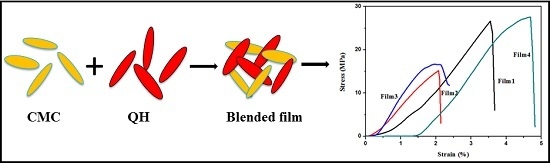
Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Quaternized Hemicelluloses
2.3. Preparation of Blended Films
| Sample Code | QH: CMC (V/V) |
|---|---|
| Film 1 | 1.0:1.0 |
| Film 2 | 1.0:1.5 |
| Film 3 | 2.0:1.0 |
| Film 4 | 1.5:1.0 |
2.4. FT-IR Spectroscopy
2.5. X-ray Diffraction
2.6. Atomic Force Microscopy (AFM)
2.7. Scanning Electron Microscopy (SEM)
2.8. Light Transmittance
2.9. Tensile Properties
2.10. Water Vapor Permeability
3. Results and Discussion
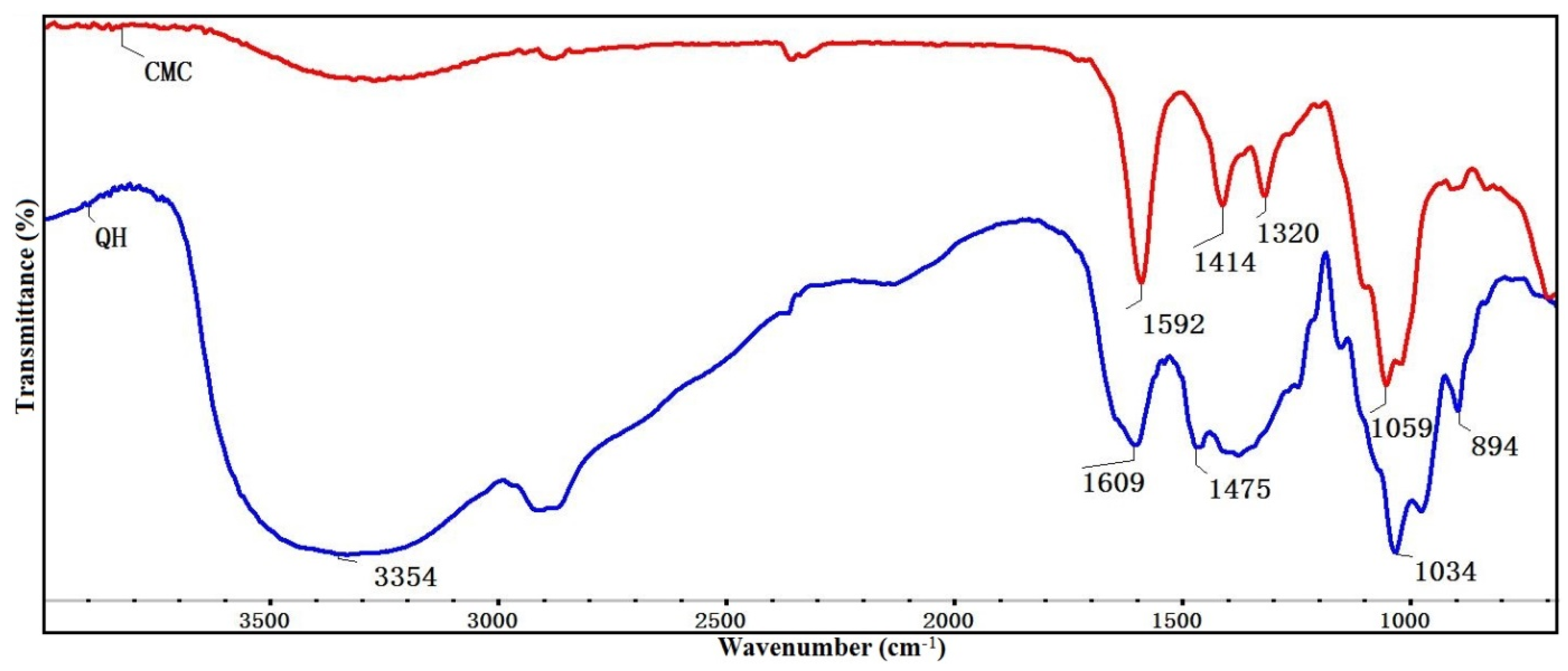
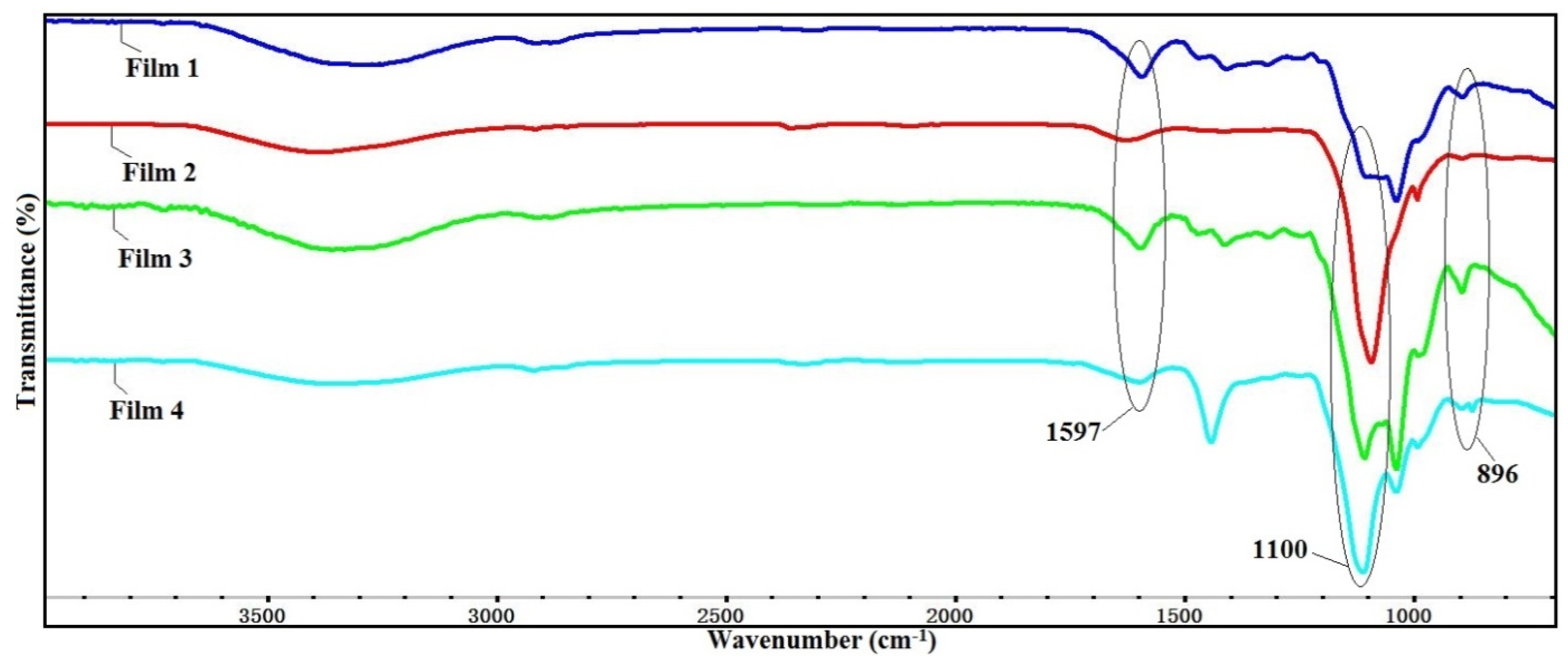
3.1. FT-IR Analysis
3.2. X-ray Analysis
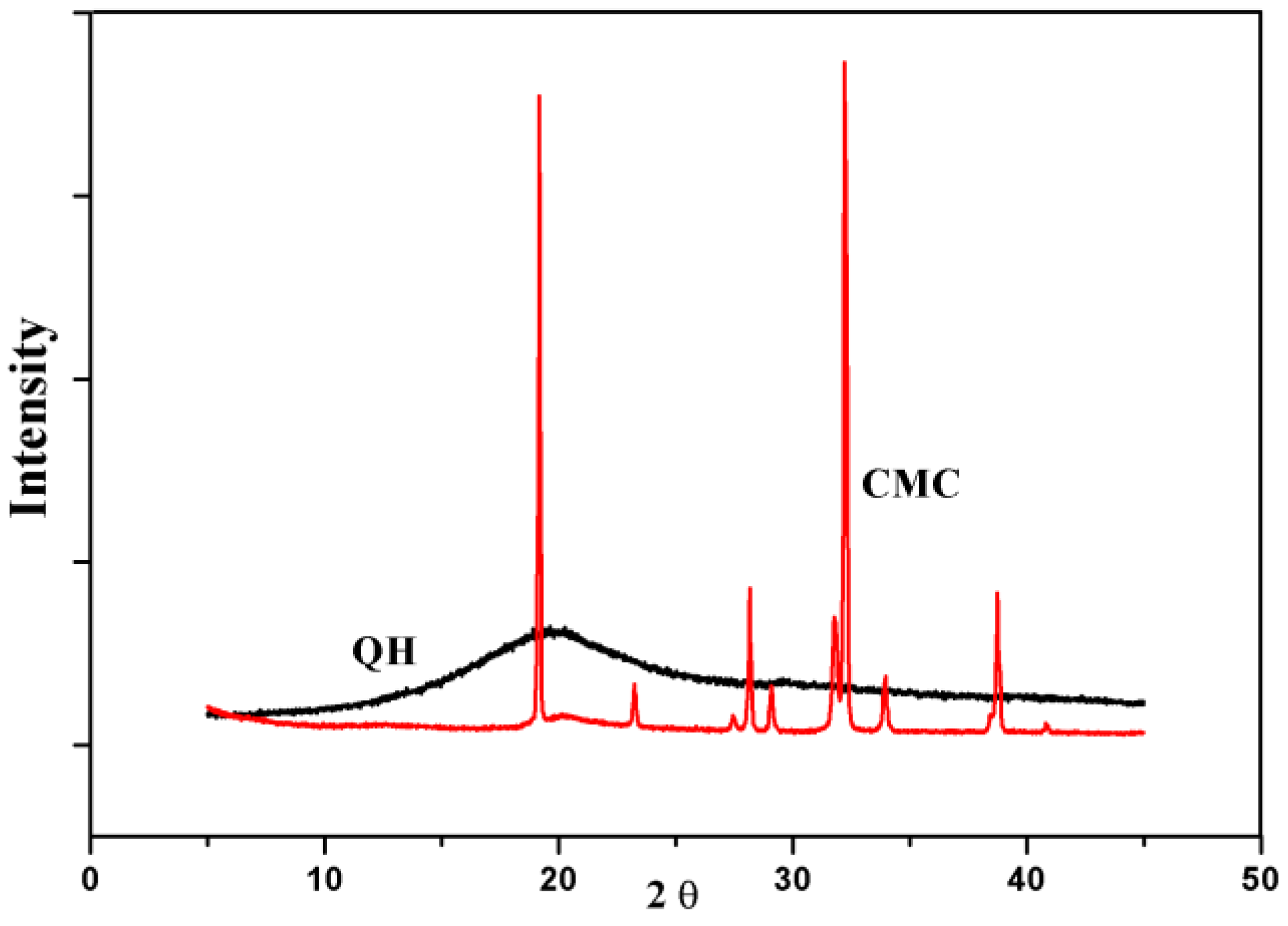
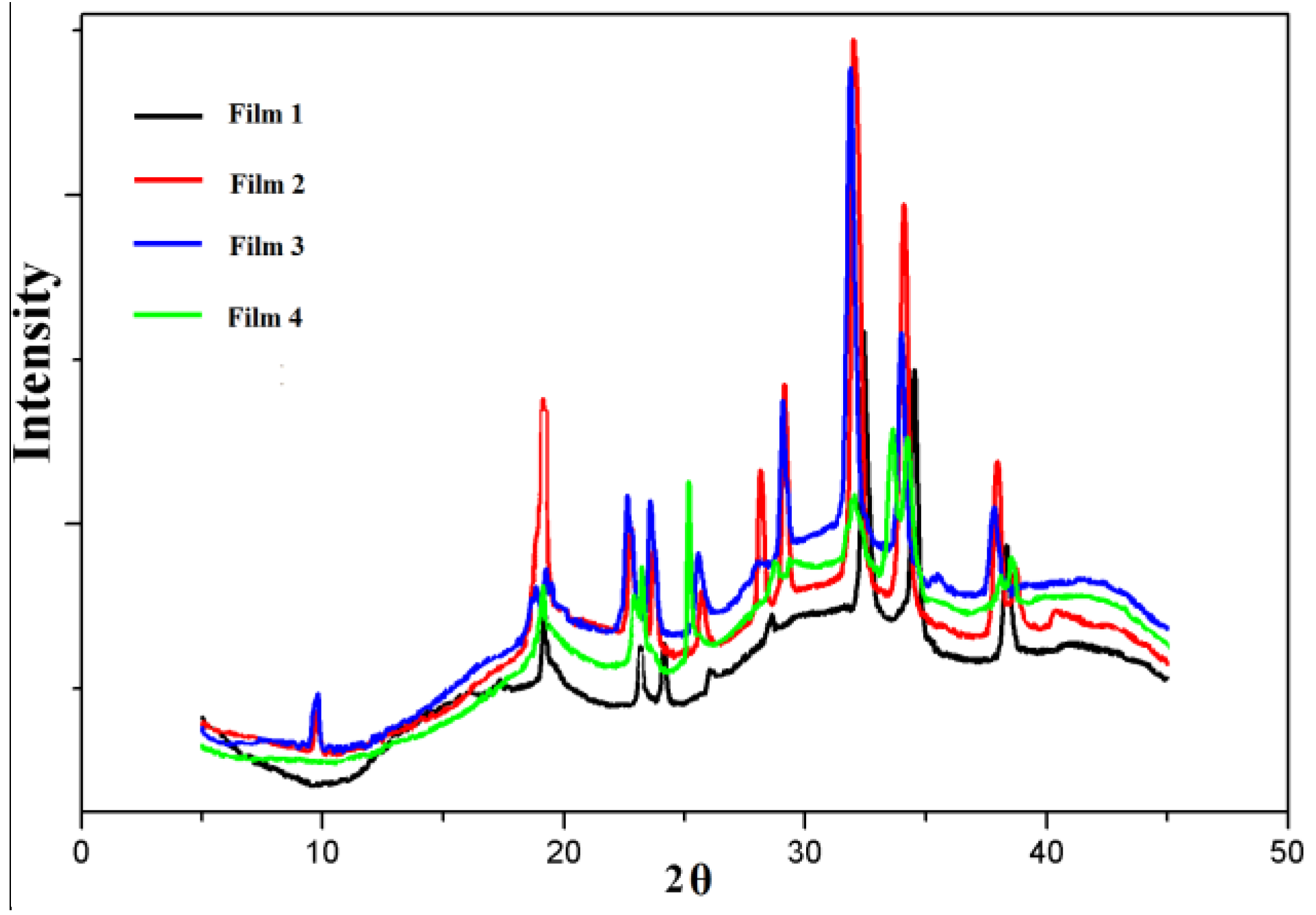
| Sample Code | QH | CMC | Film 1 | Film 2 | Film 3 | Film 4 |
|---|---|---|---|---|---|---|
| Crystallinity(%) | 9 | 84.6 | 27.7 | 33.4 | 34 | 35.9 |
3.3. Morphology of Blended films
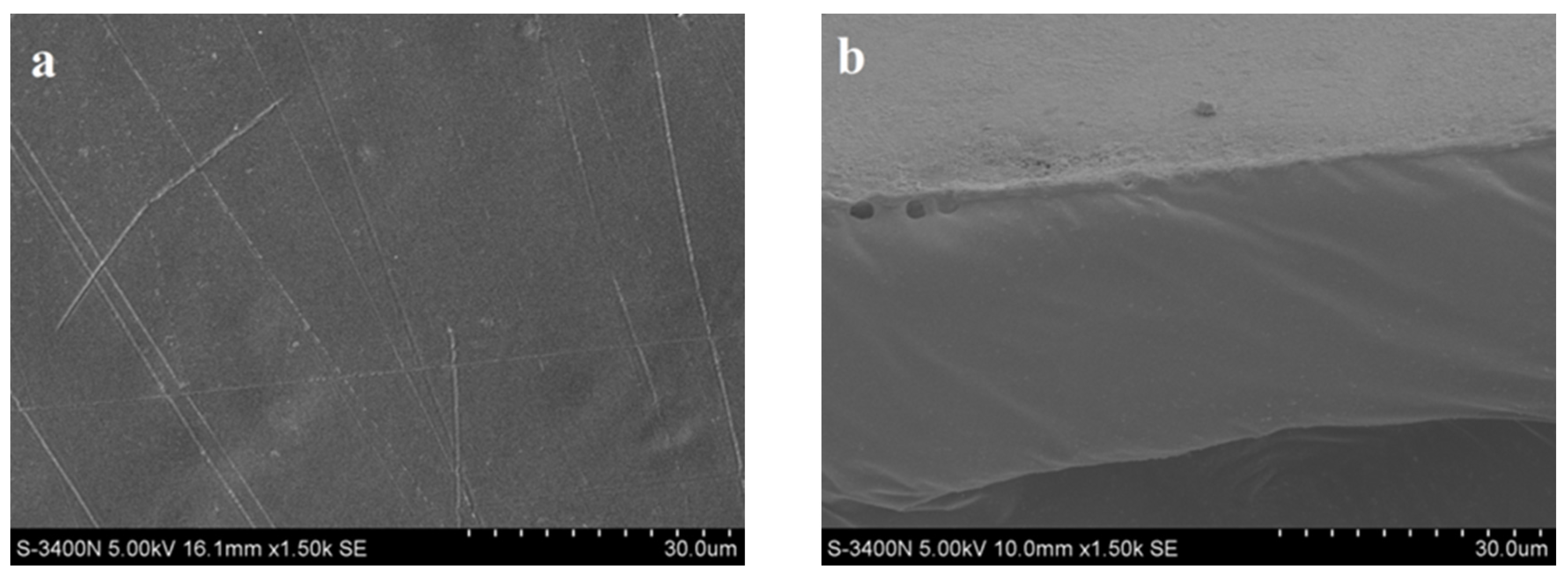
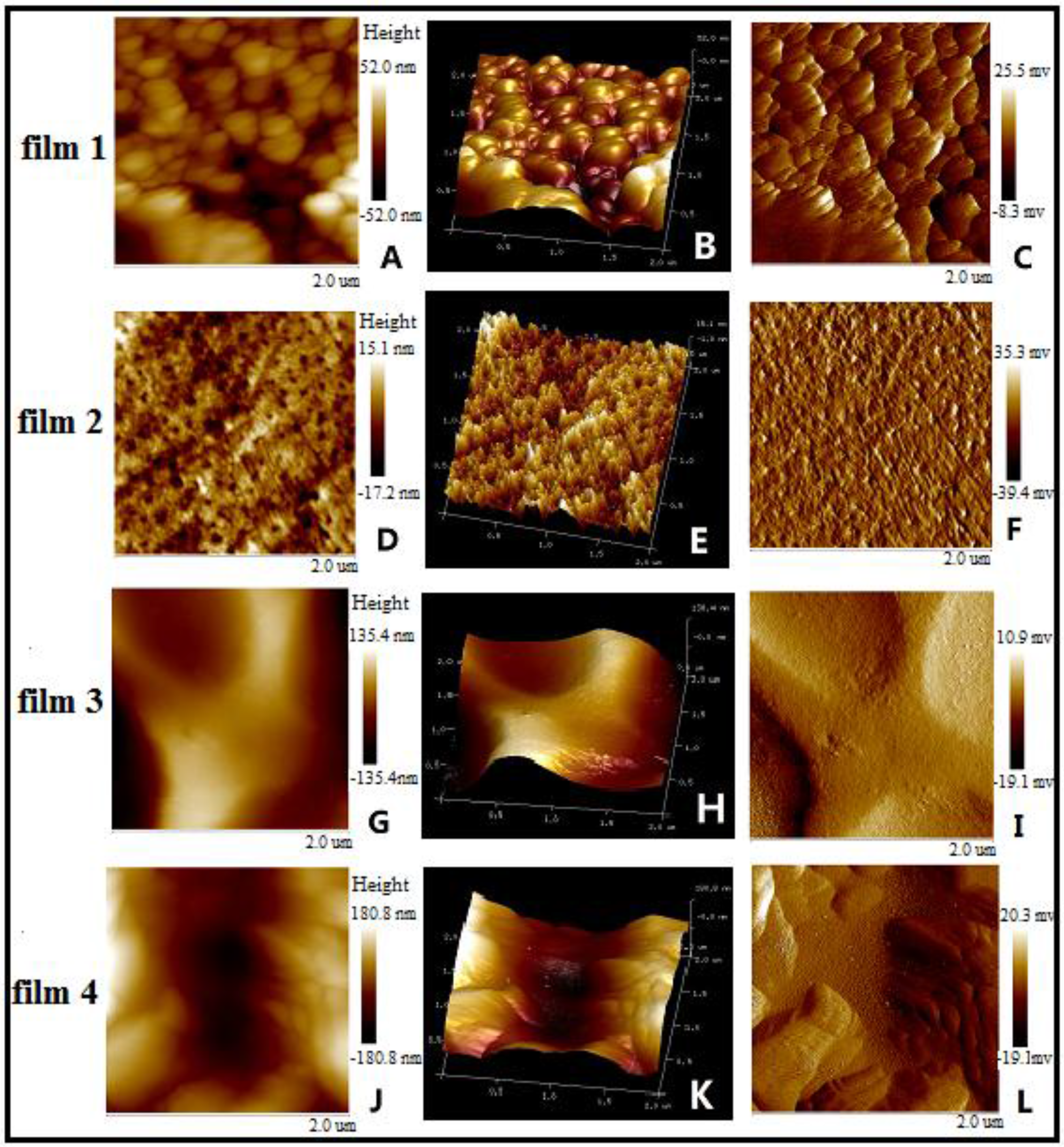
