An Experimental and Theoretical Investigation of the Electronic Structures and Photoelectrical Properties of Ethyl Red and Carminic Acid for DSSC Application
Abstract
:1. Introduction
2. Experimental and Theoretical Methods
2.1. Experiment
2.2. Theory
3. Results and Discussion
3.1. Optical Characteristics of the Dyes
3.2. FT-IR Spectra
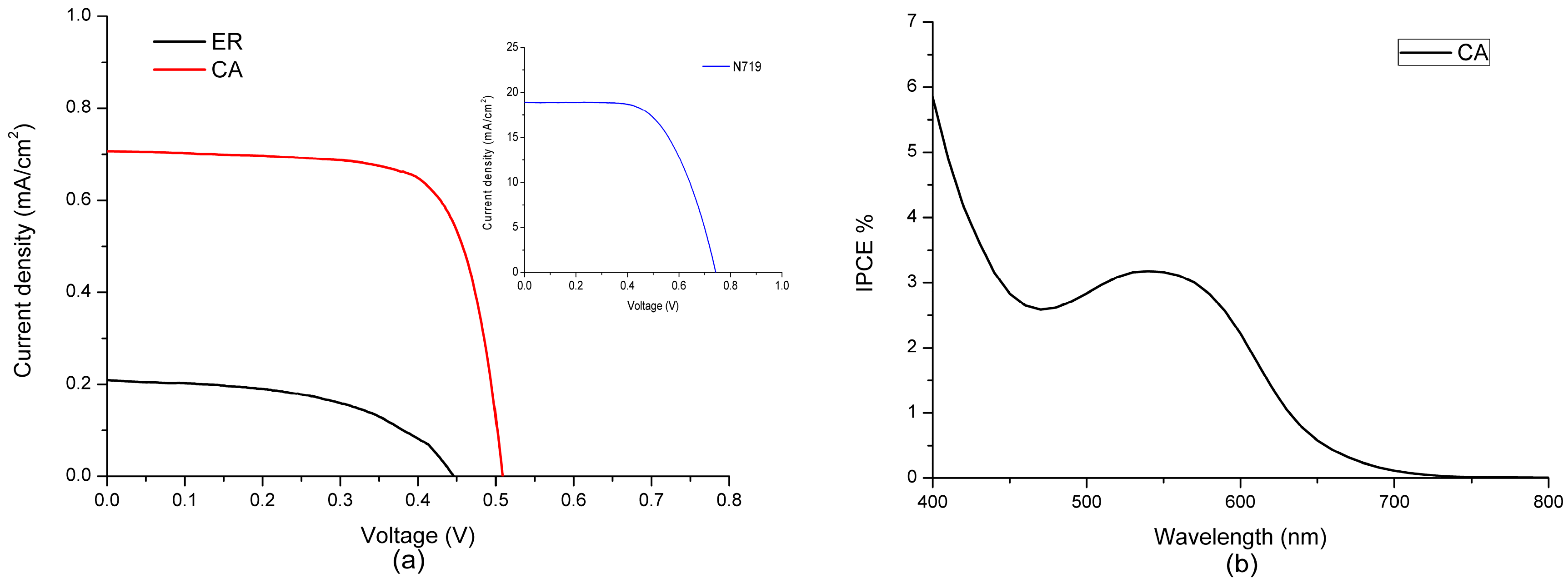
3.3. Photovoltaic Properties of Fabricated DSSCs
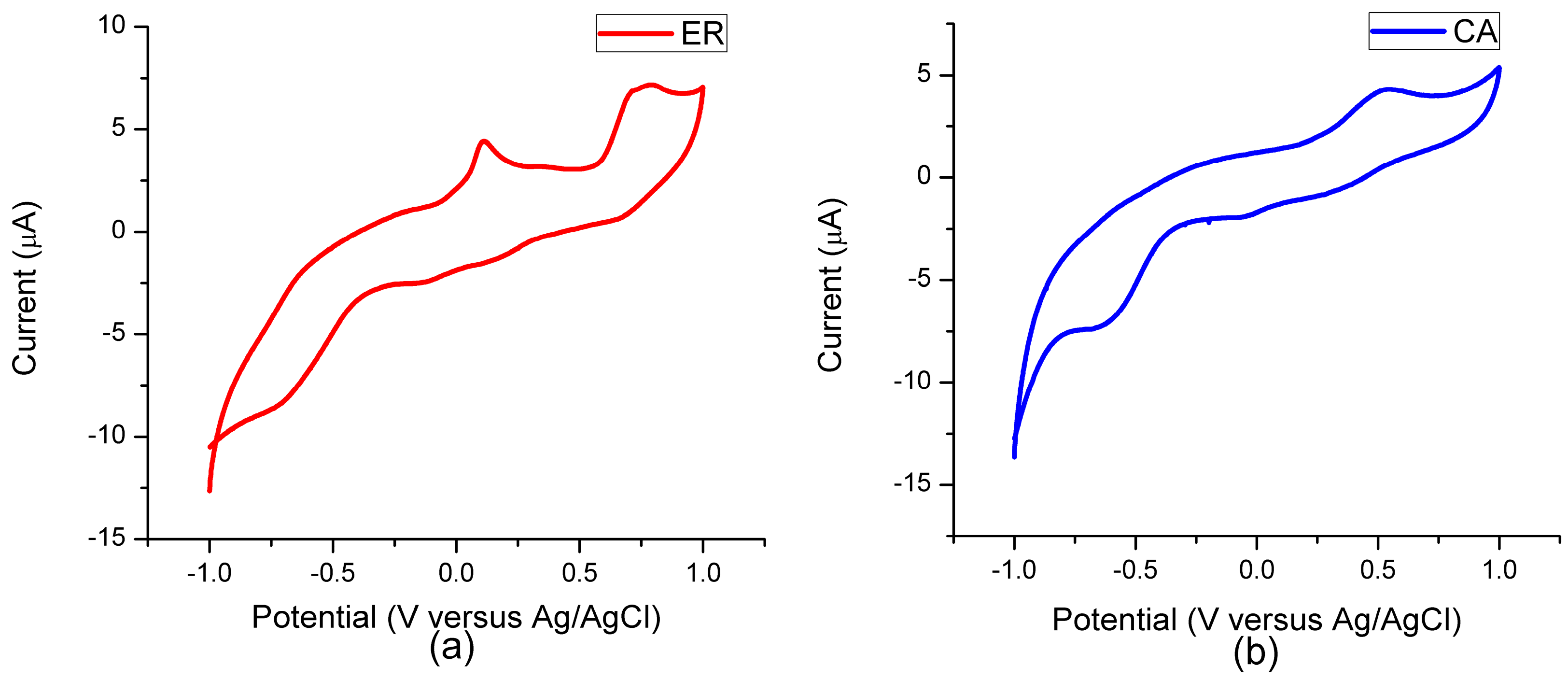
3.4. Electrochemical Characteristic
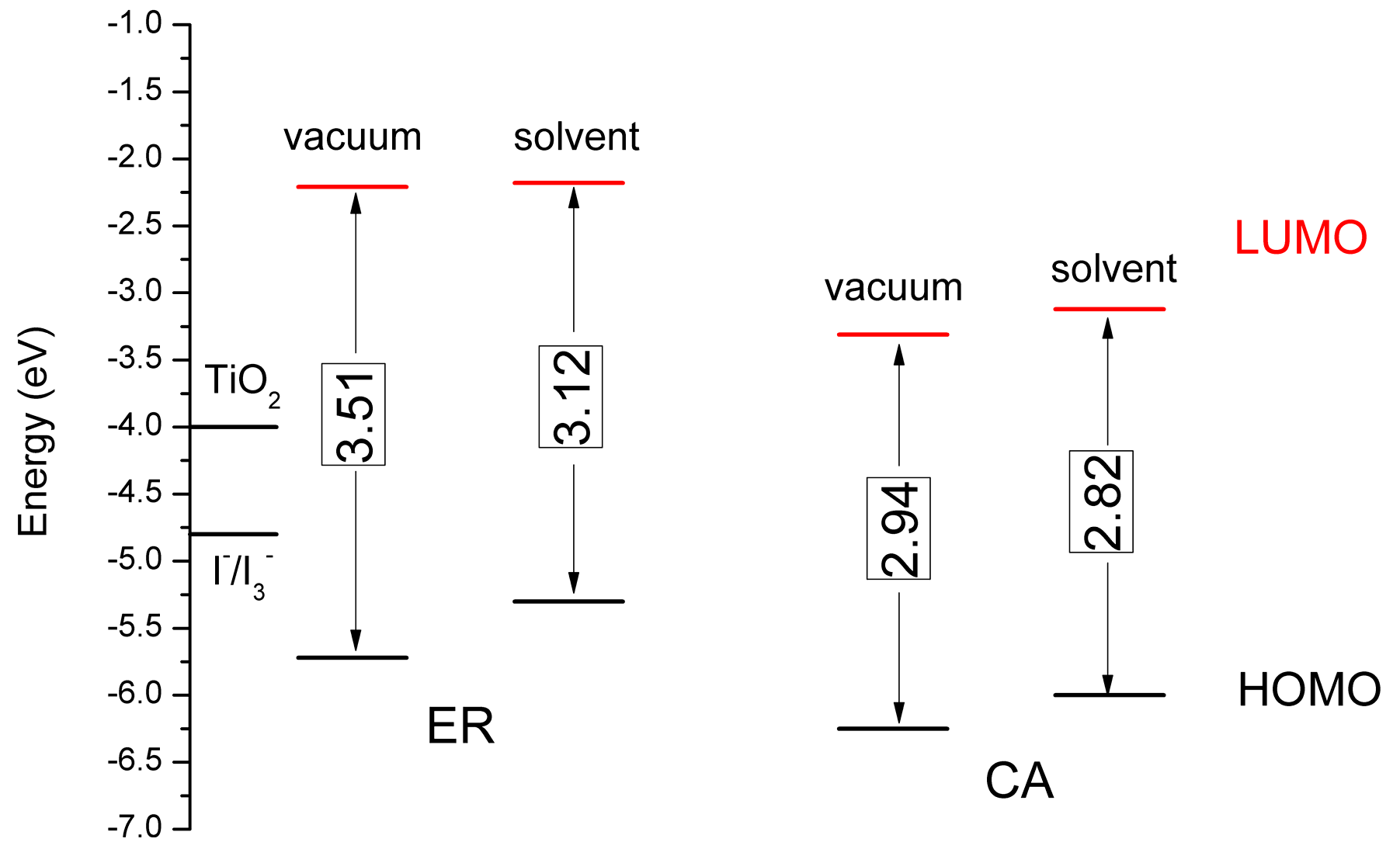
3.5. Theoretical Analysis for and
3.6. Excited State Lifetime (τ)
3.7. Total Static First Hyperpolarizability
3.8. Properties of Dye/TiO2 Complexes
3.9. Chemical Reactivity Parameters
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Yoneda, E.; Yum, J.-H.; Guglielmi, M.; Tavernelli, I.; Imai, H.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. New paradigm in molecular engineering of sensitizers for solar cell applications. J. Am. Chem. Soc. 2009, 131, 5930–5934. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; CurchodBasile, F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; NazeeruddinMd, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Y.; Jia, R.; Sun, X.; Liu, C.; Zhang, Y. Novel azobenzene nickel(II) sensitizer for dye-sensitized solar cells. J. Photochem. Photobiol. A 2016, 318, 90–96. [Google Scholar] [CrossRef]
- Massin, J.; Ducasse, L.; Toupance, T.; Olivier, C. Tetrazole as a New anchoring group for the functionalization of tio2 nanoparticles: A joint experimental and theoretical study. J. Phys. Chem. C 2014, 118, 10677–10685. [Google Scholar] [CrossRef]
- Ducasse, L.; Castet, F.; Méreau, R.; Nénon, S.; Idé, J.; Toupance, T.; Olivier, C. Structure and absorption properties of the C212 dye chemisorbed onto the TiO2 (1 0 1) anatase surface. Chem. Phys. Lett. 2013, 556, 151–157. [Google Scholar] [CrossRef]
- Zhou, N.; Prabakaran, K.; Lee, B.; Chang, S.H.; Harutyunyan, B.; Guo, P.; Butler, M.R.; Timalsina, A.; Bedzyk, M.J.; Ratner, M.A.; et al. Metal-Free Tetrathienoacene Sensitizers for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wu, H.; Li, Y.; Wang, J.; Zhang, J.; Zhang, M.; Guo, Y.; Wang, P. Dithienopicenocarbazole as the kernel module of low-energy-gap organic dyes for efficient conversion of sunlight to electricity. Energy Environ. Sci. 2015, 8, 3192–3197. [Google Scholar] [CrossRef]
