Accelerated Degradation Test and Predictive Failure Analysis of B10 Copper-Nickel Alloy under Marine Environmental Conditions
Abstract
:1. Introduction
2. Accelerated Degradation Test
| Si | Cu | P | Fe | S | Zn | Ni | Mn |
|---|---|---|---|---|---|---|---|
| <0.15 | Allowance | <0.02 | 1.20 | 0.0013 | <0.3 | 9.76 | 0.74 |
3. Observation Results and Analysis
3.1. Surface Film Observation
| Element | Original | 1 Day | 3 Day | 7 Day |
|---|---|---|---|---|
| Cu | 79.93 | 72.56 | 67.19 | 59.71 |
| Ni | 10.4 | 9.2 | 8.63 | 2.47 |
| O | 9.55 | 18.24 | 22.95 | 27.8 |
| Cl | 0.12 | 0.25 | 1.23 | 10.02 |
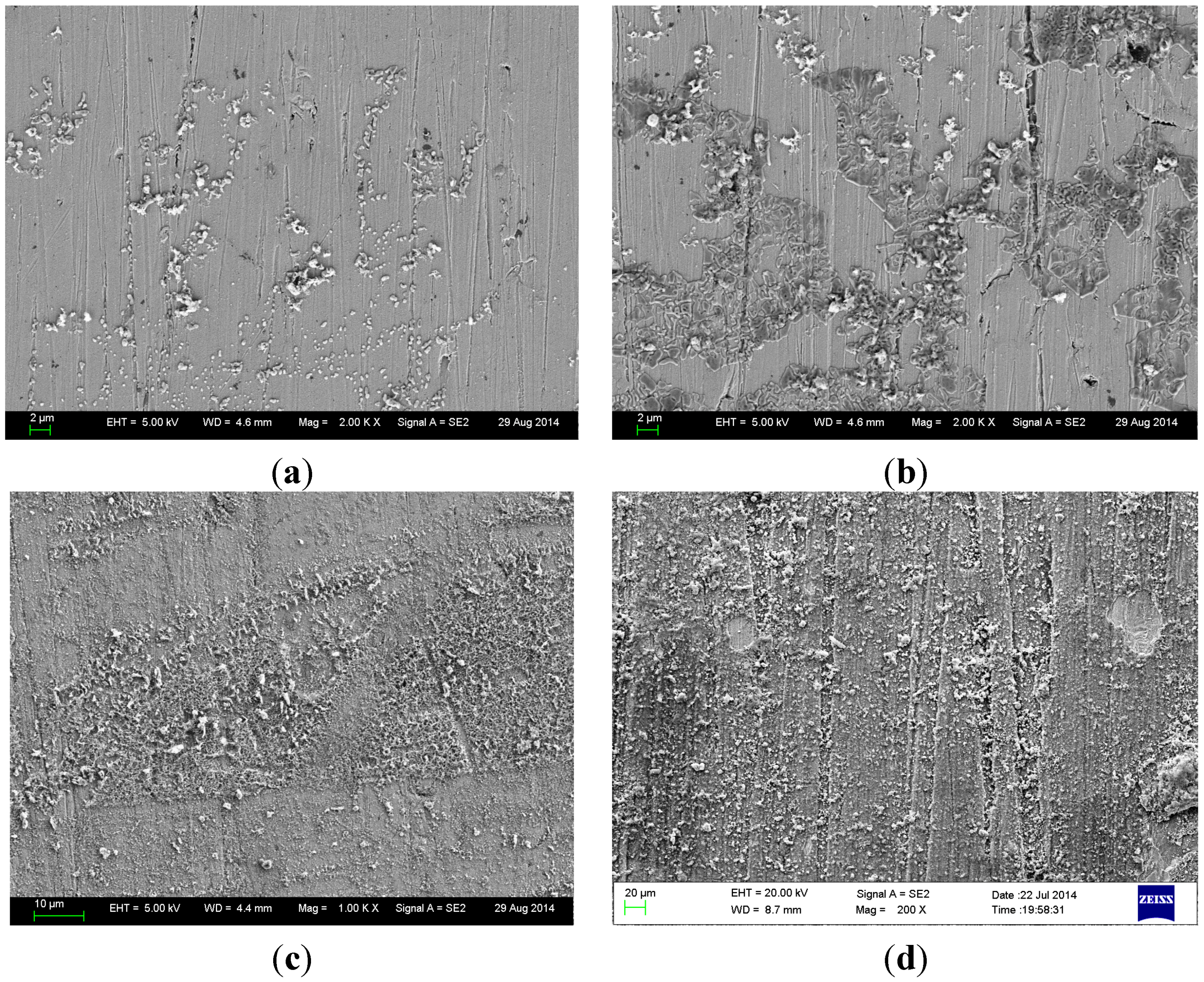
