Parameters Influencing the Growth of ZnO Nanowires as Efficient Low Temperature Flexible Perovskite-Based Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
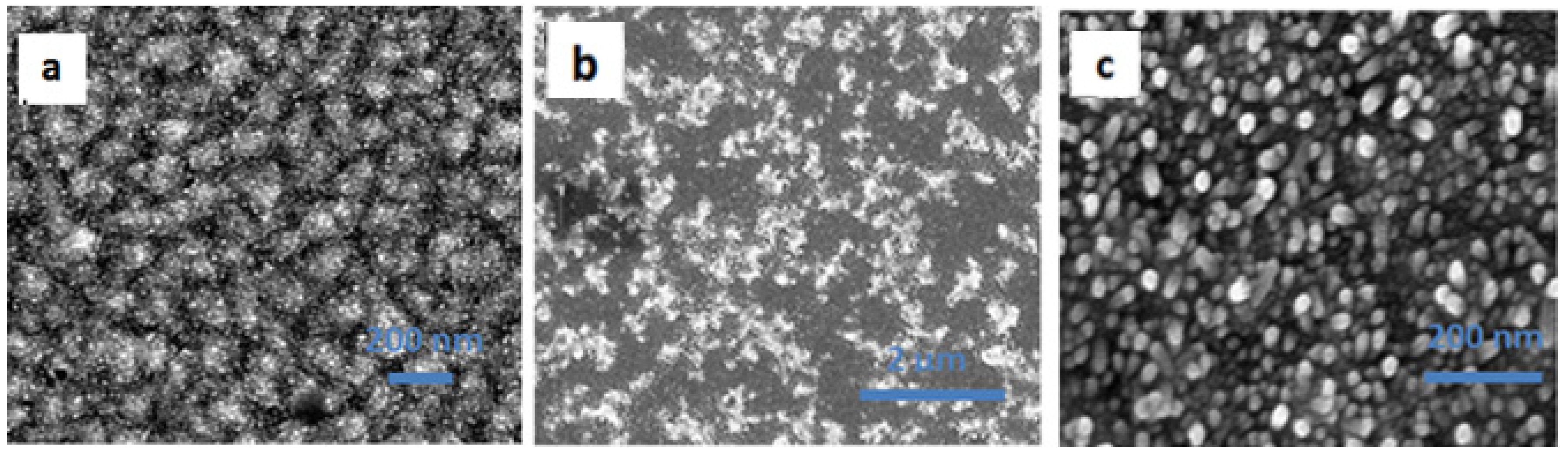
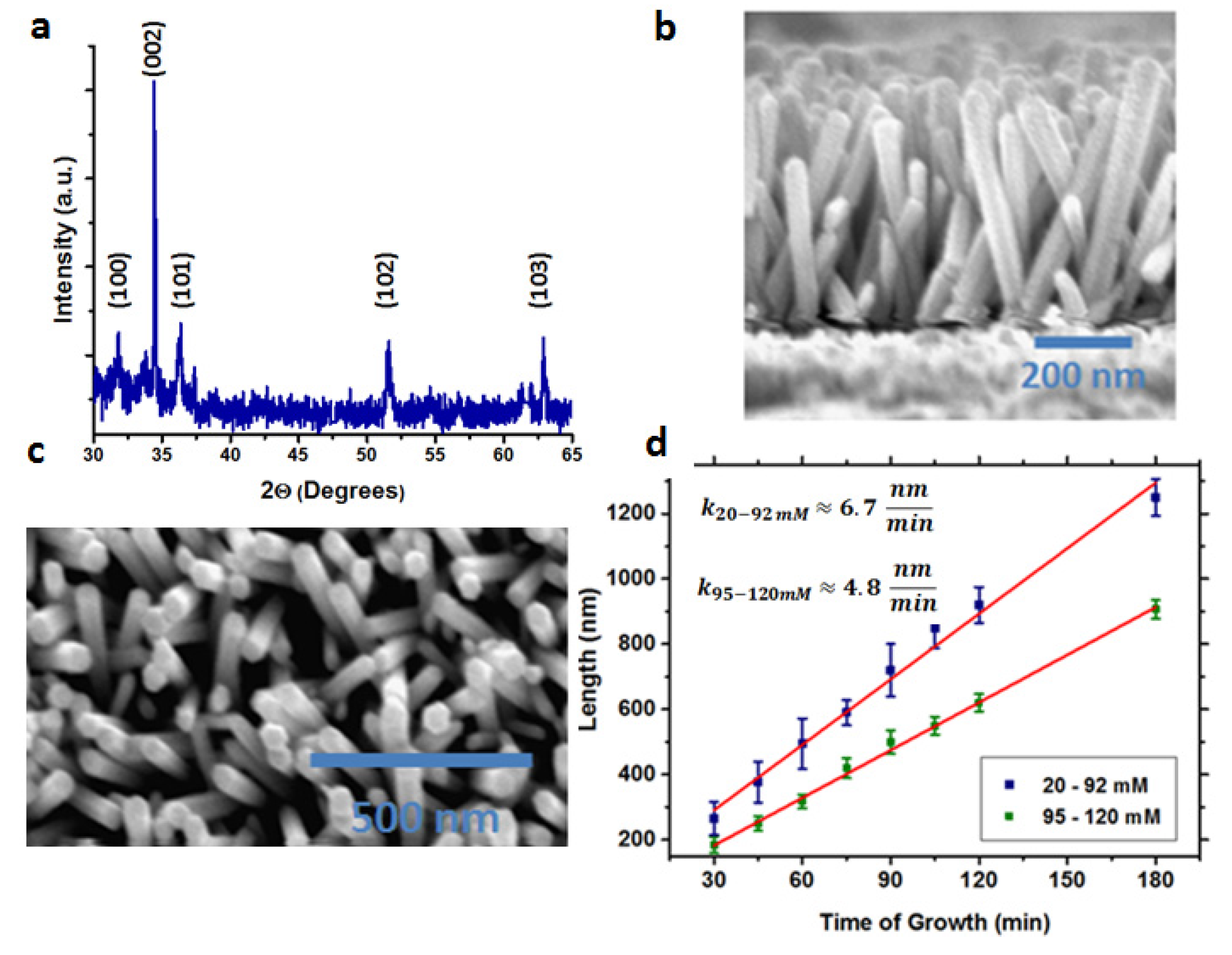
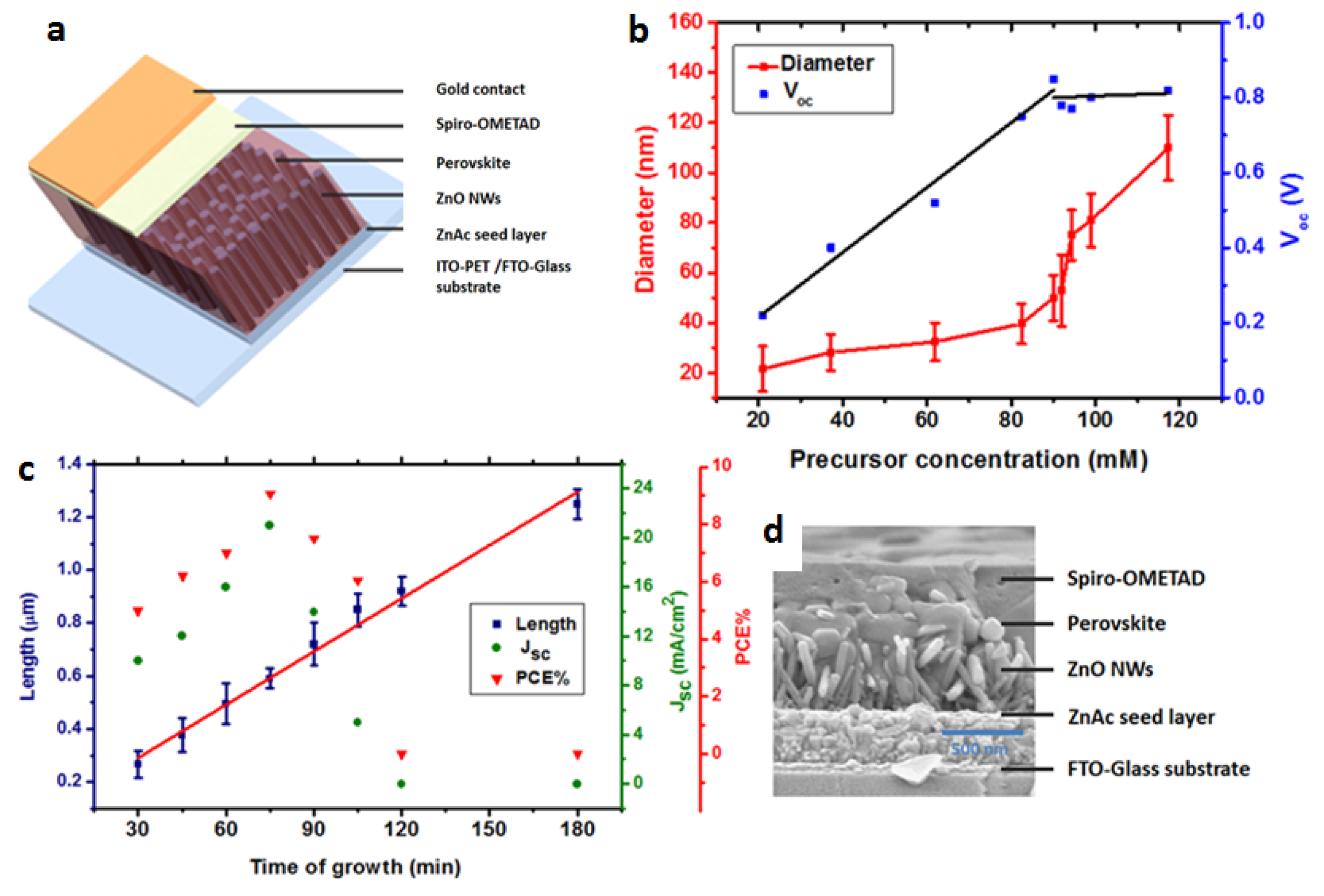
| Solar Cell Substrate | Jsc (mA/cm2) | FF (%) | VOC (V) | Efficiency (%) | Average (%) |
|---|---|---|---|---|---|
| Rigid Device (FTO-Glass) | 21.56 | 62.3 | 0.675 | 9.06 | 7.5% ± 0.6 |
| Flexible Device (ITO-PET) | 14.42 | 64.7 | 0.684 | 6.39 | 5.6% ± 0.6 |

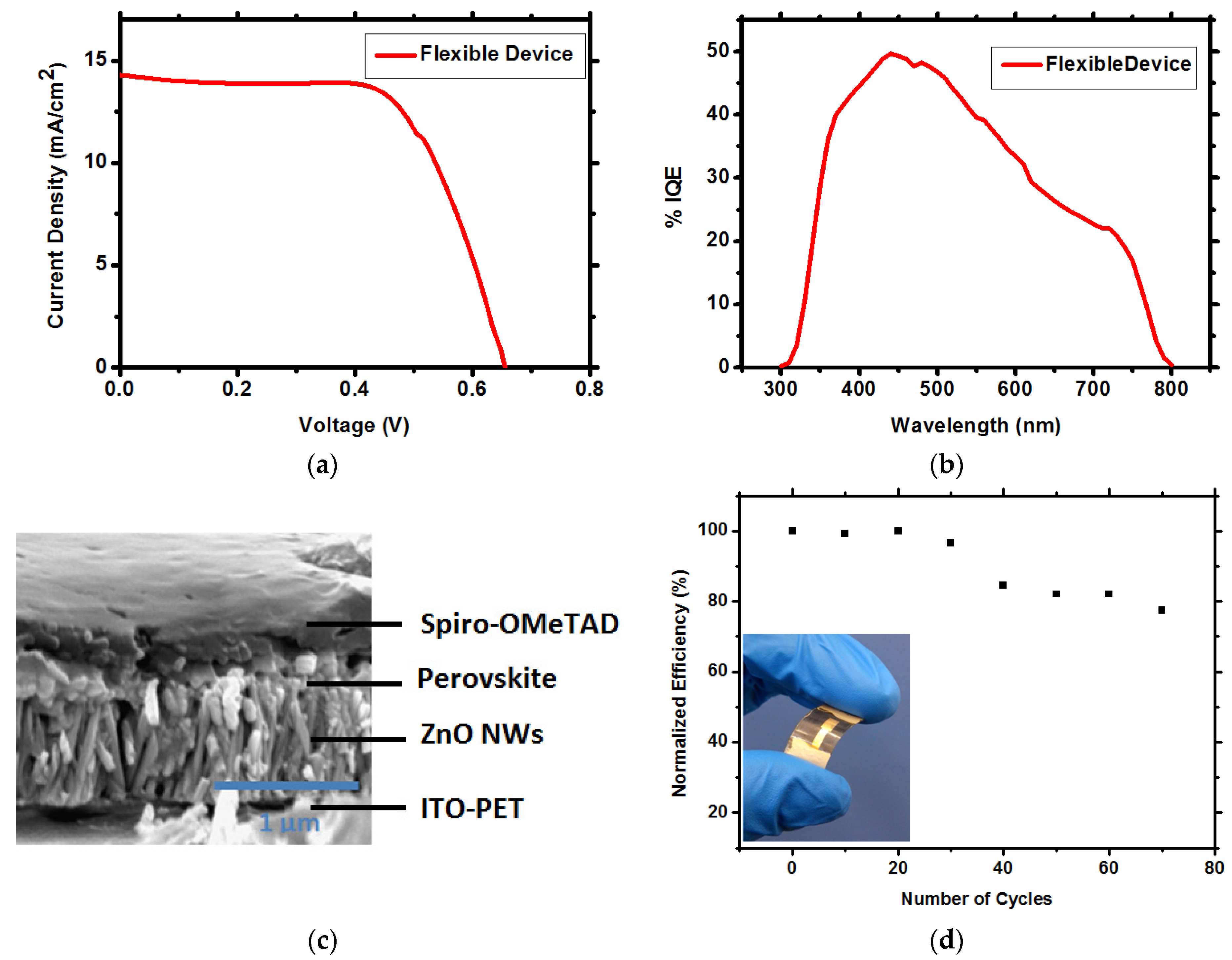
3. Experimental
3.1. ZnO NWs Growth
3.2. Solar Cell Fabrication
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Solar Simulator
3.2.3. Incident Photon to Current Efficiency (IPCE)
3.2.4. X-ray Diffraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Best Research-Cell Efficiencies. Available online: http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed on 1 December 2015).
- Gamliel, S.; Etgar, L. Organo-metal perovskite based solar cells: Sensitized versus planar architecture. RSC Adv. 2014, 4, 29012–29021. [Google Scholar] [CrossRef]
