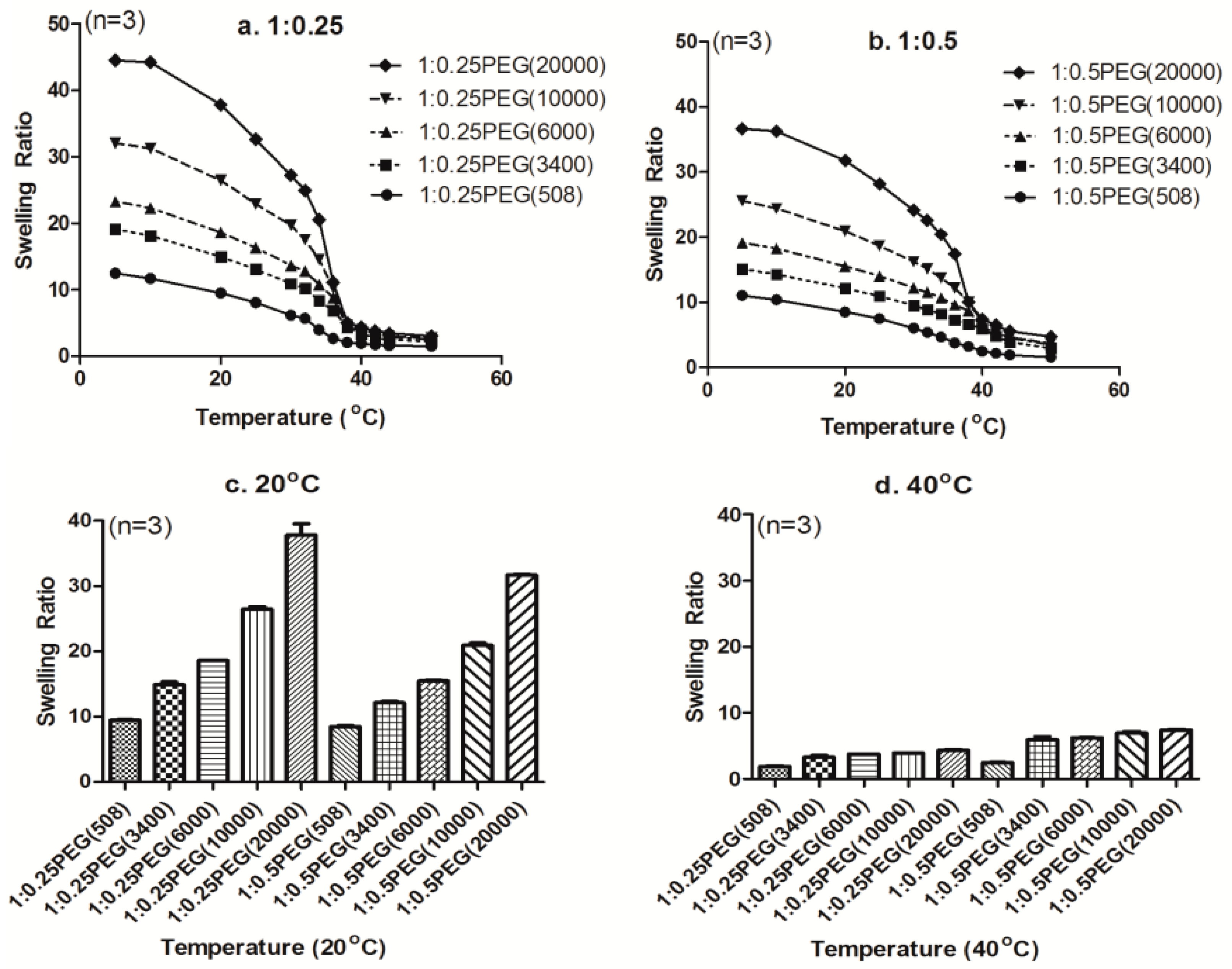
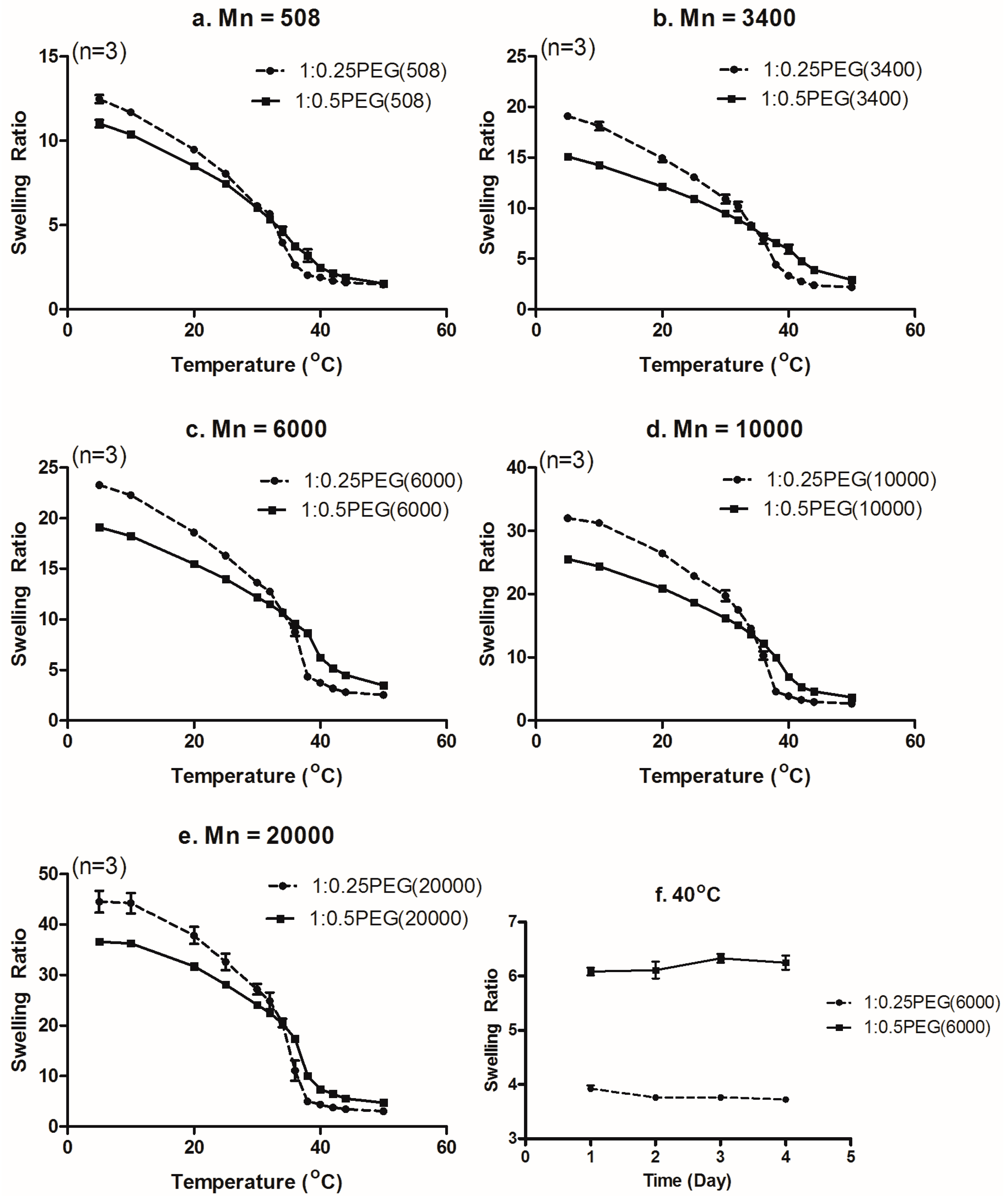
3.3. Effect of Molecular Weight of PEGDA on the Swelling Ratio
3.4. Effect of PEGDA Concentration on the Swelling Ratio
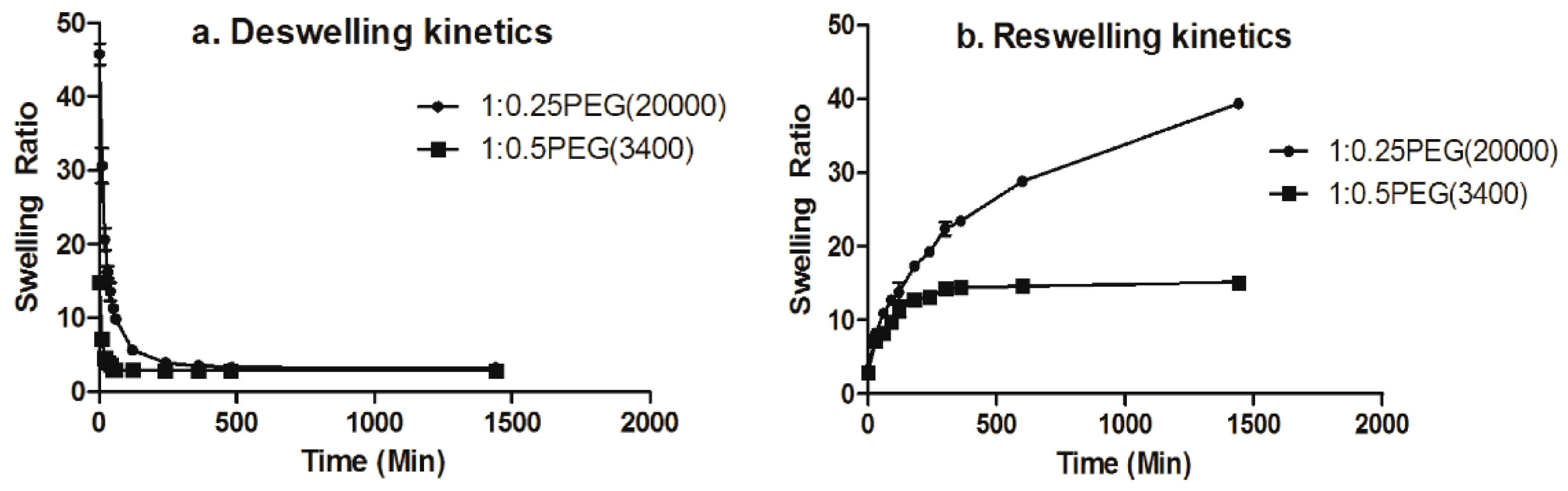
3.5. Kinetics of Deswelling/Reswelling
3.6. Combined Effect of the Molecular Weight and Concentration of PEGDA on LCST
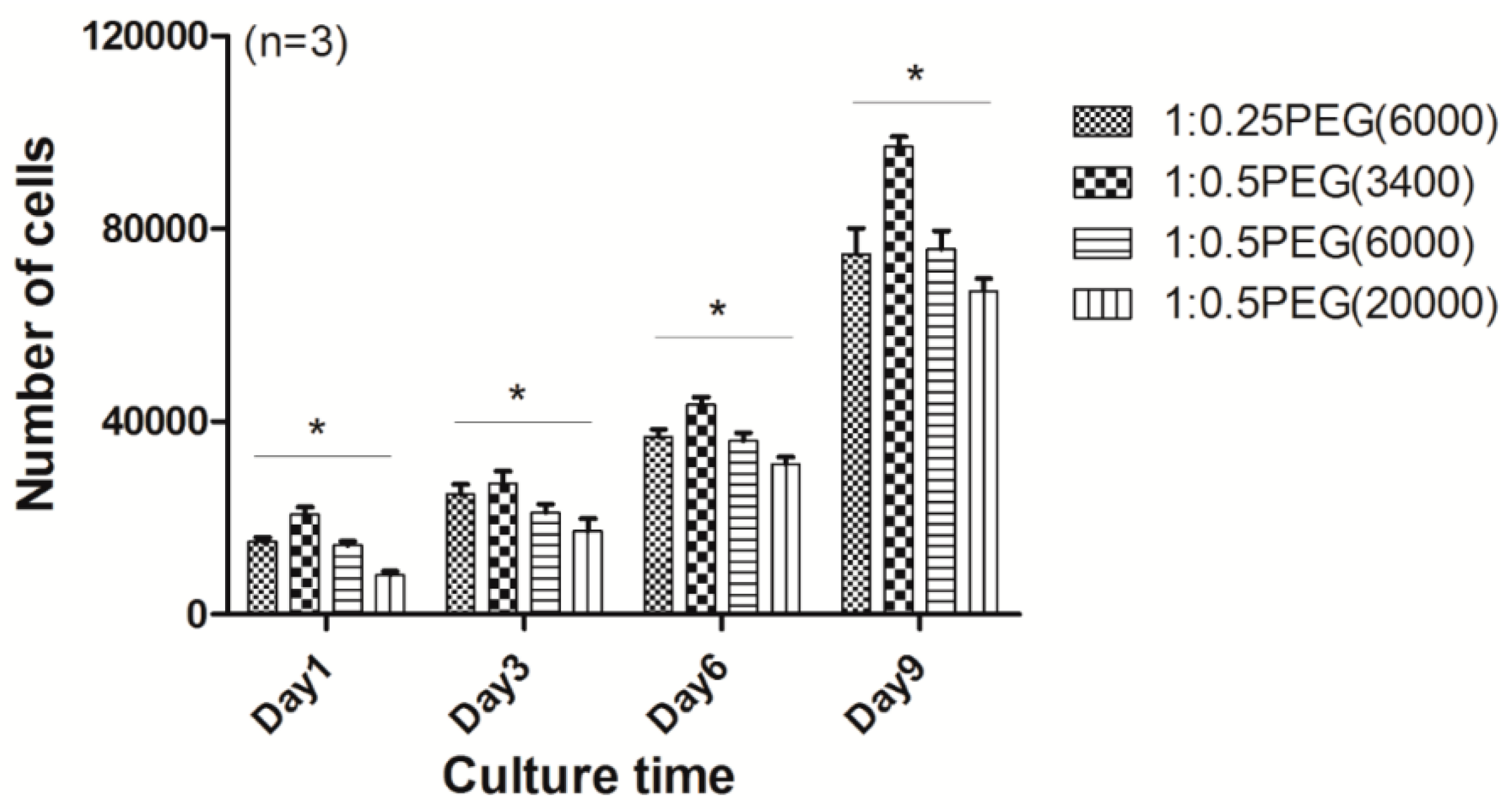
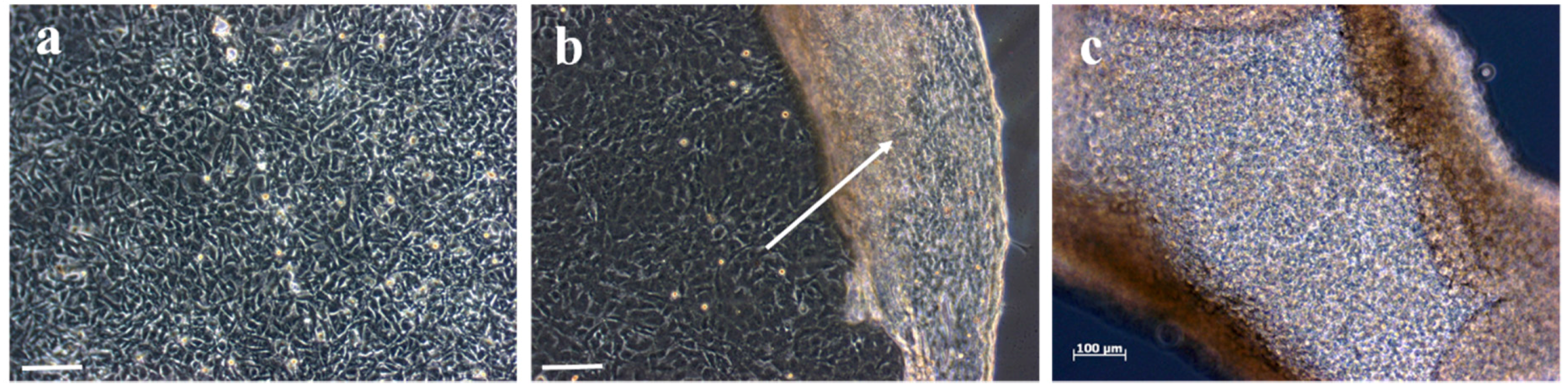
3.7. Application of the Prepared Hydrogels as 2D Monolayer Cell Culture Substrates for Cell Sheet Engineering
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 1995, 374, 240–242. [Google Scholar] [CrossRef]
- Alarcon, C.D.H.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Bae, Y.H.; Jacobs, H.; Kim, S.W. Thermally on-off switching polymers for drug permeation and release. J. Control. Release 1990, 11, 255–265. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S. Thermally reversible hydrogels: Swelling characteristics and activities of copoly(N-isopropylacrylamide-acrylamide) gels containing immobilized asparaginase. ACS Symp. Ser. 1987, 350, 236–244. [Google Scholar]