- Gao, H.-H.; Qian, X.; Chang, W.-Y.; Wang, S.-S.; Zhu, Y.-Z.; Zheng, J.-Y. Oligothiophene-linked D–π–A type phenothiazine dyes for dye-sensitized solar cells. J. Power Sources 2016, 307, 866–874. [Google Scholar] [CrossRef]
- Karlsson, K.M.; Jiang, X.; Eriksson, S.K.; Gabrielsson, E.; Rensmo, H.; Hagfeldt, A.; Sun, L. Phenoxazine dyes for dye-sensitized solar cells: Relationship between molecular structure and electron lifetime. Chem. Eur. J. 2011, 17, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, Y.; Allama, F.; Lepeltier, M.; Massin, J.; Castet, F.; Ducasse, L.; Hirsch, L.; Boubegtiten, Z.; Jonusauskas, G.; Olivier, C.; et al. New synthetic routes towards soluble and dissymmetric triphenodioxazine dyes designed for dye-sensitized solar cells. Chem. Eur. J. 2014, 20, 3678–3688. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Niu, L.; Xing, Z.; Song, R.; Liu, G.; Sun, M.; Cheng, G.; Zhong, H.; Liu, Z.; Zhang, Z.; et al. Aqueous-Processable Noncovalent Chemically Converted Graphene–Quantum Dot Composites for Flexible and Transparent Optoelectronic Films. Adv. Mater. 2010, 22, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, H. A novel application of plasmonics: Plasmon-driven surface-catalyzed reactions. Small 2012, 8, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Nurachman, Z.; Hartini, H.; Rahmaniyah, W.R.; Kurnia, D.; Hidayat, R.; Prijamboedi, B.; Suendo, V.; Ratnaningsih, E.; Panggabean, L.M.G.; Nurbaiti, S. Tropical marine Chlorella sp PP1 as a source of photosynthetic pigments for dye-sensitized solar cells. Algal Res. 2015, 10, 25–32. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Wang, Y.Q.; Li, X.; Agren, H.; Zhu, W.H.; Xie, Y.S. Porphyrins containing a triphenylamine donor and up to eight alkoxy chains for dye-sensitized solar cells: A high efficiency of 10.9%. ACS Appl. Mater. Interfaces 2015, 7, 27976–27985. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Mondal, B.; Mukherjee, K. Visible light absorption and photo-sensitizing properties of spinach leaves and beetroot extracted natural dyes. Spectrochim. Acta Part A 2015, 148, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zdyb, A.; Krawczyk, S. Adsorption and electronic states of morin on TiO2 nanoparticles. Chem. Phys. 2014, 443, 61–66. [Google Scholar] [CrossRef]