3.2. Micromorphology Observation
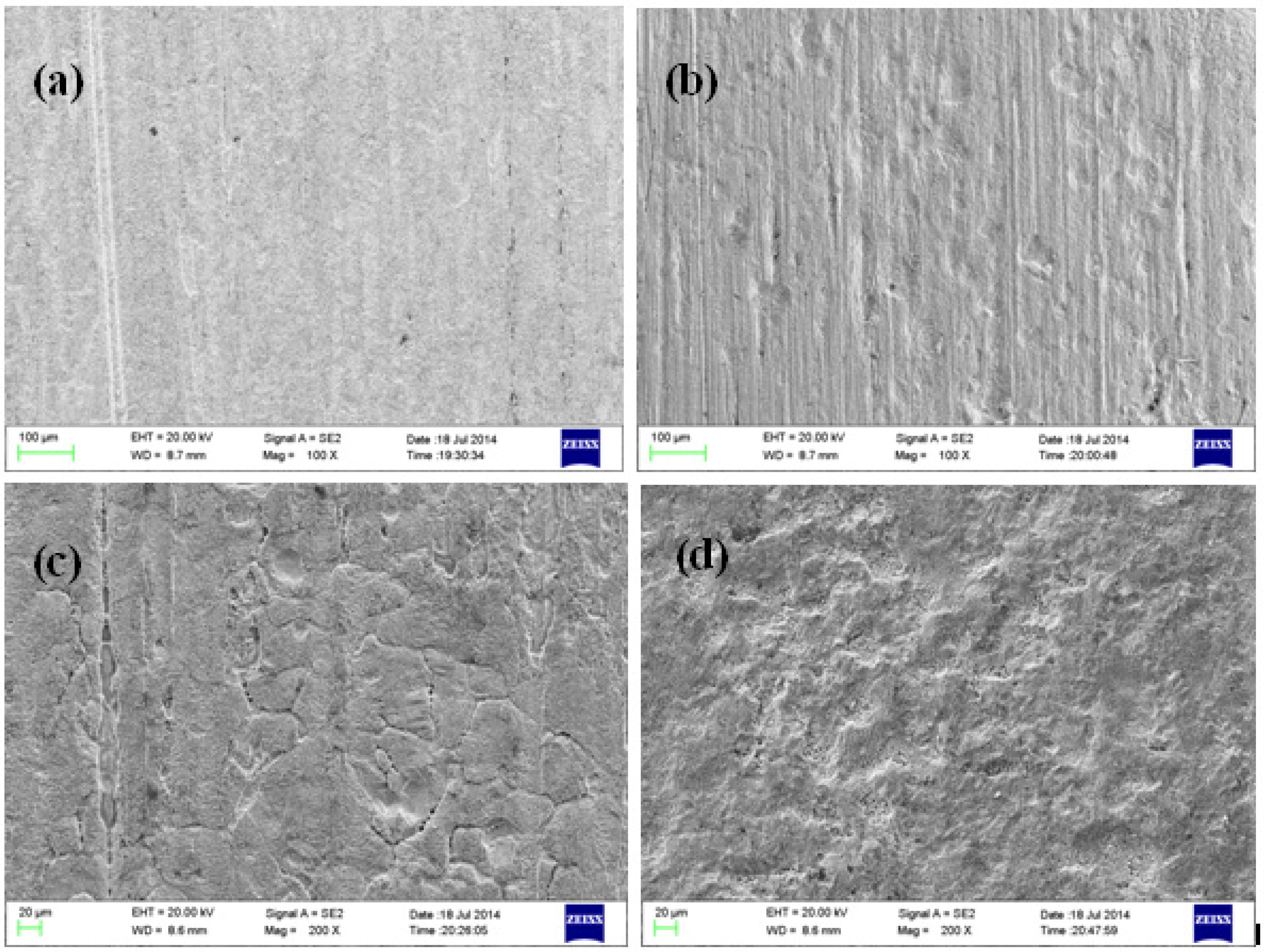
3.3. Analysis of Corrosion Products
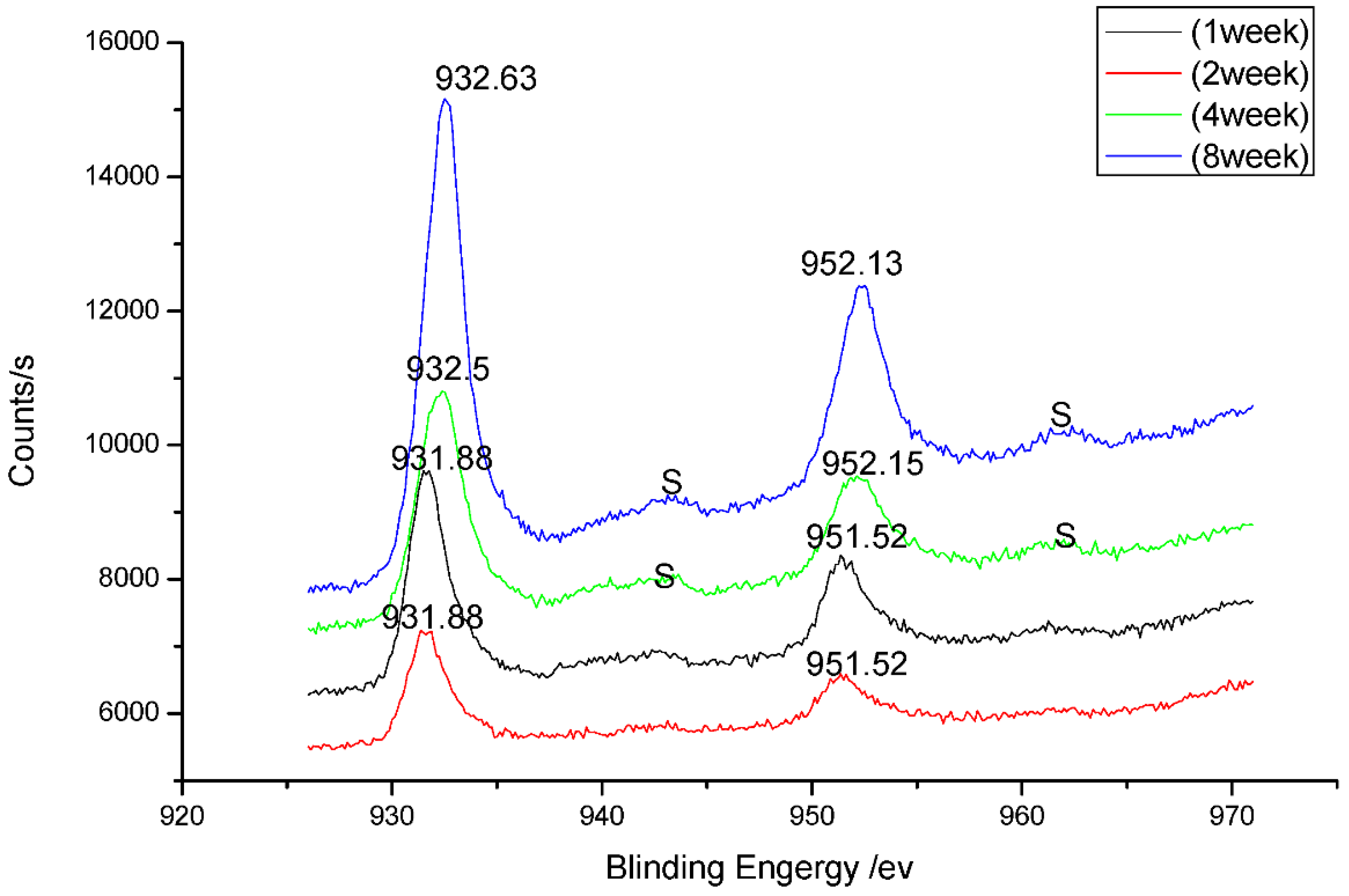
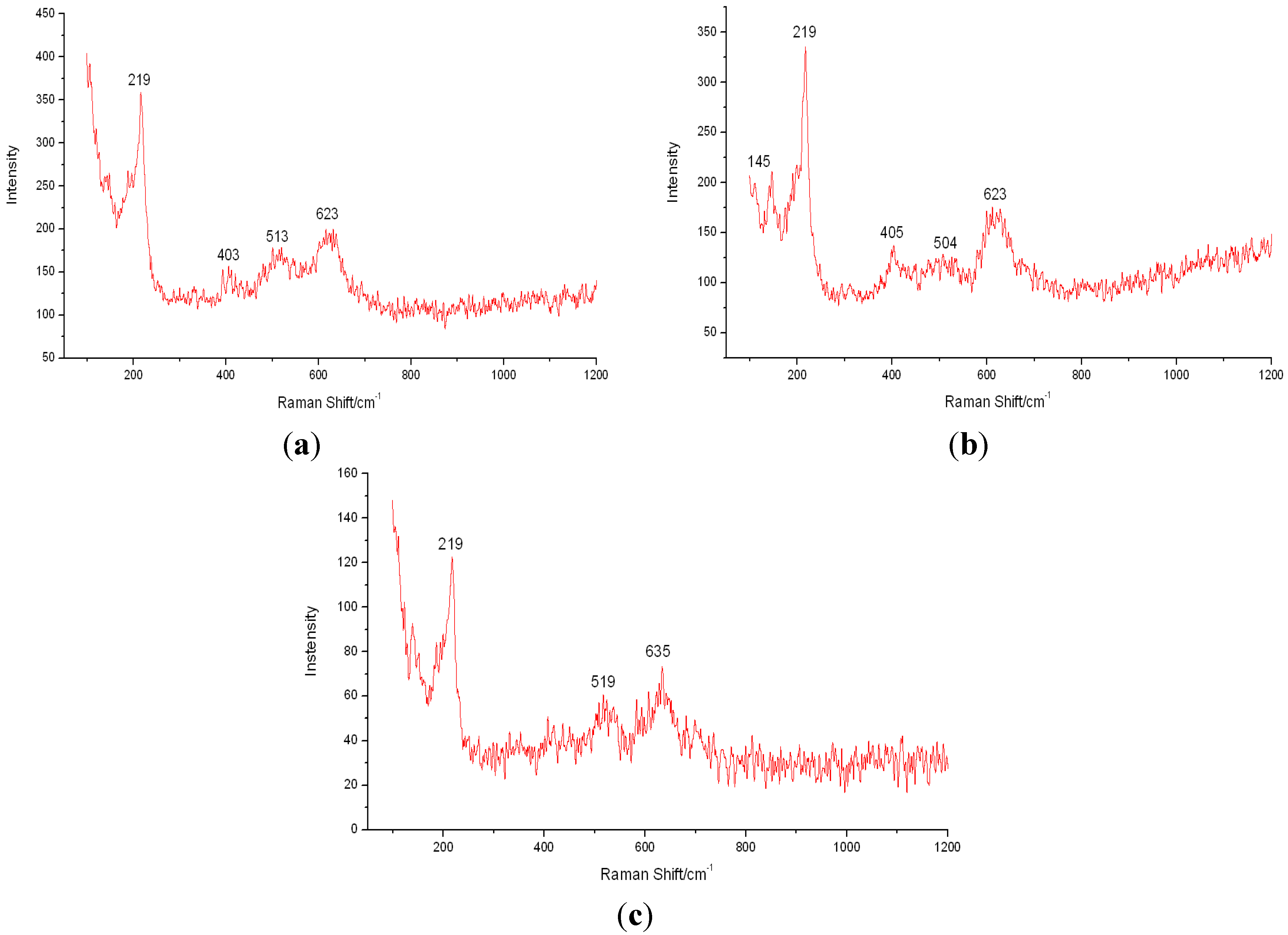
3.4. Analysis of Corrosion Mechanism
4. Experiments and Consideration on Environmental Effects
4.1. Effect of Dissolved Oxygen
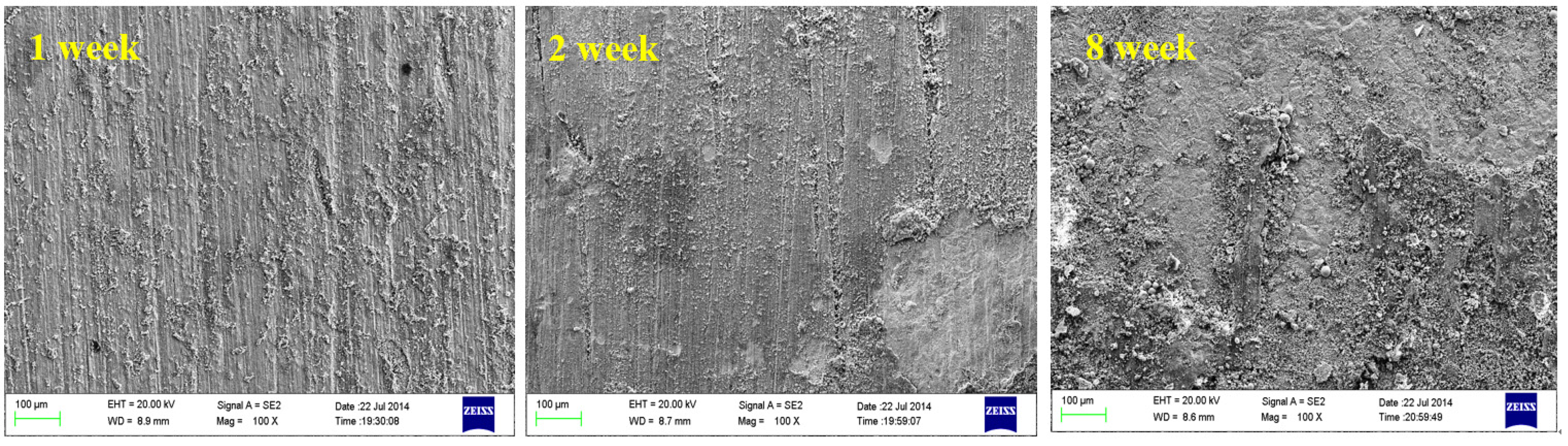
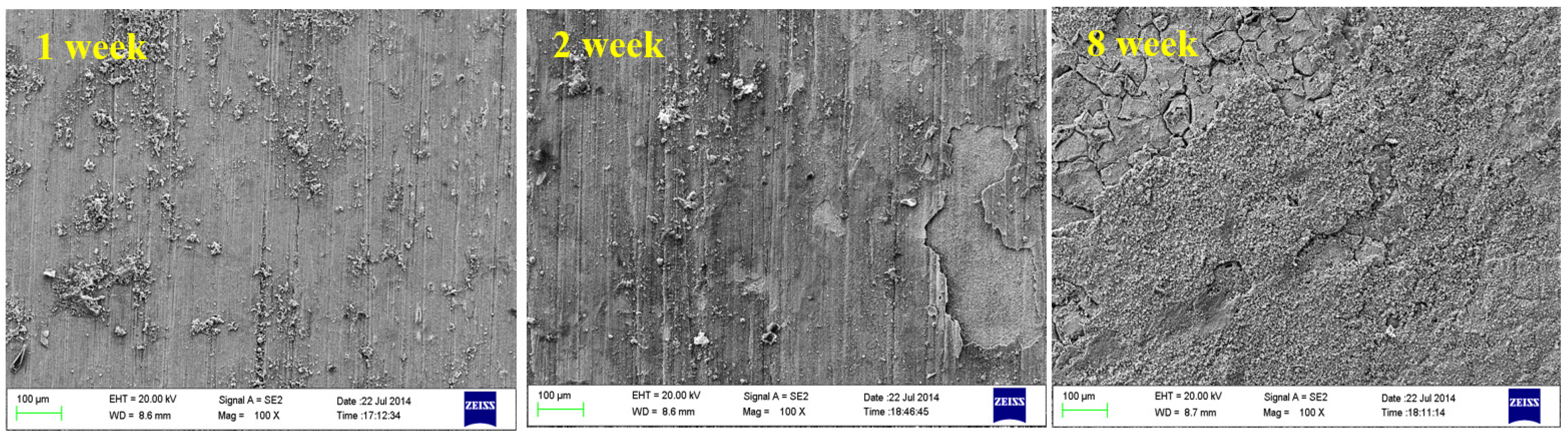
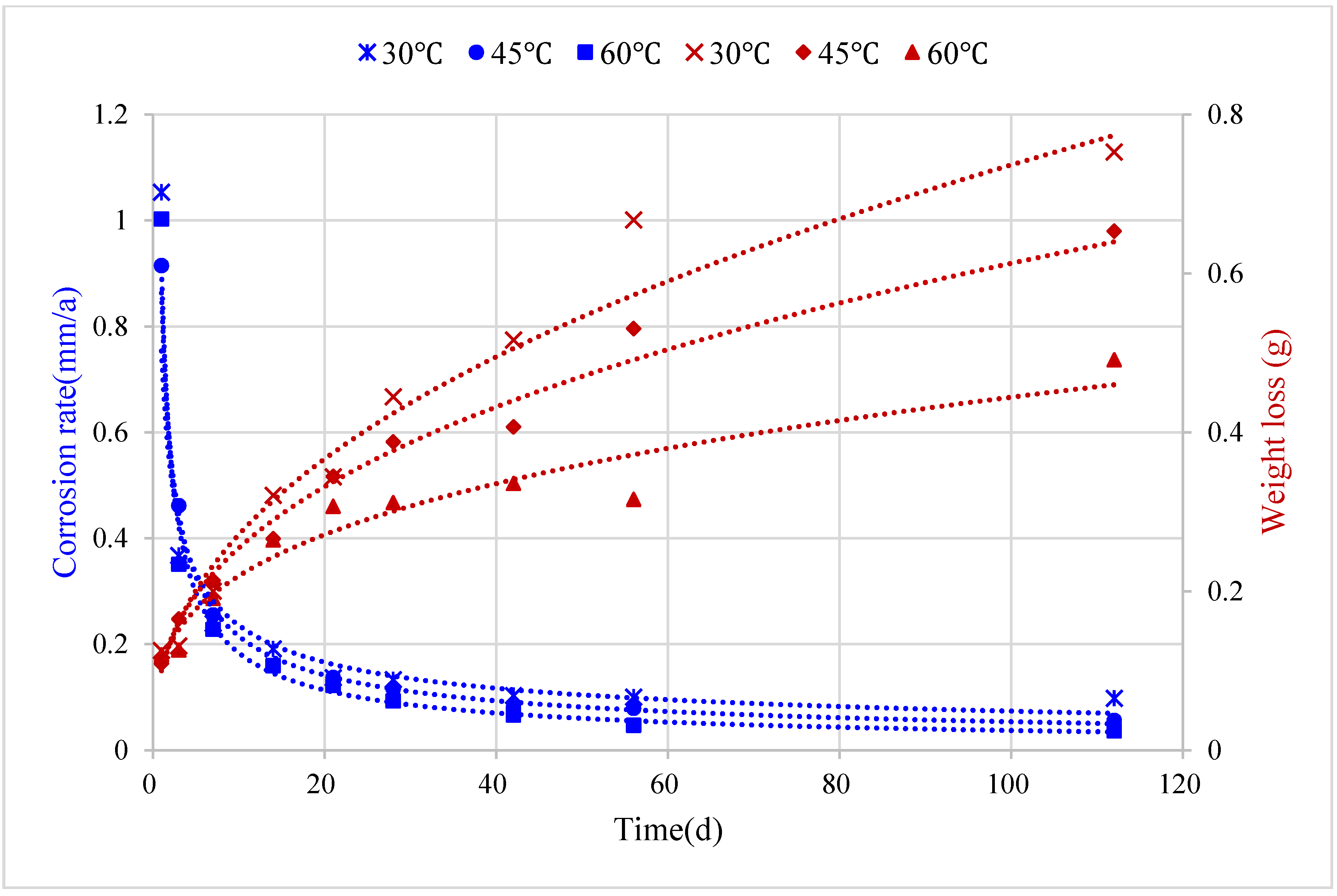
4.2. Effect of Medium Temperature
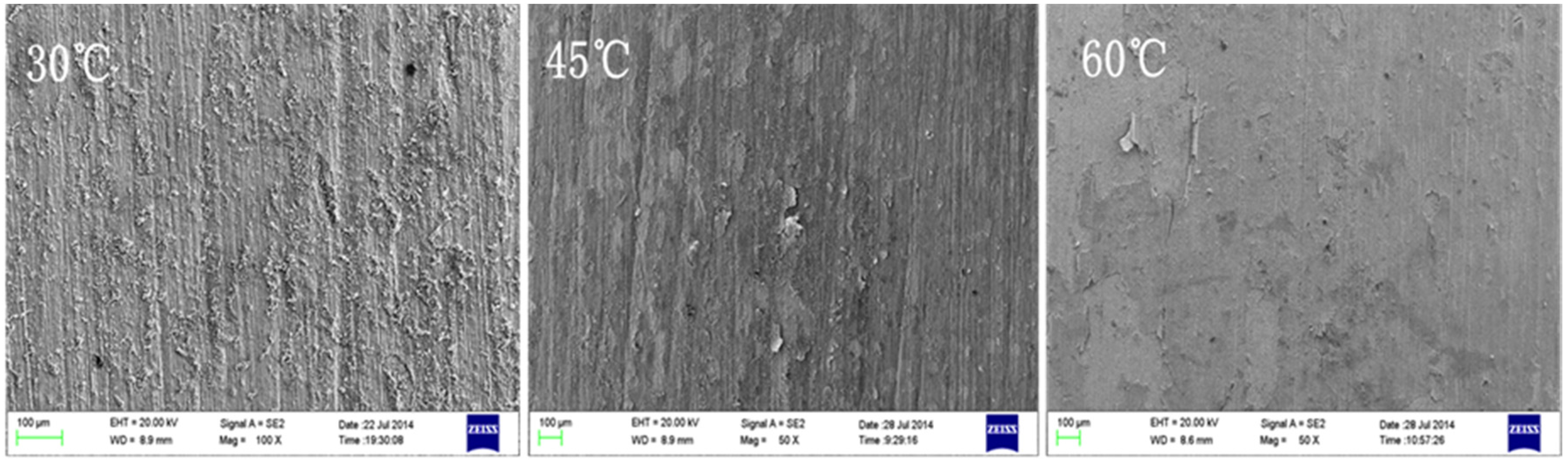

5. Corrosion Prediction and Discussion
5.1. Corrosion Prediction Considering Dissolved Oxygen
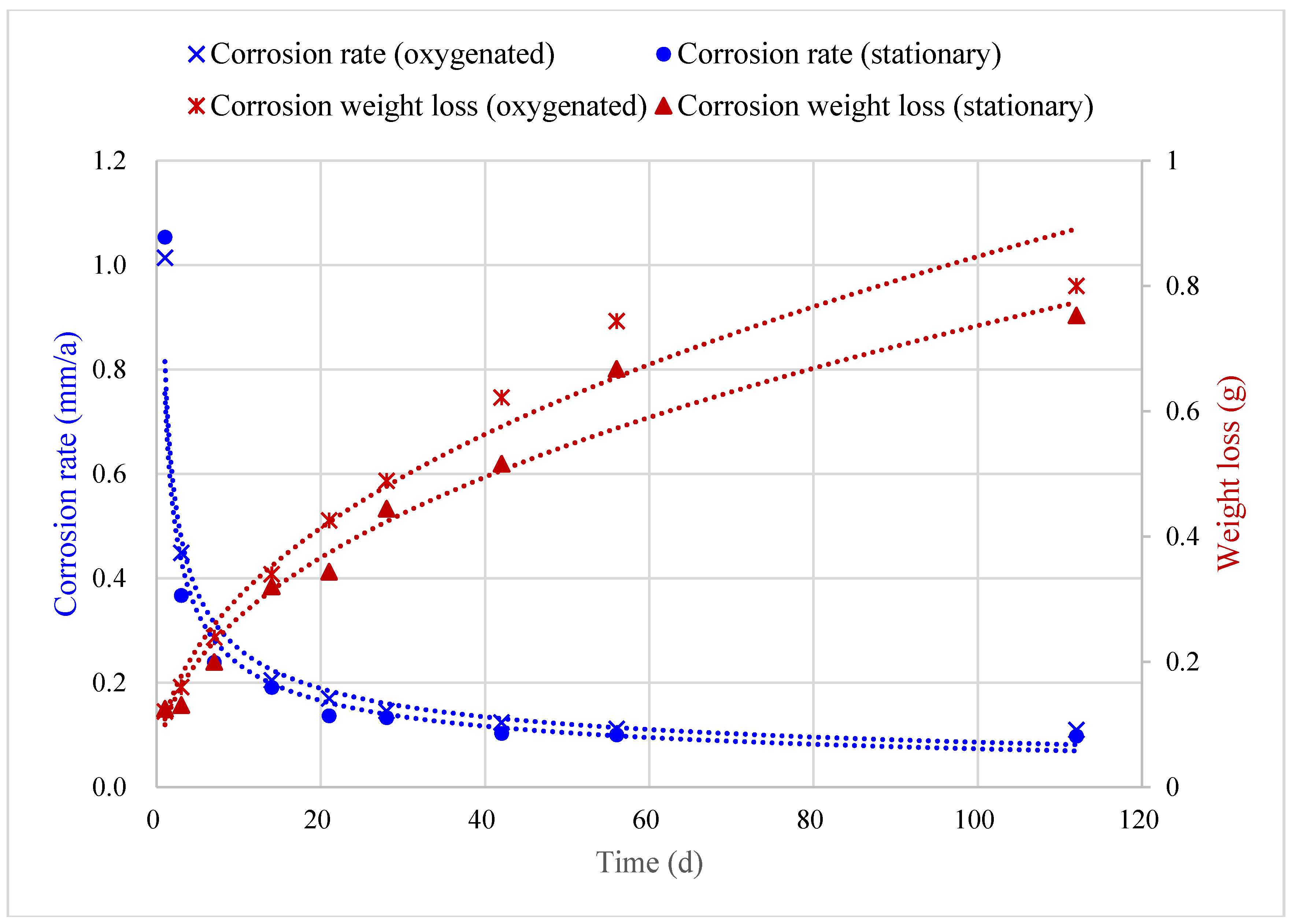