- Walther, A.; Barner-Kowollik, C.; Müller, A.H.E. Mixed, multicompartment, or janus micelles? a systematic study of thermoresponsive bis-hydrophilic block terpolymers. Langmuir 2010, 26, 12237–12246. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.H.; Chen, J.P.; Cheung, C.; Chilkoti, A.; Ding, Z.L.; Dong, L.C.; Fong, R.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar] [CrossRef]
- Kaneko, Y.; Yoshida, R.; Sakai, K.; Sakurai, Y.; Okano, T. Temperature-responsive shrinking kinetics of poly (N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J. Membr. Sci. 1995, 101, 13–22. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yang, Y.Y.; Wang, F.J.; Chung, T.S. Thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with expanded network structures and improved oscillating swelling-deswelling properties. Langmuir 2002, 18, 2013–2018. [Google Scholar] [CrossRef]
- Kim, S.; Healy, K.E. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 2003, 4, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Elisseeff, J.; McIntosh, W.; Anseth, K.; Riley, S.; Ragan, P.; Langer, R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J. Biomed. Mater. Res. 2000, 51, 164–171. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. The effects of crosslinking density on cartilage formation in photocrosslinkable hydrogels. Biomed. Sci. Instrum. 1999, 35, 309–314. [Google Scholar] [PubMed]