3.4. Mechanical Properties
| Sample | Tensile Strength (MPa) | Tensile Strain at Break (%) | Young‘s Modulus (MPa) |
|---|---|---|---|
| Film 1 | 25.2 ± 2.3 | 2.8 ± 0.7 | 1319.9 ± 102.7 |
| Film 2 | 14.2 ± 0.9 | 2.6 ± 0.9 | 842.0 ± 11.1 |
| Film 3 | 14.8 ± 1.6 | 1.7 ± 0.5 | 1177.2 ± 126.5 |
| Film 4 | 27.0 ± 1.5 | 3.9 ± 0.8 | 1118.5 ± 53.5 |
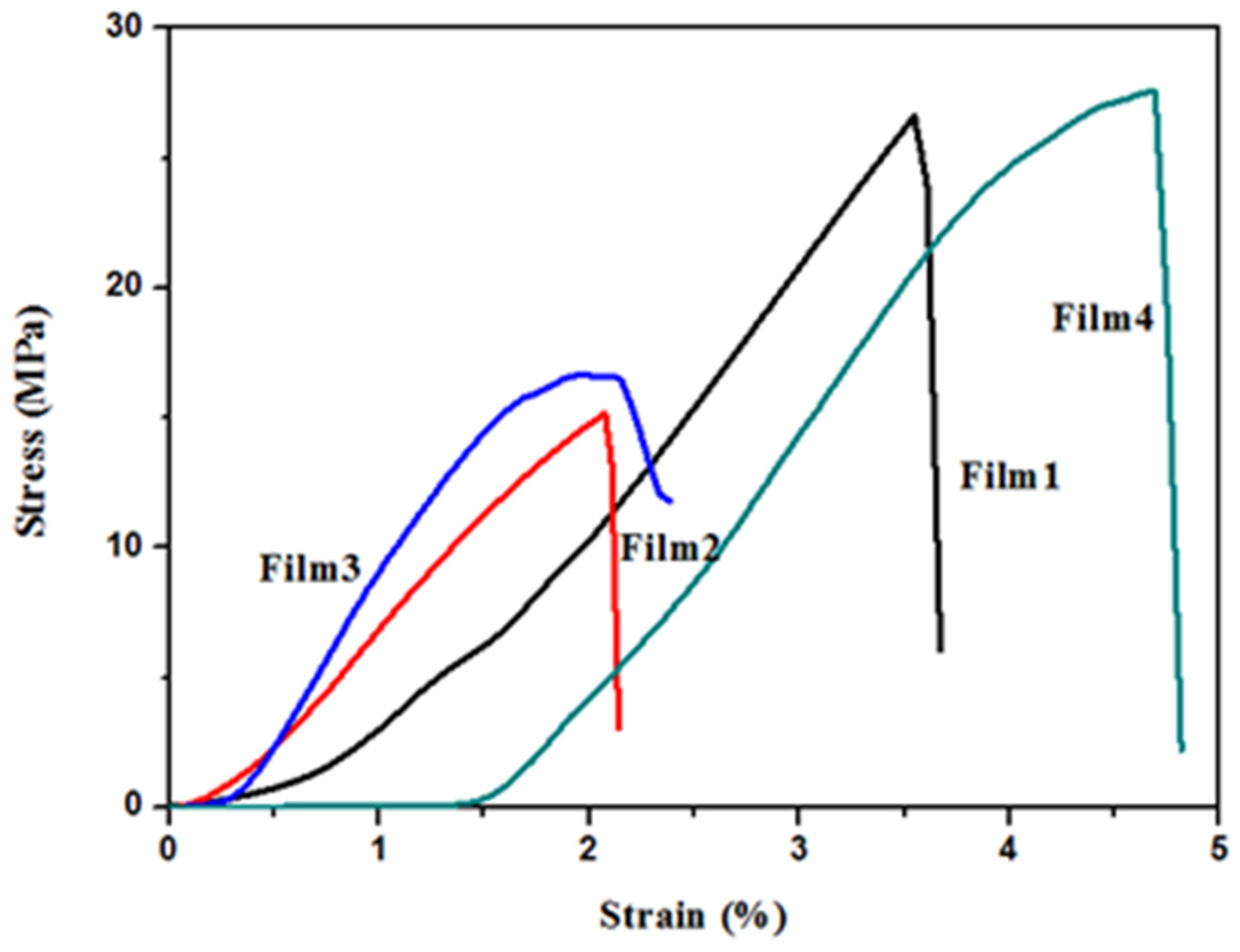
3.5. UV-Vis Transparency of Films
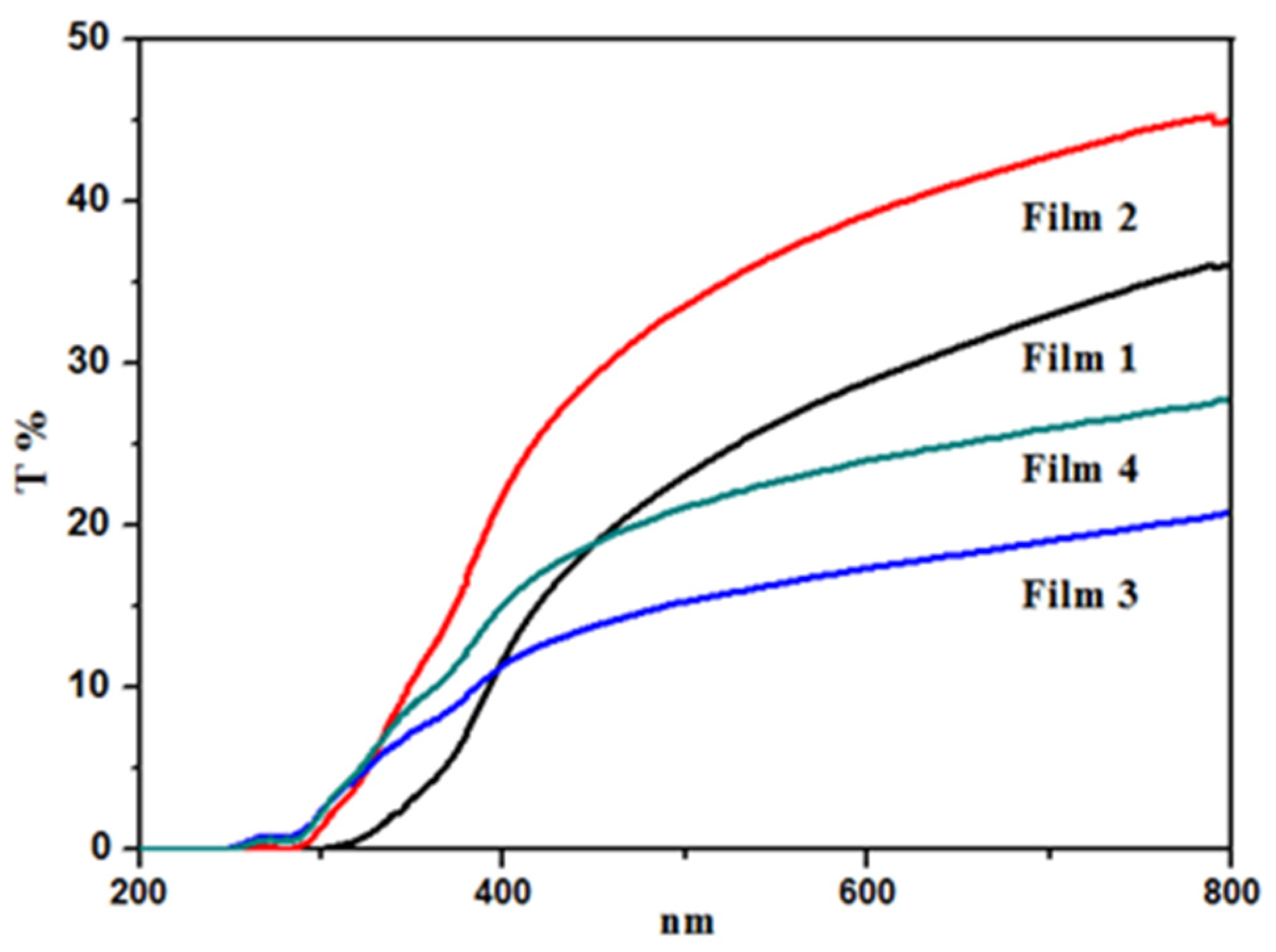
3.6. Water Vapor Permeability (WVP)
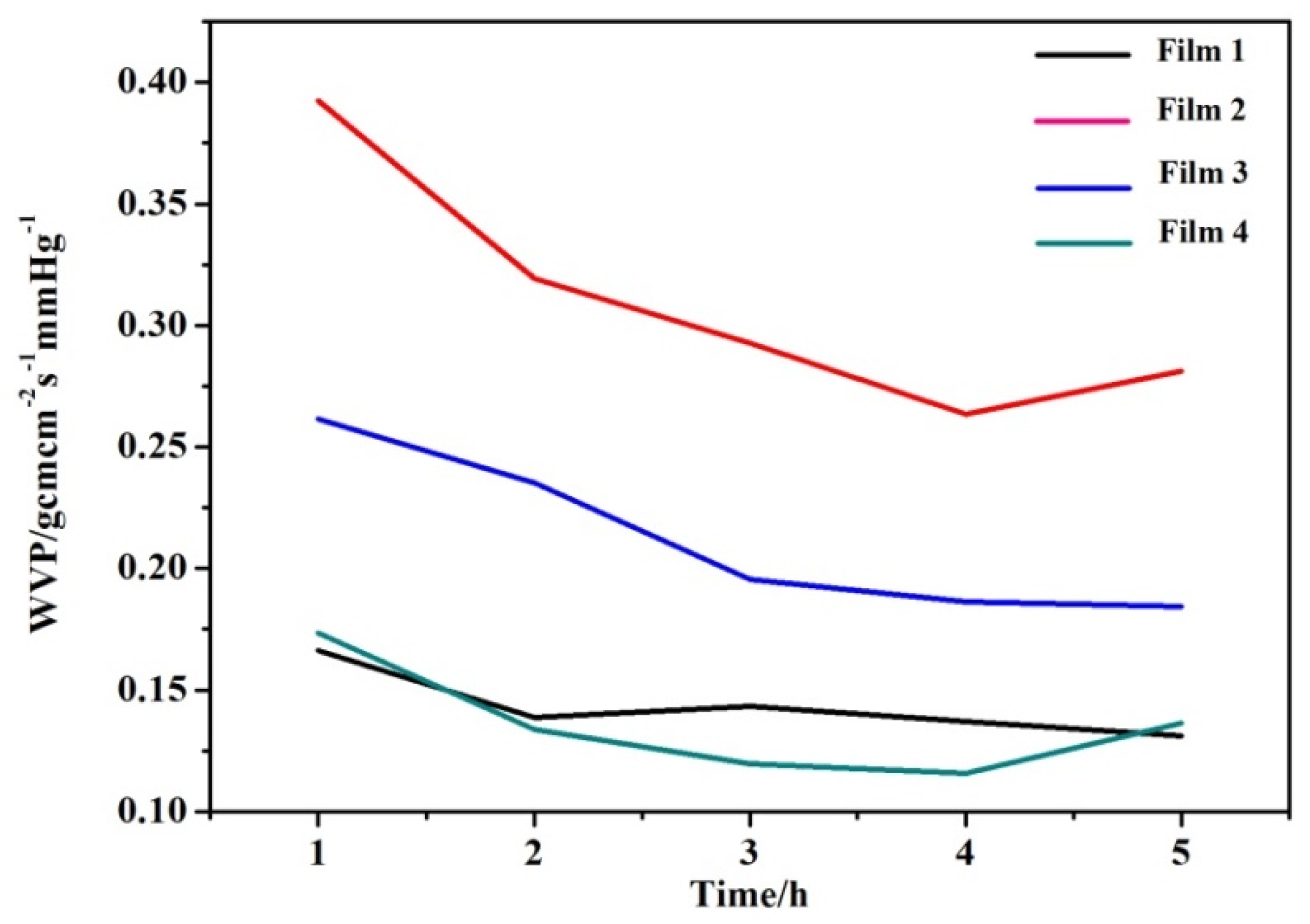
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.M.; Guan, Y.; Chen, G.G.; Zhang, B.; Ren, J.L.; Peng, F.; Sun, R.C. A non-covalent strategy for montmorillonite/xylose self-healing hydrogels. RSC Adv. 2015, 5, 41006–41012. [Google Scholar] [CrossRef]
- Edlund, U.; Ryberg, Y.Z.; Albertsson, A.C. Barrier films from renewable forestry waste. Biomacromolecules 2010, 11, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Soleimani, F. Synthesis of novel polysaccharide based superabsorbent hydrogels via graft copolymerization of vinylic monomers onto kappa carrageenan. Int. J. Chem. Eng. Appl. 2011, 2, 304–306. [Google Scholar]
- Pérez, J.; Munoz-Dorado, J.; de la Rubia, T.; Martinez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.H.; Spagnol, C.; Pereira, A.G.; Martins, A.F.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composites with a focus on hydrogels containing nanofibers or nanowhiskers of cellulose and chitin. J. Appl. Polym. Sci. 2014, 131, 39725. [Google Scholar] [CrossRef]
- Kochumalayil, J.J.; Zhou, Q.; Kasai, W.; Berglund, L.A. Regioselective modification of a xyloglucan hemicellulose for high-performance biopolymer barrier films. Carbohydr. Polym. 2013, 93, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Stevanic, J.S.; Bergström, E.M.; Gatenholm, P.; Berglund, L.; Salmén, L. Arabinoxylan/nanofibrillated cellulose composite films. J. Mater. Sci. 2012, 47, 6724–6732. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, B.; Tan, X.; Qi, X.M.; Bian, J.; Peng, F.; Sun, R.C. Organic–inorganic composite films based on modified hemicelluloses with clay nanoplatelets. ACS Sustain. Chem. Eng. 2014, 2, 1811–1818. [Google Scholar] [CrossRef]
- Stevanic, J.S.; Joly, C.; Mikkonen, K.S.; Pirkkalainen, K.; Serimaa, R.; Rémond, C.; Toriz, G.; Gatenholm, P.; Tenkanen, M.; Salmén, L. Bacterial nanocellulose-reinforced arabinoxylan films. J. Appl. Polym. Sci. 2011, 122, 1030–1039. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Urruzola, I.; Labidi, J. Xylan–cellulose films: Improvement of hydrophobicity, thermal and mechanical properties. Carbohydr. Polym. 2014, 112, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov. Food Sci. Emerg. Technol. 2010, 11, 697–702. [Google Scholar] [CrossRef]