5.2. Corrosion Prediction Considering Medium Temperature
6. Conclusions
Author Contributions
Conflicts of Interest
References
- De la Fuente, D.; Simancas, J.; Morcillo, M. Morphological study of 16-year patinas formed on copper in a wide range of atmospheric exposes. Corros. Sci. 2008, 50, 268–285. [Google Scholar] [CrossRef]
- Watanabe, M.; Toyoda, E.; Handa, T.; Ichino, T.; Kuwaki, N.; Higashi, Y.; Tanaka, T. Evolution of patinas on copper exposed in a suburban area. Corros. Sci. 2007, 49, 766–780. [Google Scholar] [CrossRef]
- Goidanich, S.; Brunk, J.; Herting, G.; Arenas, M.A.; Odnevall Wallinder, I. Atmospheric corrosion of brass in outdoor applications: Patina evolution, metal release and aesthetic appearance at urban exposure conditions. Sci. Total Environ. 2011, 412–413, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.; Odnevall Wallinder, I.; Leygraf, C.; le Bozec, N. Corrosion-induced copper runoff from naturally and pre-patinated copper in a marine environment. Corros. Sci. 2006, 48, 4316–4338. [Google Scholar] [CrossRef]
- Gianni, L.; Gigante, G.; Cavallini, M.; Adriaens, A. Corrosion of bronzes by extended wetting with single versus mixed acidic pollutants. Materials 2014, 7, 3353–3370. [Google Scholar] [CrossRef] [Green Version]