- Zhang, X.Z.; Chu, C.C. A responsive poly(N-isopropylacrylamide)/poly(ethylene glycol) diacrylate hydrogel microsphere. Colloid Polym. Sci. 2004, 282, 1415–1420. [Google Scholar] [CrossRef]
- Lee, W.F.; Lin, Y.H. Swelling behavior and drug release of NIPAAm/PEGMEA copolymeric hydrogels with different crosslinkers. J. Mater. Sci. 2006, 41, 7333–7340. [Google Scholar] [CrossRef]
- Lee, W.F.; Lin, Y.H. Thermoreversible hydrogels. XIX. Synthesis and swelling behavior and drug release behavior for the N-isopropylacrylamide/poly(ethylene glycol) methylether acrylate copolymeric hydrogels. J. Appl. Polym. Sci. 2003, 90, 1683–1691. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Nishida, K.; Ohki, T.; Kanzaki, M.; Sekine, H.; Shimizu, T.; Okano, T. Cell delivery in regenerative medicine: The cell sheet engineering approach. J. Control. Release 2006, 116, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sekine, H.; Yamato, M.; Okano, T. Cell sheet-based myocardial tissue engineering: New hope for damaged heart rescue. Curr. Pharm. Des. 2009, 15, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Hern, D.L.; Hubbell, J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Jeong, J.H.; Chan, V.; Cha, C.; Zorlutuna, P.; Dyck, C.; Hsia, K.J.; Bashir, R.; Kong, H. “Living” microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry. Adv. Mater. 