- Aharon, S.; Gamliel, S.; El Cohen, B.; Etgar, L. Depletion region effect of highly efficient hole conductor free CH3NH3PbI3 perovskite solar cells. Phys. Chem. Chem. Phys 2014, 16, 10512–10518. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Moon, S.-J.; Haggman, L.; Boschloo, G.; Yang, L.; Johansson, E.M.; Nazeeruddin, M.K.; Gratzel, M.; Hagfeldt, A. Using a two-step deposition technique to prepare perovskite (CH3NH3PbI3) for thin film solar cells based on ZrO2 and TiO2 mesostructures. RSC Adv. 2013, 3, 18762–18766. [Google Scholar] [CrossRef]
- Lv, S.; Han, L.; Xiao, J.; Zhu, L.; Shi, J.; Wei, H.; Xu, Y.; Dong, J.; Xu, X.; Li, D.; et al. Mesoscopic TiO2/CH3NH3PbI3 perovskite solar cells with new hole-transporting materials containing butadiene derivatives. Chem. Commun. 2014, 50, 6931–6934. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, Y.; Shi, J.; Wei, H.; Xiao, J.; Xu, X.; Luo, J.; Xu, J.; Li, D.; Luo, Y.; et al. Impressive enhancement in the cell performance of ZnO nanorod-based perovskite solar cells with Al-doped ZnO interfacial modification. Chem. Commun. 