Influence of Radiation Sterilization on Properties of Biodegradable Lactide/Glycolide/Trimethylene Carbonate and Lactide/Glycolide/ε-caprolactone Porous Scaffolds with Shape Memory Behavior
Abstract
:1. Introduction
2. Results and Discussion
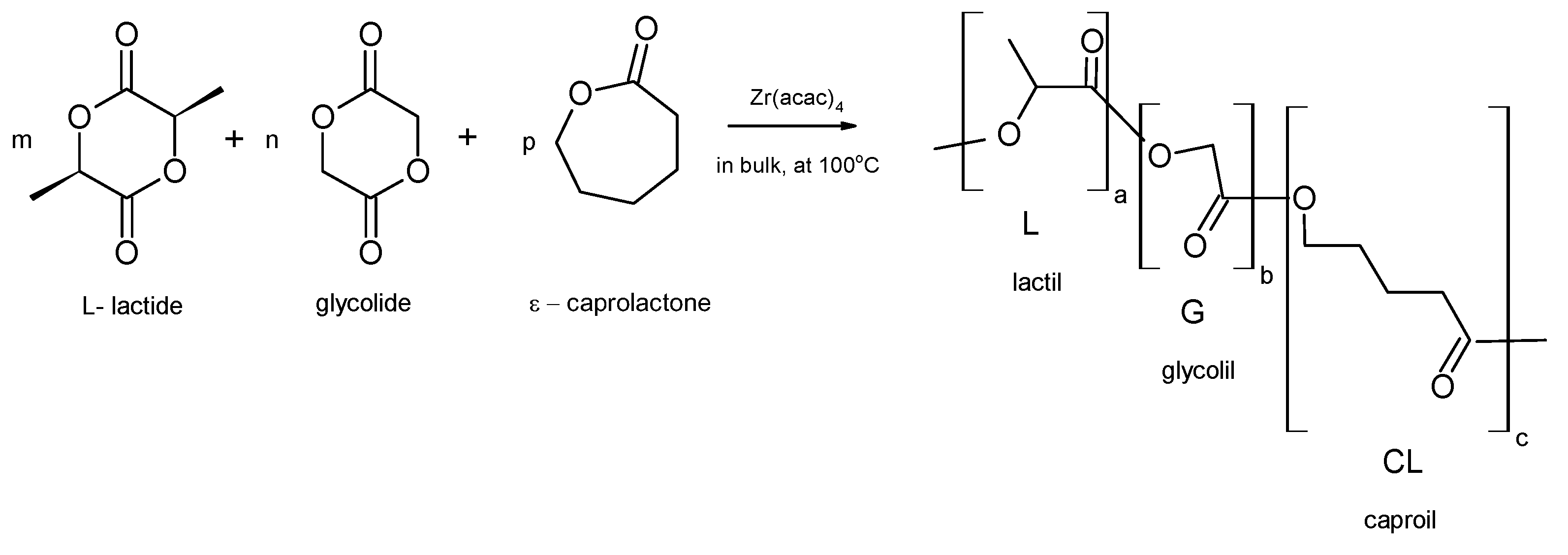
2.1. Scaffolds Formulation and Procedure of Investigations

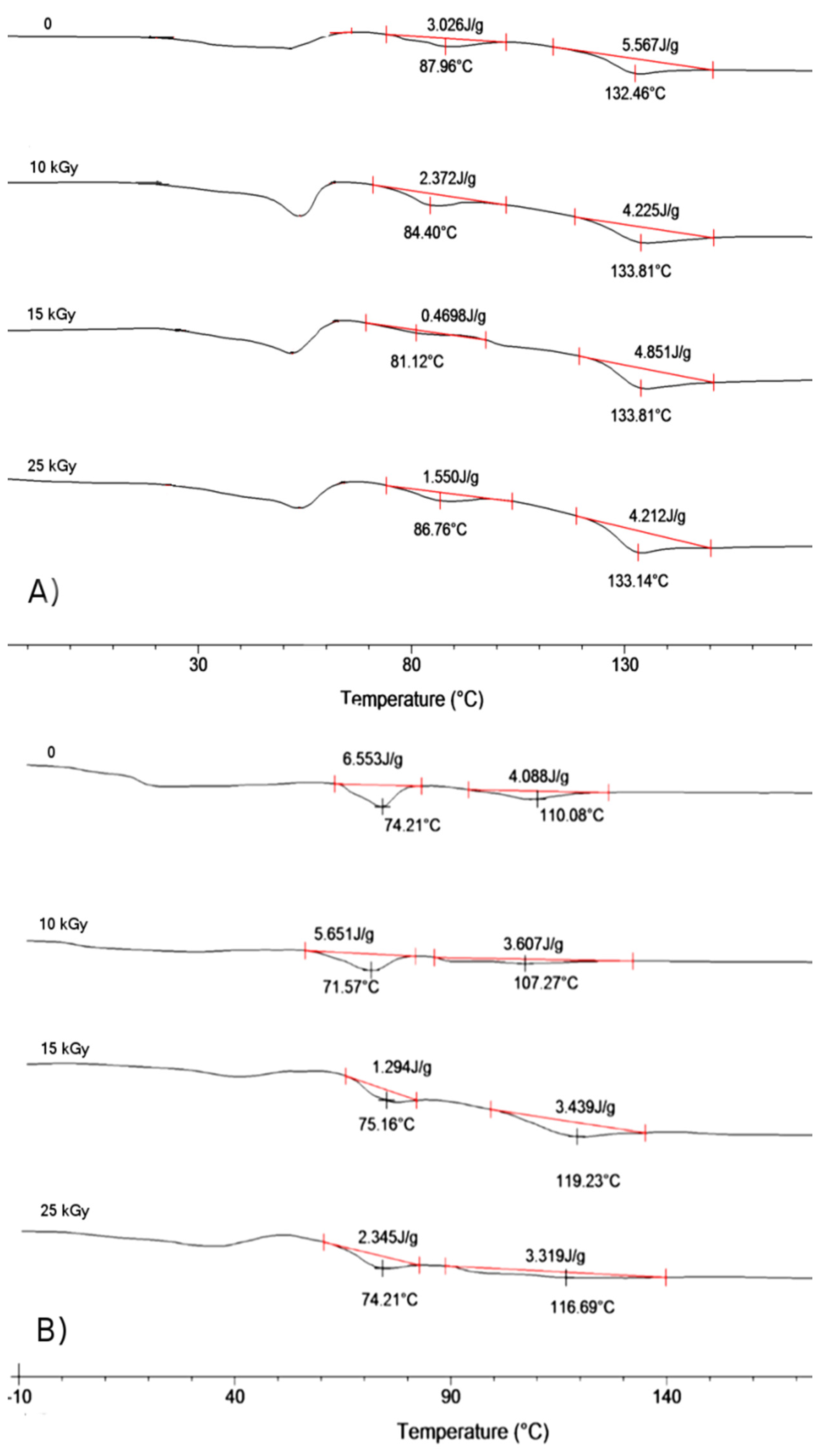
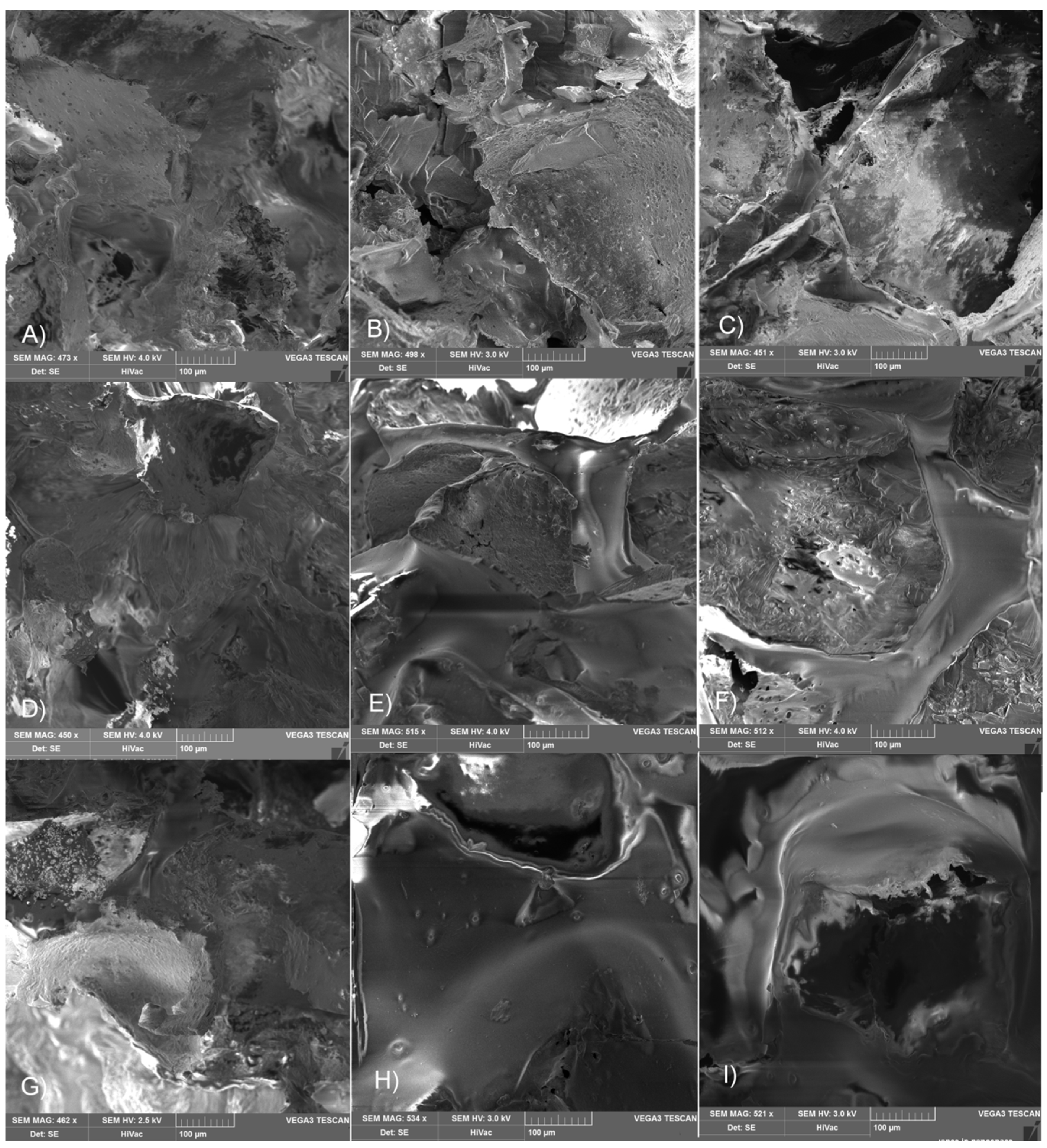
2.2. Influence of Gamma Irradiation on the Properties of Matrices and Scaffolds’ Morphology
| No. | Properties of Polymeric Matrix | Properties of Scaffold | ||||||
|---|---|---|---|---|---|---|---|---|
| Composition (mol. %) | Mn (kg/mol) | Đ | Tg (°C) | Average Porosity P (%) | Average Pore Diameter (µm) | Scaffold Diameter (mm) | Compressive Strength of Dried Scaffolds (MPa) | |
| LGT21 | l-lactide—68 Glycolide—11 TMC units—21 | 36.8 | 2.3 | 47 | 86 ± 5 | 395 ± 59 | 10.1 ± 0.2 | 0.8 ± 0.1 |
| LGT40 | l-lactide—46 Glycolide—14 TMC units40 | 23.9 | 1.9 | 28 | 88 ± 9 | 410 ± 67 | 10.0 ± 0.3 | 0.6 ± 0.1 |
| LGC | l-lactide—79 Glycolide—10 Caprolactone—11 | 35.1 | 2.2 | 55 | 83 ± 3 | 380 ± 45 | 10.2 ± 0.3 | 0.9 ± 0.2 |
| No. | Properties of Polymeric Matrix | Properties of Scaffold at Temporary Shape | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose (kGy) | Mn (kg/mol) | dMn (%) | Đ | Tg (°C) | Average Porosity P (%) | Scaffold Diameter (mm) | Compressive Strength of Dried Scaffolds (MPa) | |