- Lim, A.; Kumara, N.; Tan, A.L.; Mirza, A.H.; Chandrakanthi, R.L.N.; Petra, M.I.; Ming, L.C.; Senadeera, G.K.R.; Ekanayake, P. Potential natural sensitizers extracted from the skin of Canarium odontophyllum fruits for dye-sensitized solar cells. Spectrochim. Acta Part A 2015, 138, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Calogero, G.; Yum, J.H.; Sinopoli, A.; Di Marco, G.; Gratzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Chien, C.Y.; Hsu, B.D. Performance enhancement of dye-sensitized solar cells based on anthocyanin by carbohydrates. Sol. Energy 2014, 108, 403–411. [Google Scholar] [CrossRef]
- Wang, X.F.; Xiang, J.F.; Wang, P.; Koyama, Y.; Yanagida, S.; Wada, Y.; Hamada, K.; Sasaki, S.; Tamiaki, H. Dye-sensitized solar cells using a chlorophyll a derivative as the sensitizer and carotenoids having different conjugation lengths as redox spacers. Chem. Phys. Lett. 2005, 408, 409–414. [Google Scholar] [CrossRef]
- Yamazaki, E.; Murayama, M.; Nishikawa, N.; Hashimoto, N.; Shoyama, M.; Kurita, O. Utilization of natural carotenoids as photo sensitizers for dye-sensitized solar cells. Sol. Energy 2007, 81, 512–516. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid ul, I.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundara prabhu, R.; Prasanna, S.; Mallick, T.K.; Senthilarasu, S. Review on natural dye sensitized solar cells: Operation, materials and methods. Renew. Sustain. Energy Rev. 2015, 51, 1306–1325. [Google Scholar] [CrossRef]
- Kumara, N.; Kooh, M.R.R.; Lim, A.; Petra, M.I.; Voo, N.Y.; Lim, C.M.; Ekanayake, P. DFT/TDDFT and experimental studies of natural pigments extracted from black tea waste for DSSC application. Int. J. Photoenergy 2013. [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, H.X.; Song, P.; Sun, C.F. Photoactive layer of DSSCS based on natural dyes: A study of experiment and theory. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Geerlings, P.; Proft, F.D.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef] [PubMed]
- Martsinovich, N.; Troisi, A. Theoretical studies of dye-sensitised solar cells: From electronic structure to elementary processes. Energy Environ. Sci. 2011, 4, 4473–4495. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Taufany, F.; Nachimuthu, S.; Jiang, J.C.; Liaw, D.J. Design strategies of metal free-organic sensitizers for dye sensitized solar cells: Role of donor and acceptor monomers. Org. Electron. 2014, 15, 1205–1214. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Ji, Z.; Li, H.B.; Yong, W.; Xu, H.L.; Min, Z.; Yun, G.; Su, Z.M. Modulation on charge recombination and light harvesting towardhigh-performance benzothiadiazole-based sensitizers in dye-sensitized solar cells: A theoretical investigation. J. Power Sources 2014, 267, 300–308. [Google Scholar] [CrossRef]