2014, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Lim, D.C.; Hu, J.; Kwon, J.D.; Jeong, M.G.; Seo, H.O.; Lee, J.Y.; Jang, K.Y.; Lim, J.H.; Lee, K.H.; et al. Surface modification of a ZnO electron-collecting layer using atomic layer deposition to fabricate high-performing inverted organic photovoltaics. ACS Appl. Mater. Interfaces 2013, 5, 8718–8723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.H.; Yantara, N.; Dharani, S.; Graẗzel, M.; Mhaisalkar, S.; Boix, P.P.; Mathews, N. Low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Commun. 2013, 49, 11089–11091. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Boschloo, G.; Schwarzmüller, S.; Yang, L.; Johanssona, E.M.J.; Hagfeldt, A. Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells. Nanoscale 2013, 5, 11686–11691. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; López-Santos, M.C.; Guillen, E.; Nazeeruddin, M.K.; Grätzel, M.; Gonzalez-Elipe, A.R.; Ahmad, S. Perovskite solar cells based on nanocolumnar plasma-deposited ZnO thin films. ChemPhysChem 2014, 15, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Barboux, P.; Pauporté, T. Electrochemical design of nanostructured ZnO charge carrier layers for efficient solid-state perovskite sensitized solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Tan, D.H.; Montano, M.; Goldberger, J.; Somorjai, G.; Yang, P. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Vayssiers, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solution. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Huu, N.K.; Son, D.-Y.; Jang, I.-H.; Lee, C.-R.; Park, N.-G. Hierarchical SnO2 nanoparticle-ZnO nanorod photoanode for improving transport and life time of photoinjected electrons in dye-sensitized solar cell. ACS Appl. Mater. Interfaces 2013, 5, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Chung, J.; Kim, S.-J.; Park, N.-G. Synthesis, structure, and photovoltaic property of a nanocrystalline 2H perovskite-type novel sensitizer (CH3CH2NH3)PbI3. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Aharon, S.; Cohen, B.E.; Etgar, L. Hybrid lead halide iodide and lead halide bromide in efficient hole conductor free perovskite solar cell. J. Phys. Chem. C 2014, 118, 17160–17165. [Google Scholar] [CrossRef]
- El Cohen, B.; Gamliel, S.; Etgar, L. Parameters influencing the deposition of methylammonium lead halide iodide in hole conductor free perovskite-based solar cells. APL Mater. 2014, 2. [Google Scholar] [CrossRef]
- Noha, J.H.; Jeona, N.J.; Choia, Y.C.; Nazeeruddin, K.; Grätzel, M.; Seok, S.I. Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/co-complex as hole-transporting material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Aharon, S.; Layani, M.; El Cohen, B.; Shukrun, E.; Magdassi, S.; Etgar, L. Self-assembly of perovskite for fabrication of semitransparent perovskite solar cells. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymshits, A.; Iagher, L.; Etgar, L. Parameters Influencing the Growth of ZnO Nanowires as Efficient Low Temperature Flexible Perovskite-Based Solar Cells. Materials 2016, 9, 60. https://doi.org/10.3390/ma9010060
Dymshits A, Iagher L, Etgar L. Parameters Influencing the Growth of ZnO Nanowires as Efficient Low Temperature Flexible Perovskite-Based Solar Cells. Materials. 2016; 9(1):60. https://doi.org/10.3390/ma9010060
Chicago/Turabian StyleDymshits, Alex, Lior Iagher, and Lioz Etgar. 2016. "Parameters Influencing the Growth of ZnO Nanowires as Efficient Low Temperature Flexible Perovskite-Based Solar Cells" Materials 9, no. 1: 60. https://doi.org/10.3390/ma9010060