| LGT21 C | 0 | 36.8 | - | 2.3 | 47 | 55 ± 5 | 6.8 ± 0.3 | 1.1 ± 0.1 |
| 10 | 32.7 | 11 | 2.4 | 46.6 | 58 ± 7 | 7.3 ± 0.4 | 1.1 ± 0.2 | |
| 15 | 29.8 | 19 | 2.5 | 46.4 | 57 ± 6 | 7.4 ± 0.3 | 1.0 ± 0.2 | |
| 25 | 23.6 | 36 | 2.7 | 45.6 | 58 ± 7 | 7.4 ± 0.4 | 0.7 ± 0.1 | |
| LGT40 C | 0 | 23.9 | - | 1.9 | 28 | 58 ± 5 | 7.0 ± 0.4 | 0.6 ± 0.1 |
| 10 | 19.4 | 19 | 2.5 | 26.7 | 61 ± 7 | 7.8 ± 0.5 | 0.4 ± 0.1 | |
| 15 | 17.1 | 29 | 2.5 | 26.5 | 62 ± 4 | 7.8 ± 0.3 | 0.4 ± 0.2 | |
| 25 | 13.2 | 45 | 2.8 | 25 | 61 ± 5 | 7.9 ± 0.4 | 0.2 ± 0.1 | |
| LGC C | 0 | 35.1 | - | 2.2 | 55 | 53 ± 3 | 6.7 ± 0.3 | 0.9 ± 0.2 |
| 10 | 31.7 | 10 | 2.5 | 53.6 | 57 ± 4 | 7.4 ± 0.3 | 0.8 ± 0.2 | |
| 15 | 29,8 | 15 | 2.9 | 53.5 | 58 ± 5 | 7.6 ± 0.5 | 0.8 ± 0.1 | |
| 25 | 26,2 | 25 | 2.9 | 52.6 | 58 ± 4 | 7.4 ± 0.4 | 0.5 ± 0.2 | |

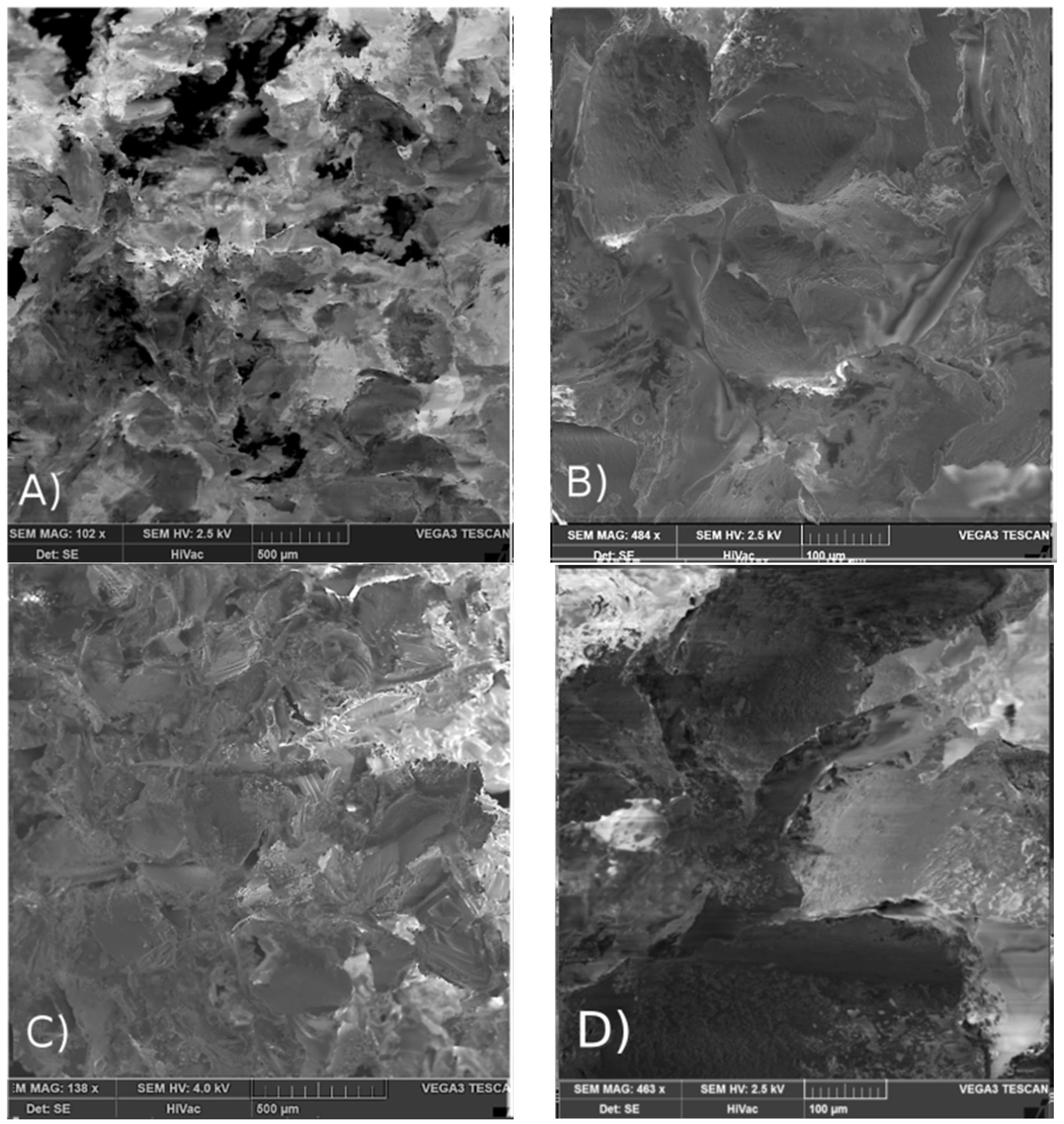
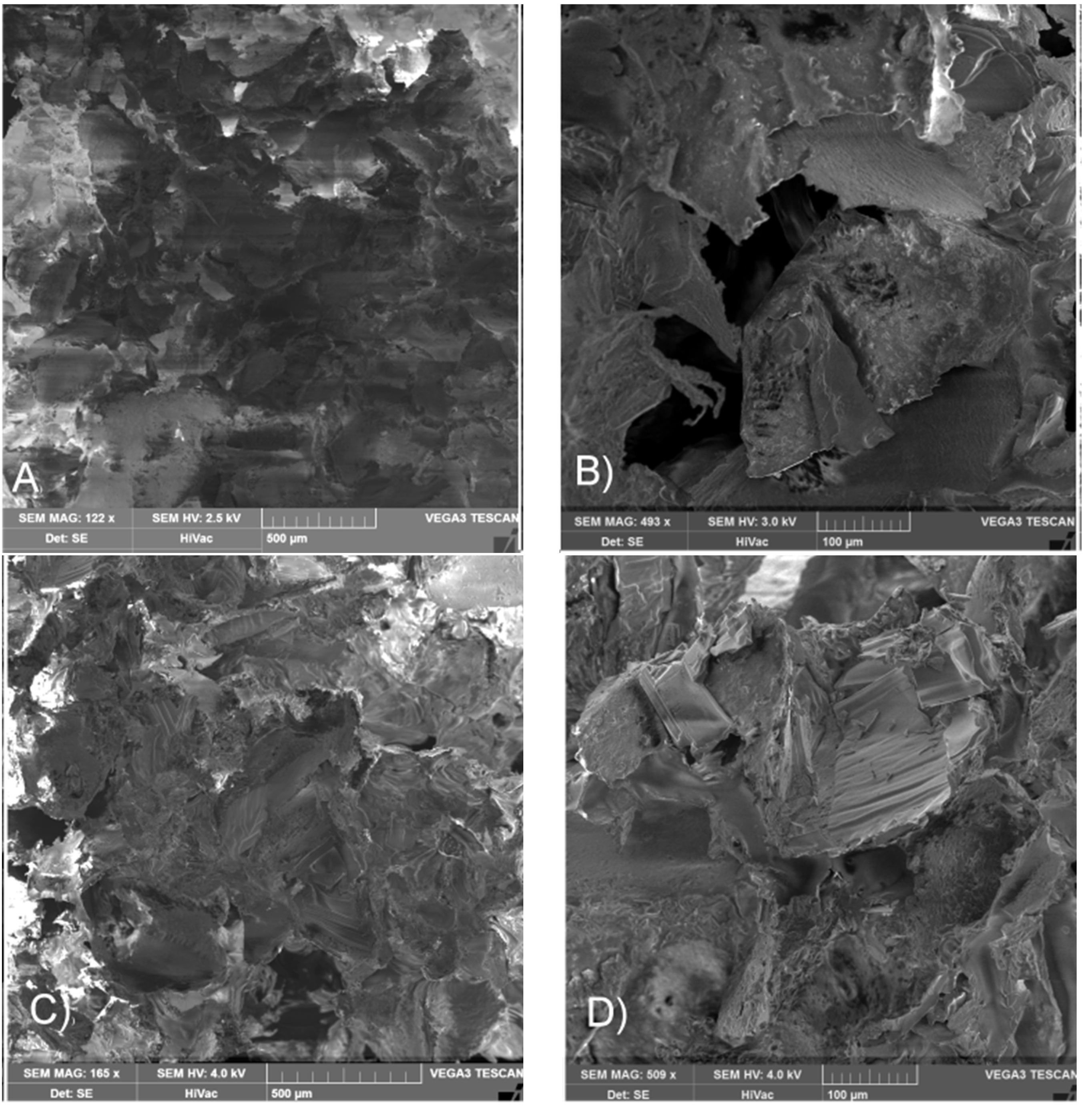
2.3. Influence of Electron Beam Sterilization on the Properties of Matrices and Scaffolds Morphology
| No. | Properties of Polymeric Matrix | Properties of Scaffold at Temporary Shape | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose (kGy) | Mn (kg/mol) | dMn (%) | Đ | Tg (°C) | Average Porosity P (%) | Scaffold Diameter (mm) | Compressive Strength of Dried Scaffolds (MPa) | |
| LGT21 C | 0 | 36.8 | - | 2.3 | 47 | 55 ± 5 | 6.8 ± 0.3 | 1.1 ± 0.1 |