- Zhang, S.W.; Liu, W.T.; Liang, J.; Li, X.Y.; Liang, W.N.; He, S.Q.; Zhu, C.S.; Mao, L.Y. Buildup mechanism of carboxymethyl cellulose and chitosan self-assembled films. Cellulose 2013, 20, 1135–1143. [Google Scholar] [CrossRef]
- Habibi, N. Preparation of biocompatible magnetite-carboxymethyl cellulose nanocomposite: Characterization of nanocomposite by FTIR, XRD, FESEM and TEM. Spectrochim. Acta A 2014, 131, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Eronen, P.; Österberg, M.; Heikkinen, S.; Tenkanen, M.; Laine, J. Interactions of structurally different hemicelluloses with nanofibrillar cellulose. Carbohydr. Polym. 2011, 86, 1281–1290. [Google Scholar] [CrossRef]
- Egüés, I.; Eceiza, A.; Labidi, J. Effect of different hemicelluloses characteristics on film forming properties. Ind. Crop. Prod. 2013, 47, 331–338. [Google Scholar] [CrossRef]
- Hansen, N.M.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Sun, R.C.; Liu, C.F. Etherification of hemicelluloses from sugarcane bagasse. J. Appl. Polym. Sci. 2007, 105, 3301–3308. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch-chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch-chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Whistler, R.L. Mechanical properties and water vapor permeability of thin film from corn hull arabinoxylan. J. Appl. Polym. Sci. 2004, 93, 2896–2902. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F. A view of etherification of hemicelluloses. J. Cellul. Sci. Technol. 2007, 2, 74–78. [Google Scholar]
- Kumaran, M. Interlaboratory comparison of the ASTM standard test methods for water vapor transmission of materials (E 96–95). J. Test. Eval. 1998, 26, 83–88. [Google Scholar]
- Hatanaka, D.; Yamamoto, K.; Kadokawa, J.I. Preparation of chitin nanofiber-reinforced carboxymethyl cellulose films. Int. J. Biol. Macromol. 2014, 69, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Peng, M.; Zhou, X.Y.; Wu, H.; Hu, J.; Xie, W.G.; Liu, S.H. Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr. Polym. 2014, 112, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Namazi, H.; Barkhordari, S. Preparation and properties of carboxymethyl cellulose/layered double hydroxide bionanocomposite films. Carbohydr. Polym. 2014, 108, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Fractional study of alkali-soluble hemicelluloses obtained by graded ethanol precipitation from sugar cane bagasse. J. Agric. Food Chem. 2009, 58, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Lin, L.; He, B.H. Synthesis and characterization of novel cationic SCB hemicelluloses with a low degree of substitution. Carbohydr. Polym. 2007, 67, 347–357. [Google Scholar] [CrossRef]
- Katsura, S.; Isogai, A.; Onabe, F.; Usuda, M. NMR analyses of polysaccharide derivatives containing amine groups. Carbohydr. Polym. 1992, 18, 283–288. [Google Scholar] [CrossRef]
- Peng, X.W.; Ren, J.L.; Zhong, L.X.; Sun, R.C. Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Tingaut, P.; Zimmermann, T.; Lopez-Suevos, F. Synthesis and characterization of bionanocomposites with tunable properties from poly (lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 2009, 11, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crop. Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.-M.; Liu, S.-Y.; Chu, F.-B.; Pang, S.; Liang, Y.-R.; Guan, Y.; Peng, F.; Sun, R.-C. Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose. Materials 2016, 9, 4. https://doi.org/10.3390/ma9010004
Qi X-M, Liu S-Y, Chu F-B, Pang S, Liang Y-R, Guan Y, Peng F, Sun R-C. Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose. Materials. 2016; 9(1):4. https://doi.org/10.3390/ma9010004
Chicago/Turabian StyleQi, Xian-Ming, Shi-Yun Liu, Fang-Bing Chu, Shuai Pang, Yan-Ru Liang, Ying Guan, Feng Peng, and Run-Cang Sun. 2016. "Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose" Materials 9, no. 1: 4. https://doi.org/10.3390/ma9010004