3D Microstructure Effects in Ni-YSZ Anodes: Prediction of Effective Transport Properties and Optimization of Redox Stability
Abstract
:1. Introduction
2. Experimental
2.1. Anode Fabrication and Redox Cycling
2.2. Electrical Conductivity Measurements
2.3. Image Acquisition by FIB-Tomography and SEM
2.4. Quantitative 3D Microstructure Analysis
2.5. Conductivities and M-Factor Simulation
3. Results
3.1. Qualitative Description of Microstructures
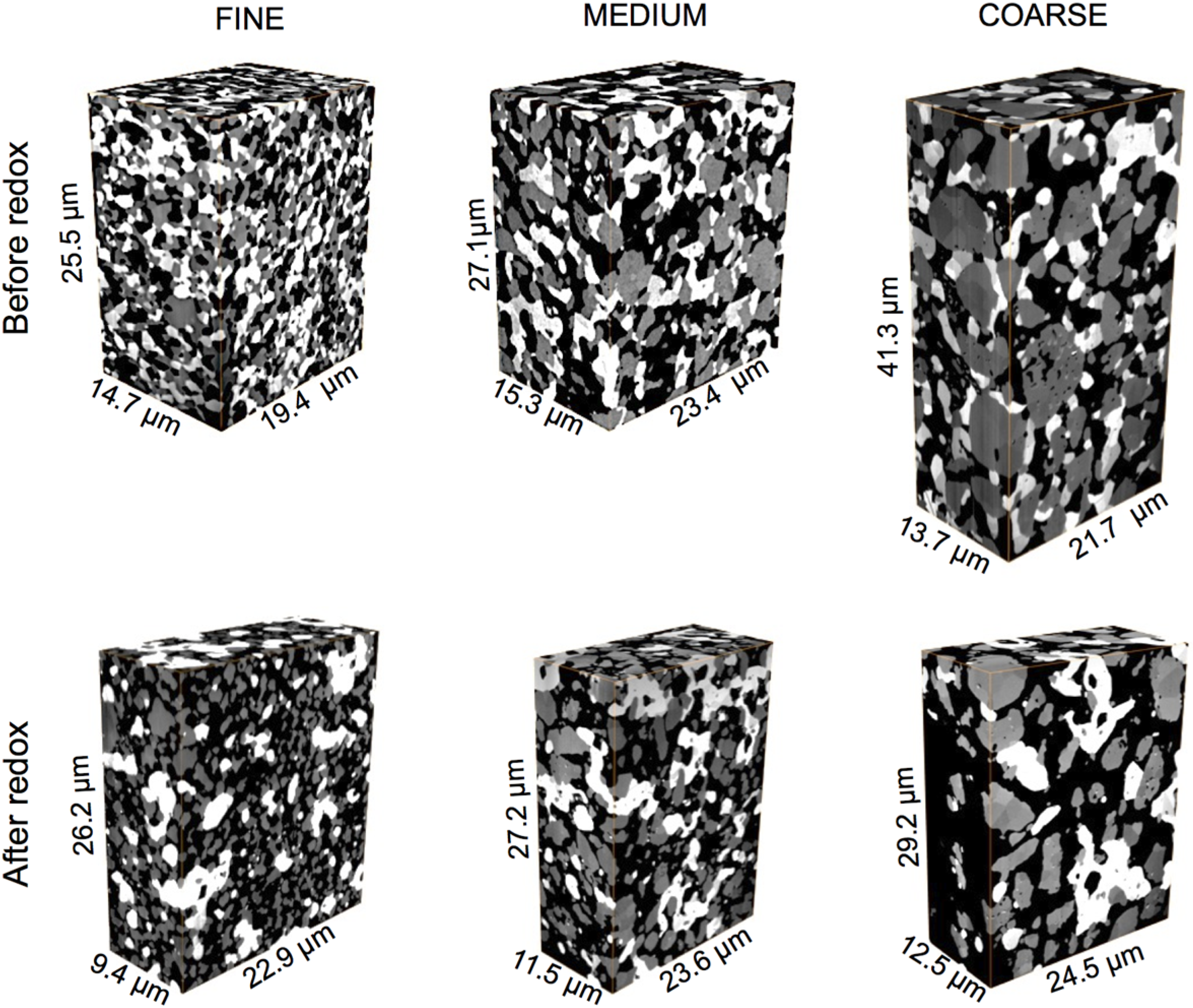
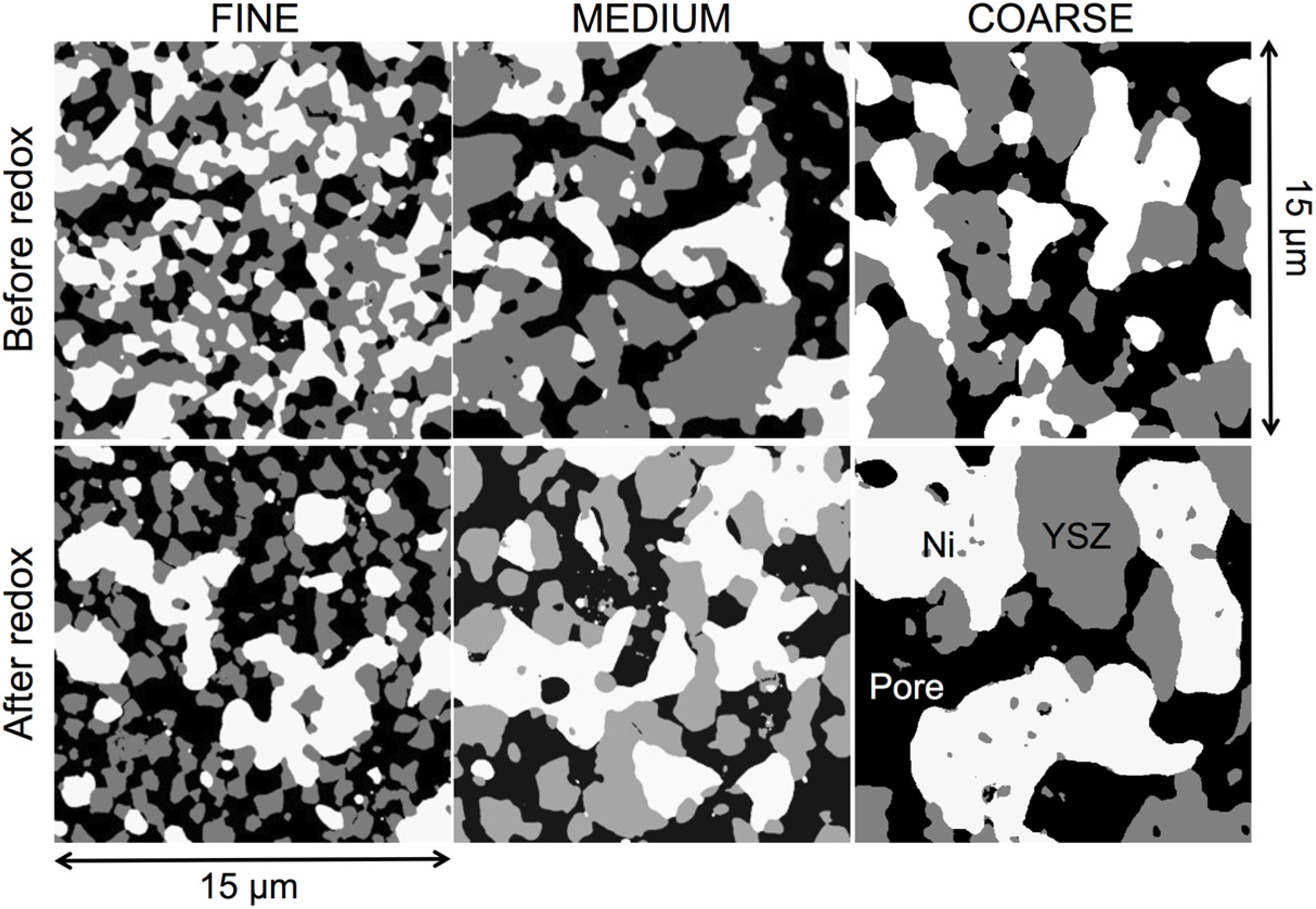