- Li, Y.Z.; Qi, D.W.; Song, P.; Ma, F.C. Fullerene-based photoactive layers for heterojunction solar cells: Structure, absorption spectra and charge transfer process. Materials 2015, 8, 42–56. [Google Scholar] [CrossRef]
- Cerezo, J.; Ferrer, F.J.A.; Santoro, F. Disentangling vibronic and solvent broadening effects in the absorption spectra of coumarin derivatives for dye sensitized solar cells. Phys. Chem. Chem. Phys. 2015, 17, 11401–11411. [Google Scholar] [CrossRef] [PubMed]
- Suresh, T.; Chitumalla, R.K.; Hai, N.T.; Jang, J.; Lee, T.J.; Kim, J.H. Impact of neutral and anion anchoring groups on the photovoltaic performance of triphenylamine sensitizers for dye-sensitized solar cells. RSC Adv. 2016, 6, 26559–26567. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Qi, D.; Song, P.; Ma, F. Effects of different functional groups on the optical and charge transport properties of copolymers for polymer solar cells. RSC Adv. 2016, 6, 61809–61820. [Google Scholar] [CrossRef]
- Feng, S.; Li, Q.S.; Yang, L.N.; Sun, Z.Z.; Niehaus, T.A.; Li, Z.S. Insights into aggregation effects on optical property and electronic coupling of organic dyes in dye sensitized solar cells. J. Power Sources 2015, 273, 282–289. [Google Scholar] [CrossRef]
- Zarate, X.; Schott-Verdugo, S.; Rodriguez-Serrano, A.; Schott, E. The nature of the donor motif in acceptor-bridge-donor dyes as an influence in the electron photo-injection mechanism in DSSCs. J. Phys. Chem. A 2016, 120, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Tsoukleris, D.S.; Arabatzis, I.M.; Chatzivasiloglou, E.; Kontos, A.I.; Belessi, V.; Bernard, M.C.; Falaras, P. 2-Ethyl-1-hexanol based screen-printed titania thin films for dye-sensitized solar cells. Sol. Energy 2005, 79, 422–430. [Google Scholar] [CrossRef]
- Fan, K.; Liu, M.; Peng, T.; Ma, L.; Dai, K. Effects of paste components on the properties of screen-printed porous TiO2 film for dye-sensitized solar cells. Renew. Energy 2010, 35, 555–561. [Google Scholar] [CrossRef]
- Cojocaru, L.; Olivier, C.; Toupance, T.; Sellier, E.; Hirsch, L. Size and shape fine-tuning of SnO2 nanoparticles for highly efficient and stable dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 13789–13799. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. 1965, 137, A1697–A1705. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Kleinman, D.A. Nonlinear dielectric polarization in optical media. Phys. Rev. 1962, 126, 1977–1979. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Matsuzawa, N.N.; Ishitani, A.; Dixon, D.A.; Uda, T. Time-dependent density functional theory calculations of photoabsorption spectra in the vacuum ultraviolet region. J. Phys. Chem. A 2001, 105, 4953–4962. [Google Scholar] [CrossRef]