| 10 | 34.1 | 7 | 2.4 | 47 | 57 ± 6 | 7.2 ± 0.5 | 1.1 ± 0.2 | |
| 15 | 32.8 | 11 | 2.4 | 46.3 | 56 ± 4 | 7.1 ± 0.3 | 1.0 ± 0.2 | |
| 25 | 25.8 | 30 | 3.2 | 45.8 | 57 ± 4 | 7.2 ± 0.4 | 0.9 ± 0.2 | |
| LGT40 C | 0 | 23.9 | - | 1.9 | 28 | 58 ± 5 | 7.0 ± 0.4 | 0.6 ± 0.1 |
| 10 | 22.0 | 8 | 2.0 | 25 | 57 ± 6 | 7.1 ± 0.5 | 0.6 ± 0.2 | |
| 15 | 20.2 | 15 | 2.0 | 25 | 60 ± 7 | 8.3 ± 0.5 | 0.7 ± 0.3 | |
| 25 | 19.2 | 20 | 2.3 | 24.5 | 63 ± 4 | 8.5 ± 0.5 | 0.5 ± 0.2 | |
| LGC C | 0 | 35.1 | - | 2.2 | 55 | 53 ± 3 | 6.7 ± 0.3 | 0.9 ± 0.2 |
| 10 | 33.7 | 4 | 2.2 | 53 | 54 ± 4 | 7.1 ± 0.2 | 0.9 ± 0.1 | |
| 15 | 33.1 | 6 | 2.3 | 53.5 | 59 ± 4 | 7.4 ± 0.3 | 0.9 ± 0.2 | |
| 25 | 30.8 | 12 | 2.7 | 52.6 | 60 ± 6 | 7.6 ± 0.4 | 0.7 ± 0.3 | |

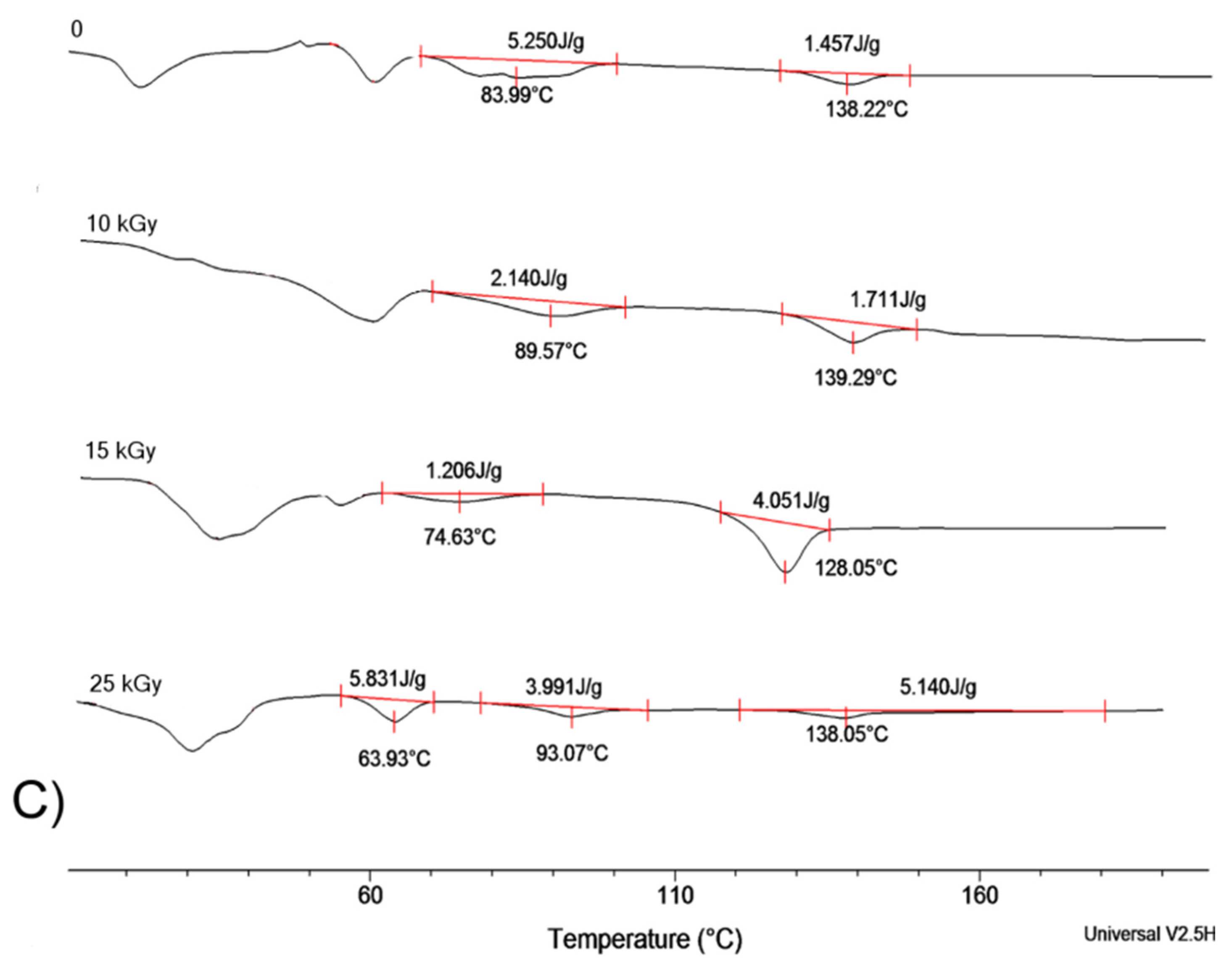
2.4. Effect of Sterilization on Shape Memory Properties
| The Type of Sterilization | No. | Dose (kGy) | Tr (°C) | em (mm) | Ds (mm) | er (mm) | tR (s) | Rr (%) |
|---|---|---|---|---|---|---|---|---|
| Unsterilized | LGT21C | 0 | 37 | 10.1 | 6.8 | 9.7 | 600 | 96 |
| LGT40 C | 0 | 30 | 10.0 | 7.0 | 9.6 | 320 | 96 | |
| LGC C | 0 | 41 | 10.2 | 6.7 | 9.4 | 340 | 94 | |
| Gamma Irradiation | LGT21 C | 25 | 37 | 10.1 | 7.4 | 9.1 | 690 | 89 |
| LGT40 C | 25 | 30 | 10.0 | 7.9 | 8.6 | 520 | 86 | |
| LGC C | 25 | 41 | 10.2 | 7.4 | 9.2 | 380 | 90 | |
| Electron Beam | LGT21 C | 25 | 37 | 10.1 | 7.2 | 9.6 | 620 | 95 |