3.2. Electrical Conductivity Measurements

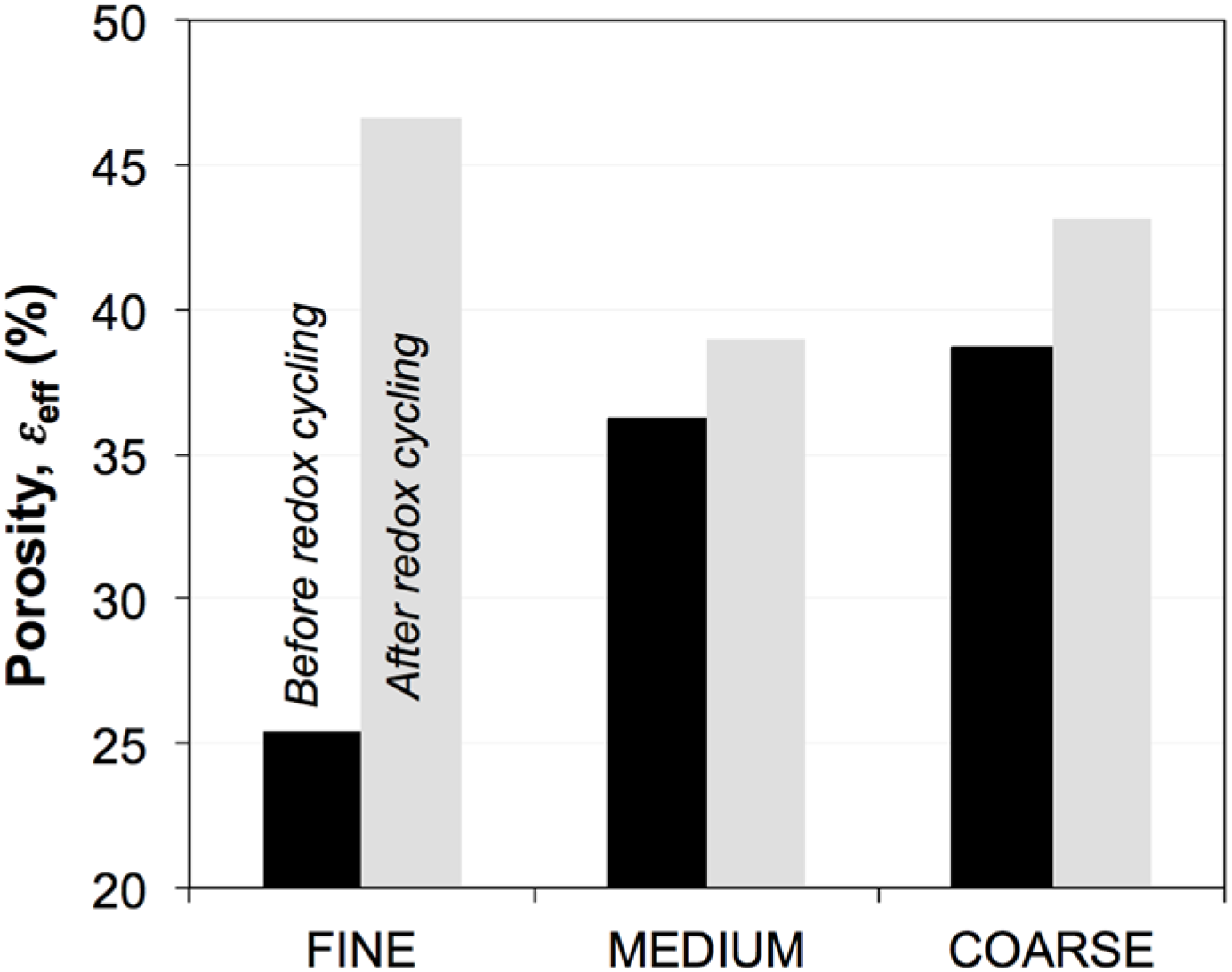
3.3. Effective Volume Fractions (Φeff, εeff) and Percolation (P)

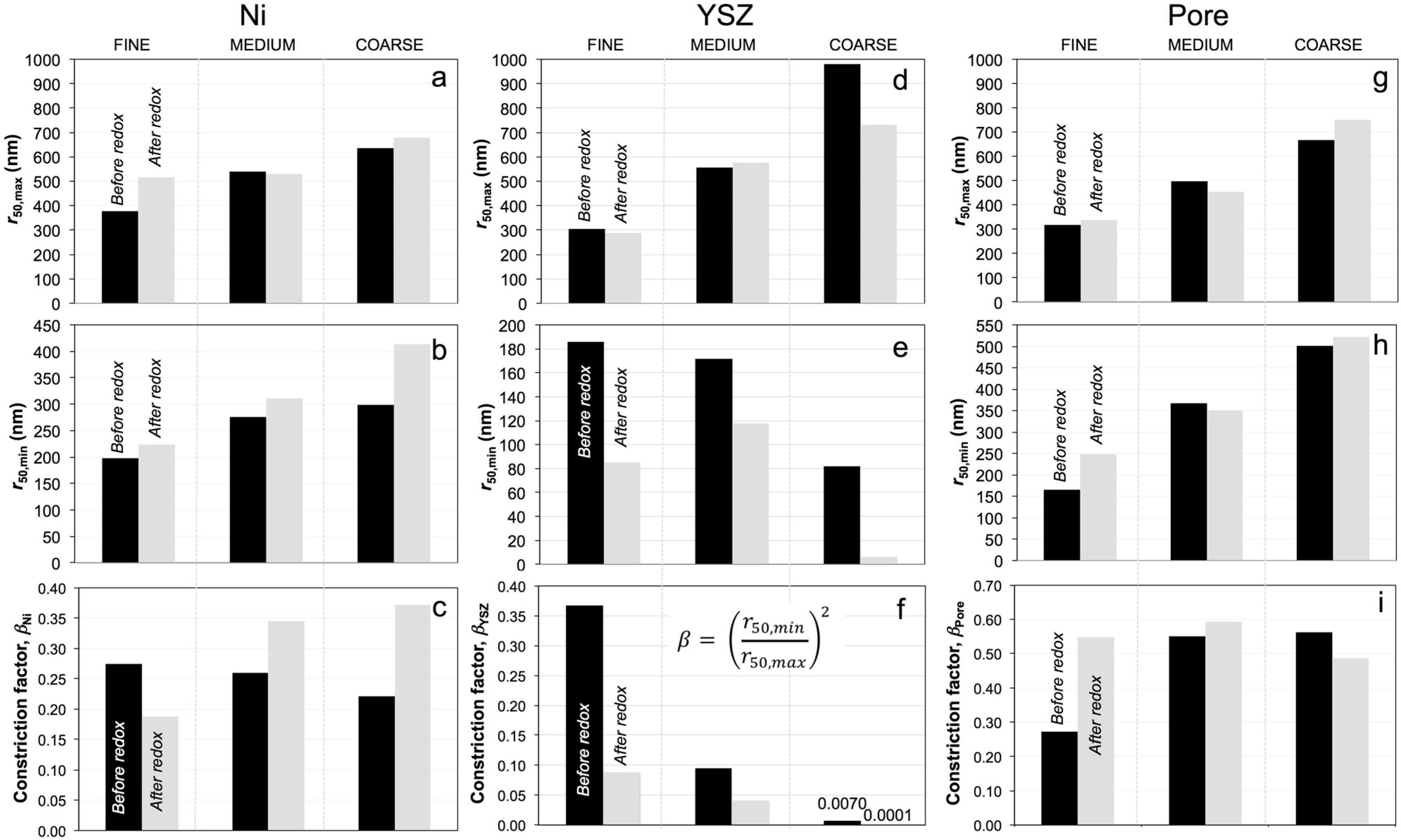