- Ordon, P.; Tachibana, A. Investigation of the role of the C-PCM solvent effect in reactivity indices. J. Chem. Sci. 2005, 117, 583–589. [Google Scholar] [CrossRef]
- Peng, B.; Yang, S.; Li, L.; Cheng, F.; Chen, J. A density functional theory and time-dependent density functional theory investigation on the anchor comparison of triarylamine-based dyes. J. Chem. Phys. 2010, 132, 034305. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-Armas, R.; Oviedo López, J.; San-Miguel, M.A.; Sanz, J.F.; Ordejón, P.; Pruneda, M. Real-Time TD-DFT simulations in dye sensitized solar cells: The electronic absorption spectrum of alizarin supported on TiO2 nanoclusters. J. Chem. Theory Comput. 2010, 6, 2856–2865. [Google Scholar]
- Zhang, J.; Li, H.-B.; Sun, S.-L.; Geng, Y.; Wu, Y.; Su, Z.-M. Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- Sun, M. Control of structure and photophysical properties by protonation and subsequent intramolecular hydrogen bonding. J. Chem. Phys. 2006, 124, 054903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Zhao, X.; Chen, M. Electronic structure and optical properties of dianionic and dicationic π-Dimers. J. Phys. Chem. A 2010, 114, 6972–6977. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grätzel, M. Recent Advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Bhargava, P. Effect of amount of dye in the TiO2 photoanode on electron transport, recombination, Jsc and Voc of dye-sensitized solar cells. RSC Adv. 2013, 3, 2655–2661. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Cong, J.; Chen, R.; Teng, C.; Liu, J.; Hao, Y.; Wang, L.; Sun, L. Effect of different electron donating groups on the performance of dye-sensitized solar cells. Dyes Pigm. 2010, 84, 62–68. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, S.; Rajarajan, G.; Gnanendra, T.S.; Anbarasan, P.M.; Aroulmoji, V. Structural and optical properties of Purpurin for dye-sensitized solar cells. Spectrochim. Acta, Part A 2015, 149, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kou, L.; Diao, L.; Zhang, Q.; Li, Z.; Wu, Q.; Lu, W.; Pan, D.; Wei, Z. Theoretical study of WS-9-Based organic sensitizers for unusual vis/NIR absorption and highly efficient dye-sensitized solar cells. J. Phys. Chem. C 2015, 119, 9782–9790. [Google Scholar] [CrossRef]