2012, 24, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Bucio, E.; Burillo, G. Radiation polymerization and crosslinking of (N-isopropylacrylamide) in solution and in solid state. Polym. Bull. 2007, 58, 565–573. [Google Scholar] [CrossRef]
- Truong, V.X.; Barker, I.A.; Tan, M.; Mespouille, L.; Dubois, P.; Dove, A.P. Preparation of in situ-forming poly(5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one)-poly(ethylene glycol) hydrogels with tuneable swelling, mechanical strength and degradability. J. Mater. Chem. B 2013, 1, 221–229. [Google Scholar] [CrossRef]
- Truong, V.X.; Blakey, I.; Whittaker, A.K. Hydrophilic and amphiphilic polyethylene glycol-based hydrogels with tunable degradability prepared by “click” chemistry. Biomacromolecules 2012, 13, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, N.C.; Chatterji, P.R. Structural characteristics and swelling behavior of poly(ethylene glycol) diacrylate hydrogels. Macromolecules 1996, 29, 1976–1979. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A 2009, 90, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive terpolymers based on methacrylate monomers: Effect of architecture and composition. J. Polym. Sci. Part A 2010, 48, 775–783. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.L.; Schluter, A.D.; Zhang, A.F. Synthesis of an oligo(ethylene glycol)-based third-generation thermoresponsive dendronized polymer. J. Polym. Sci. Part A 2009, 47, 6630–6640. [Google Scholar] [CrossRef]