- Burleigh, T.D.; Gierke, C.G.; Fredj, N.; Boston, P. Copper Tube Pitting in Santa Fe Municipal Water Caused by Microbial Induced Corrosion. Materials 2014, 7, 4321–4334. [Google Scholar] [CrossRef]
- Efird, K.D.; Anderson, D.B. Sea water corrosion of 90–10 and 70–30 Cu-Ni: 14 year exposures. Mater. Perform. 1975, 14, 37–40. [Google Scholar]
- Parvizi, M.S.; Aladjem, A.; Castle, J.E. Behaviour of 90–10 cupronickel in sea water. Int. Mater. Rev. 1988, 33, 169–200. [Google Scholar] [CrossRef]
- Kear, G.; Barkaer, B.D.; Stokes, K.; Walsh, F.C. Electrochemical corrosion behavior of 90–10 Cu-Ni alloy in chloride-based electrolytes. Appl. Electrochem. 2004, 34, 659–662. [Google Scholar] [CrossRef]
- Melchers, R.E. Temperature effect on seawater immersion corrosion of 90:10 copper-nickel alloy. Corrosion 2001, 57, 440–451. [Google Scholar] [CrossRef]
- Melchers, R.E. Bi-modal trends in the long-term corrosion of copper and high copper alloys. Corros. Sci. 2015, 95, 51–61. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study between the Micro and Macro Scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Li, Z.; Zhu, A.Y.; Luo, L.Y.; Liang, J. Corrosion behavior of novel imitation-gold copper alloy with rare earth in 3.5% NaCl solution. Mater. Des. 2012, 34, 618–629. [Google Scholar] [CrossRef]