- Marinado, T.; Hagberg, D.P.; Hedlund, M.; Edvinsson, T.; Johansson, E.M.J.; Boschloo, G.; Rensmo, H.; Brinck, T.; Sun, L.; Hagfeldt, A. Rhodanine dyes for dye-sensitized solar cells: Spectroscopy, energy levels and photovoltaic performance. Phys. Chem. Chem. Phys. 2009, 11, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef] [PubMed]
- Hasselman, G.M.; Watson, D.F.; Stromberg, J.R.; Bocian, D.F.; Holten, D.; Lindsey, J.S.; Meyer, G.J. Theoretical solar-to-electrical energy-conversion efficiencies of Perylene−Porphyrin light-harvesting arrays. J. Phys. Chem. B 2006, 110, 25430–25440. [Google Scholar] [CrossRef] [PubMed]
- Preat, J.; Michaux, C.; Jacquemin, D.; Perpète, E.A. Enhanced Efficiency of Organic Dye-Sensitized Solar Cells: Triphenylamine Derivatives. J. Phys. Chem. C 2009, 113, 16821–16833. [Google Scholar] [CrossRef]
- Asbury, J.B.; Wang, Y.-Q.; Hao, E.; Ghosh, H.N.; Lian, T. Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res. Chem. Intermed. 2001, 27, 393–406. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Yoshihara, T.; Hara, K.; Fujihashi, G.; Takano, S.; Murata, S.; Arakawa, H.; Tachiya, M. Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J. Phys. Chem. B 2004, 108, 4818–4822. [Google Scholar] [CrossRef]
- Islam, A.; Sugihara, H.; Arakawa, H. Molecular design of ruthenium(II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J. Photochem. Photobiol. A 2003, 158, 131–138. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye regeneration kinetics in dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef] [PubMed]
- Marinado, T.; Nonomura, K.; Nissfolk, J.; Karlsson, M.K.; Hagberg, D.P.; Sun, L.; Mori, S.; Hagfeldt, A. How the nature of triphenylamine-polyene dyes in dye-sensitized solar cells affects the open-circuit voltage and electron lifetimes. Langmuir 2010, 26, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Preat, J.; Jacquemin, D.; Perpete, E.A. Towards new efficient dye-sensitised solar cells. Energy Environ. Sci. 2010, 3, 891–904. [Google Scholar] [CrossRef]
- Rühle, S.; Greenshtein, M.; Chen, S.G.; Merson, A.; Pizem, H.; Sukenik, C.S.; Cahen, D.; Zaban, A. Molecular adjustment of the electronic properties of nanoporous electrodes in dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 18907–18913. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, A.S.; El Mahdy, A.M.; Taha, H.O.; Soliman, K.A. The effects of macrocycle and anchoring group replacements on the performance of porphyrin based sensitizer: DFT and TD-DFT study. J. Phys. Chem. Solids 2015, 76, 22–33. [Google Scholar] [CrossRef]