| LTG40 C | 25 | 30 | 10.0 | 8.5 | 8.5 | >1000 | nd | |
| LGC C | 25 | 41 | 10.2 | 7.6 | 9.1 | 410 | 89 |
3. Experimental Section
3.1. Terpolymers Synthesis and Characterization
3.1.1. Materials and Chemicals
3.1.2. Synthesis of l-lactide/glycolide/trimethylene Carbonate Terpolymers
3.1.3. Synthesis of l-lactide/glycolide/ε-caprolactone Terpolymer
3.1.4. Terpolymers and Scaffolds Characterization
3.2. Formulation of Scaffolds and Methods of Their Characterization
3.3. Deformation Scaffolds to Temporary Shape
3.4. Measurements of Scaffolds' Shape Memory Behavior
3.5. Conditions of Sterilization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Athanasiou, K.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Shearer, H.; Ellis, M.J.; Perera, S.P.; Chaudhuri, J.B. Effects of common sterilization methods on the structure and properties of poly(d,l-lactic-co-glycolic acid) scaffolds. Tissue Eng. 2006, 12, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Cottam, E.; Hukins, D.W.L.; Lee, K.; Hewitt, C.; Jenkins, M.J. Effect of sterilisation by gamma irradiation on the ability of polycaprolactone (PCL) to act as a scaffold material. Med. Eng. Phys. 2009, 31, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainer, A.; Centola, M.; Spadaccio, C.; Gherardi, G.; Genovese, J.; Licoccia, S.; Trombetta, M. Comparative study of different techniques for the sterilization of poly-l-lactide electrospun microfibers: Effectiveness vs. material degradation. Int. J. Artif. Organs 2010, 33, 76–85. [Google Scholar] [PubMed]