- Kojima, C.; Yoshimura, K.; Harada, A.; Sakanishi, Y.; Kono, K. Temperature-sensitive hyperbranched poly(glycidol)s with oligo(ethylene glycol) monoethers. J. Polym. Sci. Part A 2010, 48, 4047–4054. [Google Scholar] [CrossRef]
- Li, W.; Zhang, A.; Schluter, A.D. Thermoresponsive dendronized polymers with tunable lower critical solution temperatures. Chem. Commun. 2008, 5523–5525. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, A.; Feldman, K.; Walde, P.; Schluter, A.D. Thermoresponsive dendronized polymers. Macromolecules 2008, 41, 3659–3667. [Google Scholar] [CrossRef]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J. Biomech. 2008, 41, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid deswelling response of poly(N-isopropylacrylamide) hydrogels by the formation of water release channels using poly(ethylene oxide) graft chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Wei, D.; Ge, L.; Guo, R. Binding characteristics between poly(ethylene glycol) and hydrophilic modified ibuprofen in aqueous solution. J. Phys. Chem. B 2010, 114, 3472–3481. [Google Scholar] [CrossRef] [PubMed]

| Sample Codes | Molecular Weights of PEGDA | Weight Ratios (NIPAm:PEGDA) | Molar Ratios (NIPAm:PEGDA) |
|---|---|---|---|
| 1:0.25PEG(508)/1:0.5PEG(508) | 508 | 1:0.25/1:0.5 | 1:0.0557/1:0.1114 |
| 1:0.25PEG(3400)/1:0.5PEG(3400) | 3400 | 1:0.25/1:0.5 | 1:0.0083/1:0.0166 |
| 1:0.25PEG(6000)/1:0.5PEG(6000) | 6000 | 1:0.25/1:0.5 | 1:0.0047/1:0.0094 |
| 1:0.25PEG(10,000)/1:0.5PEG(10,000) | 10,000 | 1:0.25/1:0.5 | 1:0.0028/1:0.0057 |
| 1:0.25PEG(20,000)/1:0.5PEG(20,000) | 20,000 | 1:0.25/1:0.5 | 1:0.0014/1:0.0028 |
| NIPAM:PEGDA Weight Ratio | Molecular Weight of PEGDA | ||||
|---|---|---|---|---|---|
| 508 | 3400 | 6000 | 10,000 | 20,000 | |
| 1:0.25 | 34.0 ± 0.3 °C | 36.3 ± 0.3 °C | 36.3 ± 0.3 °C | 35.9 ± 0.1 °C | 35.3 ± 0.2 °C |
| 1:0.5 | 34.3 ±0.3 °C | 41.4 ± 0.3 °C | 39.1 ± 0.3 °C | 38.5 ± 0.5 °C | 36.9 ± 0.4 °C |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, K.H.; Lee, J.W. Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering. Materials 2016, 9, 854. https://doi.org/10.3390/ma9100854
Son KH, Lee JW. Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering. Materials. 2016; 9(10):854. https://doi.org/10.3390/ma9100854
Chicago/Turabian StyleSon, Kuk Hui, and Jin Woo Lee. 2016. "Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering" Materials 9, no. 10: 854. https://doi.org/10.3390/ma9100854