- Duncan, W.R.; Prezhdo, O.V. Theoretical studies of photoinduced electron transfer in dye-sensitized TiO2. Annu. Rev. Phys. Chem. 2007, 58, 143–184. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-Armas, R.; Oviedo, J.; San Miguel, M.Á.; Sanz, J.F. Direct vs indirect mechanisms for electron injection in dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 11293–11301. [Google Scholar] [CrossRef]
- Sánchez-de-Armas, R.; San-Miguel, M.A.; Oviedo, J.; Sanz, J.F. Direct vs. indirect mechanisms for electron injection in DSSC: Catechol and alizarin. Comput. Theor. Chem. 2011, 975, 99–105. [Google Scholar] [CrossRef]
- Oviedo, M.B.; Zarate, X.; Negre, C.F.A.; Schott, E.; Arratia-Pérez, R.; Sánchez, C.G. Quantum dynamical simulations as a tool for predicting photoinjection mechanisms in dye-sensitized TiO2 solar cells. J. Phys. Chem. Lett. 2012, 3, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Martínez, J. Local reactivity descriptors from degenerate frontier molecular orbitals. Chem. Phys. Lett. 2009, 478, 310–322. [Google Scholar] [CrossRef]
- Soto-Rojo, R.; Baldenebro-Lopez, J.; Glossman-Mitnik, D. Study of chemical reactivity in relation to experimental parameters of efficiency in coumarin derivatives for dye sensitized solar cells using DFT. Phys. Chem. Chem. Phys. 2015, 17, 14122–14129. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and electroaccepting powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef] [PubMed]



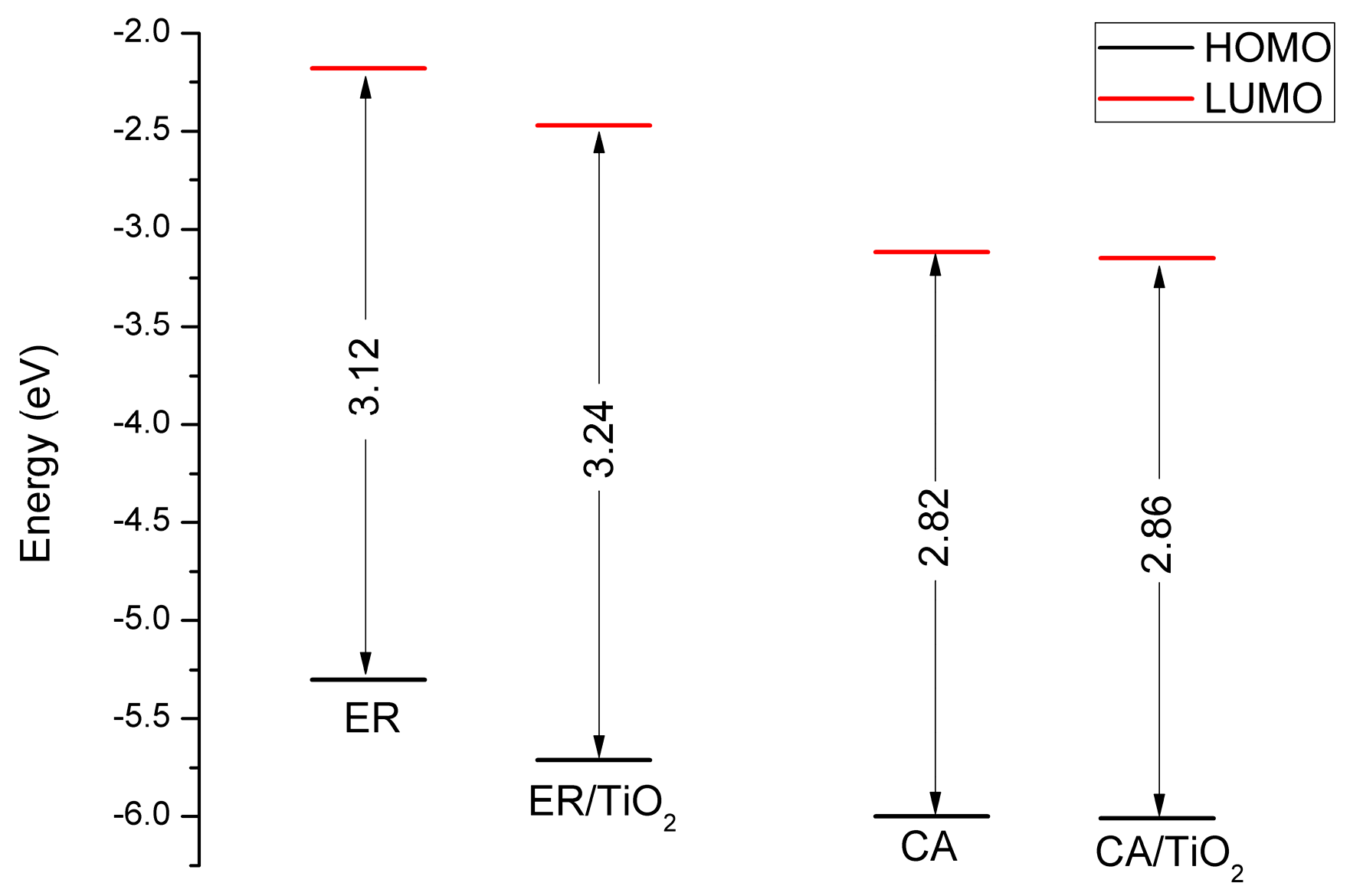
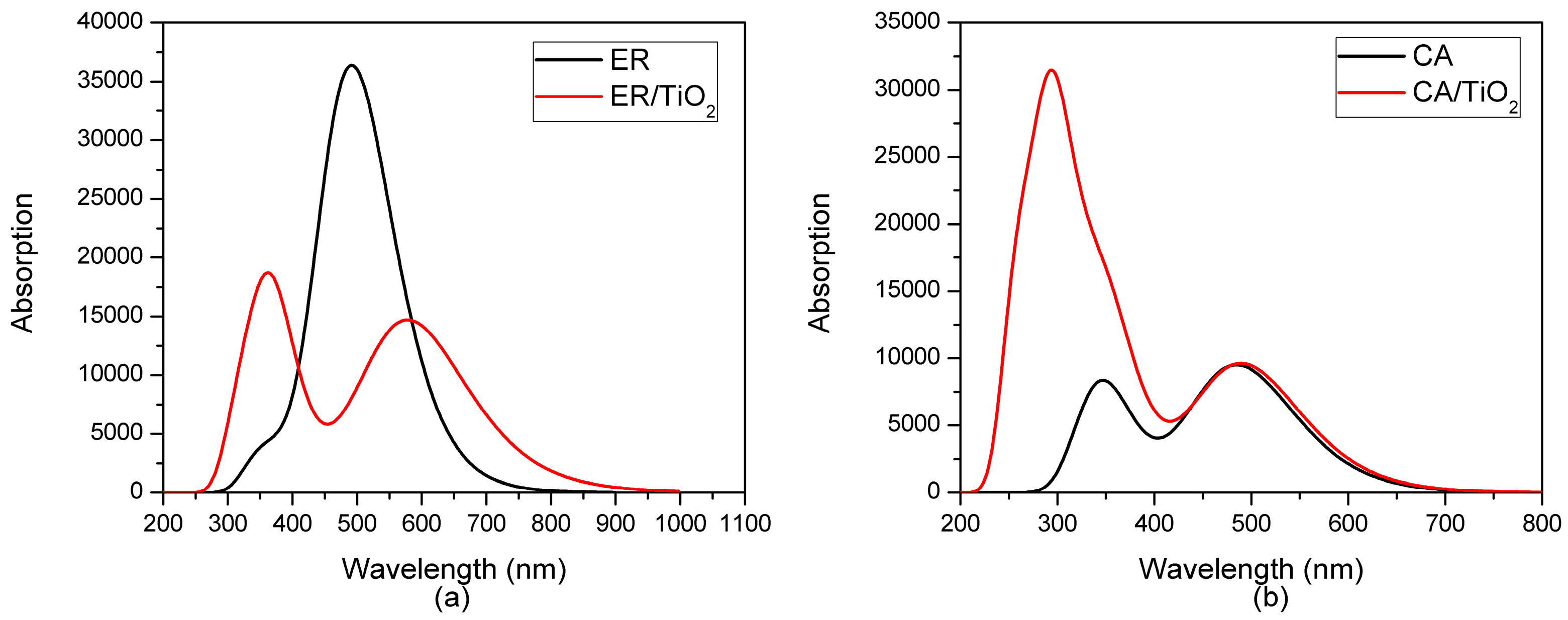
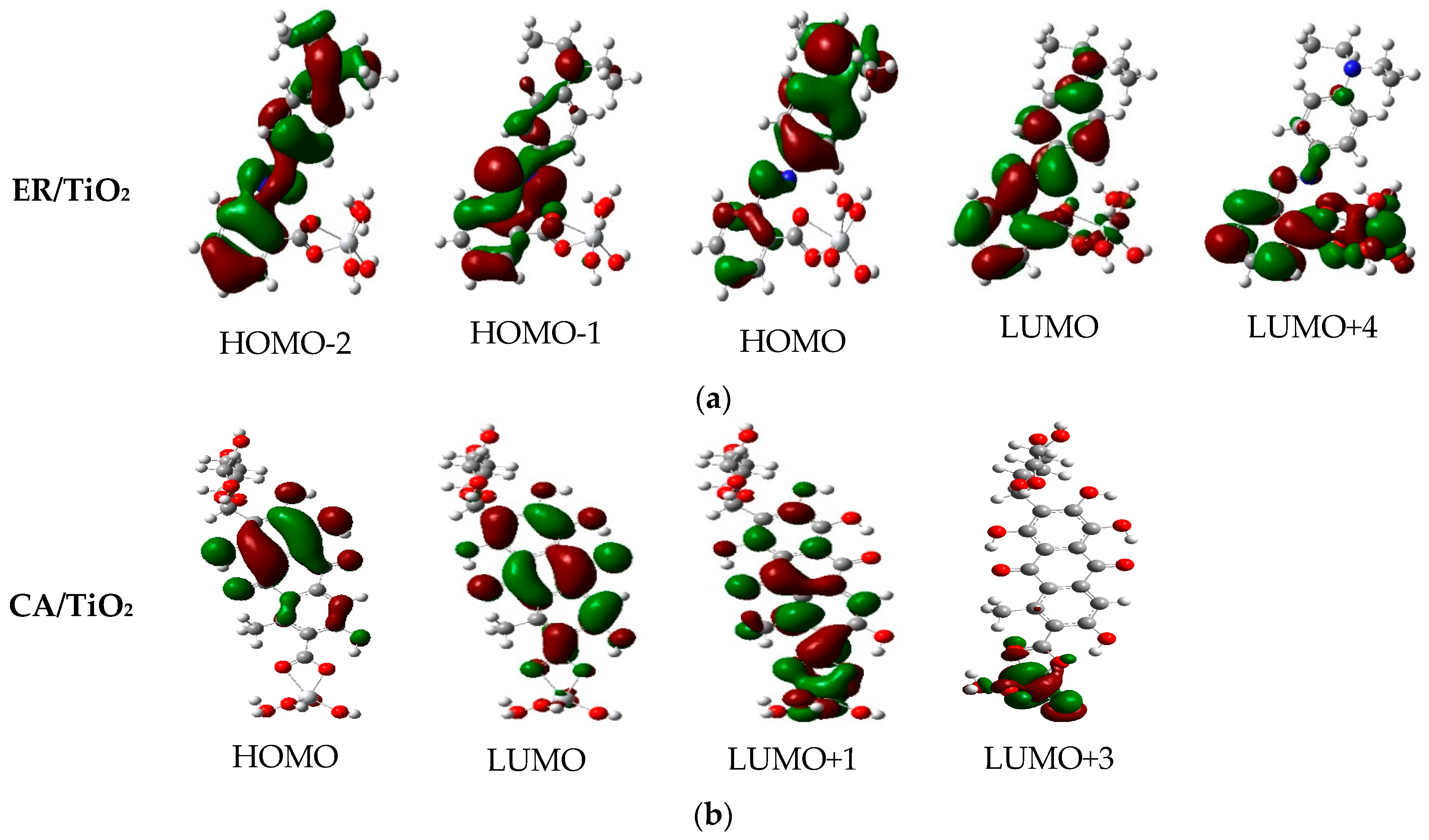

| Dye | State | (nm/eV) | Contribution MO | Strength f | Exp. b | |
|---|---|---|---|---|---|---|
| ER | S1 | 516.42/2.4009 | (0.55904)H-1L | 0.2843 | 502.50 | 499.00 |
| S2 | 483.18/2.5660 | (0.54705)HL | 0.6403 | |||
| S3 | 396.25/3.1289 | (0.66579)HL+1 | 0.0578 | |||
| S4 | 364.76/3.3991 | (0.53490)H-2L | 0.0182 | |||
| S5 | 353.83/3.5040 | (0.54898)H-1L | 0.0242 | |||
| S6 | 341.12/3.6346 | (0.60041)H-4L | 0.0353 | |||
| CA | S1 | 485.78/2.5523 | (0.70012)HL | 0.2331 | 499.00 | 531.00 |
| S2 | 394.10/3.1460 | (0.67917)H-1L | 0.0111 | |||
| S3 | 379.66/3.2657 | (0.59339)H-4L | 0.0001 | |||
| S4 | 352.82/3.5141 | (0.61934)H-2L | 0.1365 | |||
| S5 | 340.32/3.6432 | (0.65265)H-9L | 0.0003 | |||
| S6 | 333.64/3.7161 | (0.55417)HL+1 | 0.0785 |