- De Nardo, L.; Alberti, R.; Cigada, A.; Yahia, L.; Tanzi, M.C.; Farè, S. Shape memory polymer foams for cerebral aneurysm reparation: Effects of plasma sterilization on physical properties and cytocompatibility. Acta Biomater. 2009, 5, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M.; Dobrzynski, P.; Kasperczyk, J.; Bero, M. Shape memory behavior of novel (l-Lactide-Glycolide-Trimethylene carbonate) terpolymers. Biomacromolecules 2007, 8, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Smola, A.; Dobrzynski, P.; Cristea, A.; Kasperczyk, J.; Sobota, M.; Gebarowska, K.; Janeczek, H. Bioresorbable terpolymers based on l-lactide, glycolide and trimethylene carbonate with shape memory behaviour. Polym. Chem. 2014, 5, 2442–2452. [Google Scholar] [CrossRef]
- Mendes, G.C.C.; Brandão, T.R.S.; Silva, C.L.M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, L.; Moscatelli, M.; Silvi, F.; Tanzi, M.C.; Yahia, L.; Farè, S. Chemico-physical modifications induced by plasma and ozone sterilizations on shape memory polyurethane foams. J. Mater. Sci. Mater. Med. 2010, 21, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.F.; Mather, P.T.; Henderson, J.H. Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater. 2013, 9, 8790–8801. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, L.; Bertoldi, S.; Cigada, A.; Tanzi, M.C.; Haugen, H.J.; Farè, S. Preparation and characterization of shape memory polymer scaffolds via solvent casting/particulate leaching. J. Appl. Biomater. Funct. Mater. 2012, 10, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Holy, C.E.; Cheng, C.; Davies, J.E.; Shoichet, M.S. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 2001, 22, 25–31. [Google Scholar] [CrossRef]
- Odelius, K.; Plikk, P.; Albertsson, A.C. The influence of composition of porous copolyester scaffolds on reactions induced by irradiation sterilization. Biomaterials 2008, 29, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yakacki, C.M.; Lyons, M.B.; Rech, B.; Gall, K.; Shandas, R. Cytotoxicity and thermomechanical behavior of biomedical shape-memory polymer networks post-sterilization. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Rychter, P.; Pamula, E.; Orchel, A.; Posadowska, U.; Krok-Borkowicz, M.; Kaps, A.; Smigiel-Gac, N.; Smola, A.; Kasperczyk, J.; Prochwicz, W.; et al. Scaffolds with shape memory behavior for the treatment of large bone defects. J. Biomed. Mater. Res. Part A 2015, 103, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Wawryło, L.; Rychter, P.; Prochwicz, W.; Smola, A.; Dobrzyński, P. Hydrolitic degradation of biodegradable scaffolds based on l-lactide/glycolide/TMC and l-lactide/glycolide/e-caprolactone ter polymers. Eng. Biomater. 2015, 18, 10–19. [Google Scholar]