- Sanchez, M.; Gamby, J.; Perrot, H.; Rose, D.; Vivier, V. Micro-electrochemistry of copper in NaCl solution: Comparison between conventional microelectrode and micro-electrochemical cell. Electrochem. Commun. 2010, 12, 1230–1232. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Igonin, T.N.; Kovtanyuk, V.V.; Nikolaeva, L.A. Long-term forecast of corrosion mass losses of technically important metals in various world regions using a power function. Corros. Sci. 2014, 88, 306–316. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Ye, T.; Feng, Q.; Yao, J.; Wei, M. Accelerated Degradation Test and Predictive Failure Analysis of B10 Copper-Nickel Alloy under Marine Environmental Conditions. Materials 2015, 8, 6029-6042. https://doi.org/10.3390/ma8095290
Sun B, Ye T, Feng Q, Yao J, Wei M. Accelerated Degradation Test and Predictive Failure Analysis of B10 Copper-Nickel Alloy under Marine Environmental Conditions. Materials. 2015; 8(9):6029-6042. https://doi.org/10.3390/ma8095290
Chicago/Turabian StyleSun, Bo, Tianyuan Ye, Qiang Feng, Jinghua Yao, and Mumeng Wei. 2015. "Accelerated Degradation Test and Predictive Failure Analysis of B10 Copper-Nickel Alloy under Marine Environmental Conditions" Materials 8, no. 9: 6029-6042. https://doi.org/10.3390/ma8095290