| Dyes | A × 10−8 (mol·cm−2) a | (V) | (mA·cm−2) | ff | |
|---|---|---|---|---|---|
| ethyl red (ER) | 1.16 | 0.46 | 0.21 | 0.54 | 0.05 |
| carminic acid (CA) | 3.08 | 0.53 | 0.66 | 0.84 | 0.30 |
| N719 | – | 0.74 | 18.91 | 0.62 | 8.74 |
| Dyes | (eV) | (eV) | (eV) | (eV) | (eV) | (Debye) | (eV) | τ (ns) |
|---|---|---|---|---|---|---|---|---|
| ER | −5.30 | 2.57 | −2.73 | −1.27 | −0.50 | 2.20 | 0.337 | 14.05 |
| CA | −6.00 | 2.55 | −3.45 | −0.55 | −1.20 | 7.77 | 0.410 | 15.20 |
| Condition | Dyes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vacuum | ER | 4715.641 | −146.531 | −118.831 | 61.315 | −28.349 | −2.465 | 6.070 | −102.532 | −18.574 | −20.764 | 4495.681 |
| CA | 2199.959 | −367.325 | 304.207 | 120.870 | 116.514 | −8.887 | −43.438 | 7.2242 | −33.653 | 13.928 | 2528.461 | |
| solvent | ER | 23111.926 | −374.194 | −533.766 | 127.514 | 149.044 | 297.519 | 47.8815 | −202.700 | 54.757 | −11.201 | 22377.028 |
| CA | 2742.967 | −1456.004 | 388.367 | 459.224 | −303.563 | −65.006 | 125.558 | −90.987 | −32.281 | −99.709 | 3221.771 |
| Dyes | State | E (eV) | (nm) | Contribution MO | Strength f |
|---|---|---|---|---|---|
| ER | S1 | 2.1153 | 586.13 | (0.67992)HL | 0.3376 |
| S10 | 3.2715 | 378.98 | (0.62913)H-2L | 0.2385 | |
| S15 | 3.5159 | 352.64 | (0.66223)H-1L+4 | 0.0528 | |
| CA | S1 | 2.5252 | 490.98 | (0.70029)HL | 0.2347 |
| S5 | 3.5909 | 345.28 | (0.65835)HL+1 | 0.2007 | |
| S12 | 4.1885 | 296.01 | (0.46691)HL+3 | 0.3310 |
| Dye | A | I | h | ω | ω− | ω+ |
|---|---|---|---|---|---|---|
| ER | 2.45 | 5.15 | 1.35 | 5.35 | 7.42 | 3.62 |
| CA | 3.36 | 5.78 | 1.21 | 8.63 | 11.07 | 6.50 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Li, Y.; Song, P.; Ma, F. An Experimental and Theoretical Investigation of the Electronic Structures and Photoelectrical Properties of Ethyl Red and Carminic Acid for DSSC Application. Materials 2016, 9, 813. https://doi.org/10.3390/ma9100813
Sun C, Li Y, Song P, Ma F. An Experimental and Theoretical Investigation of the Electronic Structures and Photoelectrical Properties of Ethyl Red and Carminic Acid for DSSC Application. Materials. 2016; 9(10):813. https://doi.org/10.3390/ma9100813
Chicago/Turabian StyleSun, Chaofan, Yuanzuo Li, Peng Song, and Fengcai Ma. 2016. "An Experimental and Theoretical Investigation of the Electronic Structures and Photoelectrical Properties of Ethyl Red and Carminic Acid for DSSC Application" Materials 9, no. 10: 813. https://doi.org/10.3390/ma9100813