- Dobrzynski, P. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. III. Synthesis and chain-microstructure analysis of terpolymer obtained from l-lactide, glycolide, and ε-caprolactone initiated by zirconium(IV) acetylacetonate. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3129–3143. [Google Scholar] [CrossRef]
- Pamuła, E.; Dobrzyński, P.; Bero, M.; Paluszkiewicz, C. Hydrolytic degradation of porous scaffolds for tissue engineering from terpolymer of l-lactide, ε-caprolactone and glycolide. J. Mol. Struct. 2005, 744–747, 557–562. [Google Scholar] [CrossRef]
- Cairns, M.L.; Dickson, G.R.; Orr, J.F.; Farrar, D.; Hardacre, C.; Sa, J.; Lemoine, P.; Mughal, M.Z.; Buchanan, F.J. The potential of electron beam radiation for simultaneous surface modification and bioresorption control of PLLA. J. Biomed. Mater. Res. Part A 2012, 100, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Gębarowska, K.; Kasperczyk, J.; Dobrzyński, P.; Scandola, M.; Zini, E.; Li, S. NMR analysis of the chain microstructure of biodegradable terpolymers with shape memory properties. Eur. Polym. J. 2011, 47, 1315–1327. [Google Scholar] [CrossRef]
- Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry; ASTM E 1356; ASTM International: West Conshohocken, PA, USA, 2014.
- Pamula, E.; Menaszek, E. In vitro and in vivo degradation of poly(l-lactide-co-glycolide) films and scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams; ASTM D3574-08; ASTM International: West Conshohocken, PA, USA, 2008.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychter, P.; Śmigiel-Gac, N.; Pamuła, E.; Smola-Dmochowska, A.; Janeczek, H.; Prochwicz, W.; Dobrzyński, P. Influence of Radiation Sterilization on Properties of Biodegradable Lactide/Glycolide/Trimethylene Carbonate and Lactide/Glycolide/ε-caprolactone Porous Scaffolds with Shape Memory Behavior. Materials 2016, 9, 64. https://doi.org/10.3390/ma9010064
Rychter P, Śmigiel-Gac N, Pamuła E, Smola-Dmochowska A, Janeczek H, Prochwicz W, Dobrzyński P. Influence of Radiation Sterilization on Properties of Biodegradable Lactide/Glycolide/Trimethylene Carbonate and Lactide/Glycolide/ε-caprolactone Porous Scaffolds with Shape Memory Behavior. Materials. 2016; 9(1):64. https://doi.org/10.3390/ma9010064
Chicago/Turabian StyleRychter, Piotr, Natalia Śmigiel-Gac, Elżbieta Pamuła, Anna Smola-Dmochowska, Henryk Janeczek, Wojciech Prochwicz, and Piotr Dobrzyński. 2016. "Influence of Radiation Sterilization on Properties of Biodegradable Lactide/Glycolide/Trimethylene Carbonate and Lactide/Glycolide/ε-caprolactone Porous Scaffolds with Shape Memory Behavior" Materials 9, no. 1: 64. https://doi.org/10.3390/ma9010064