3.4. Phase Size Distributions (PSD), Average Radii (r50,max, r50,min), and Constriction Factor (β)
3.5. Geodesic Tortuosities (τ)
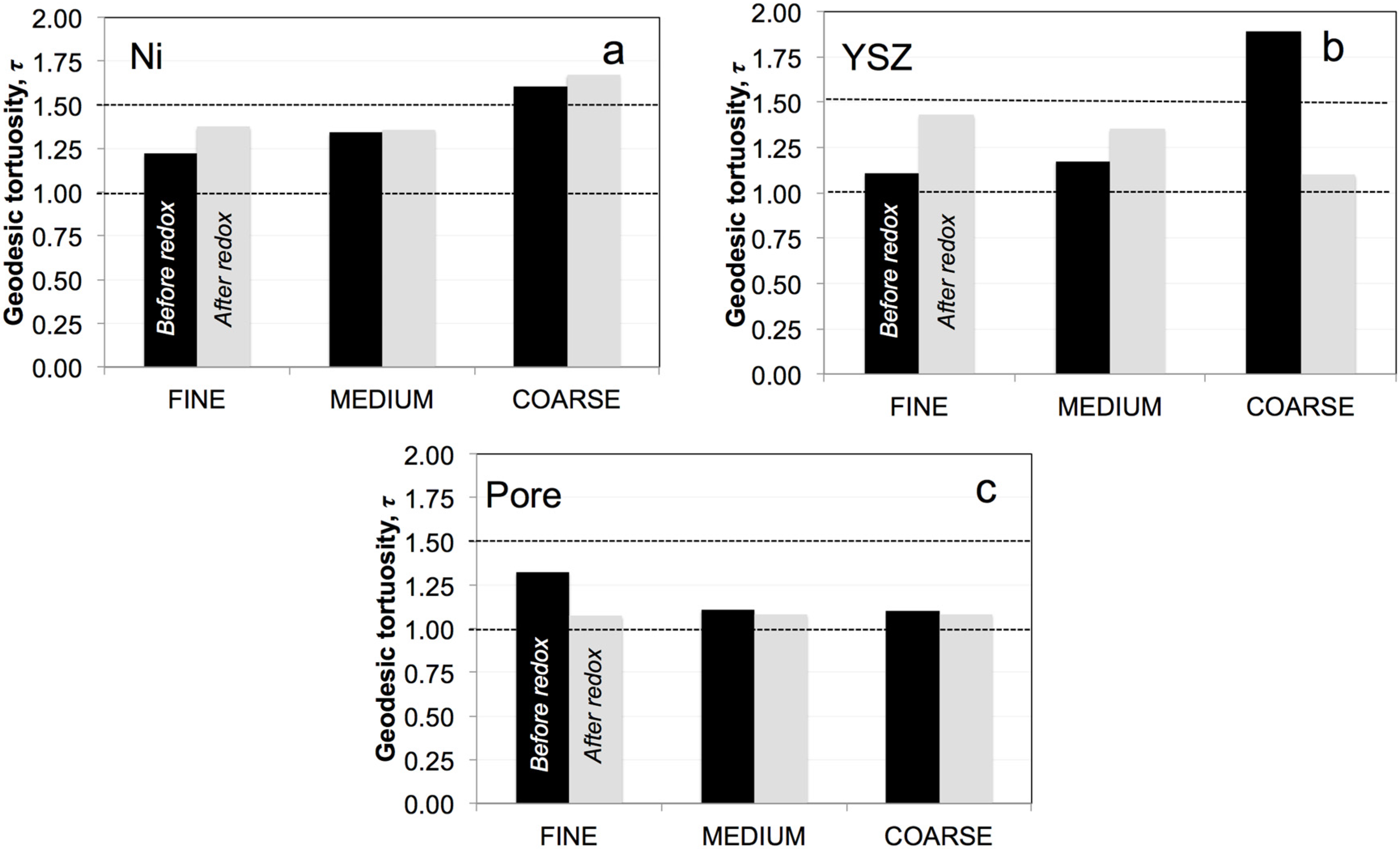
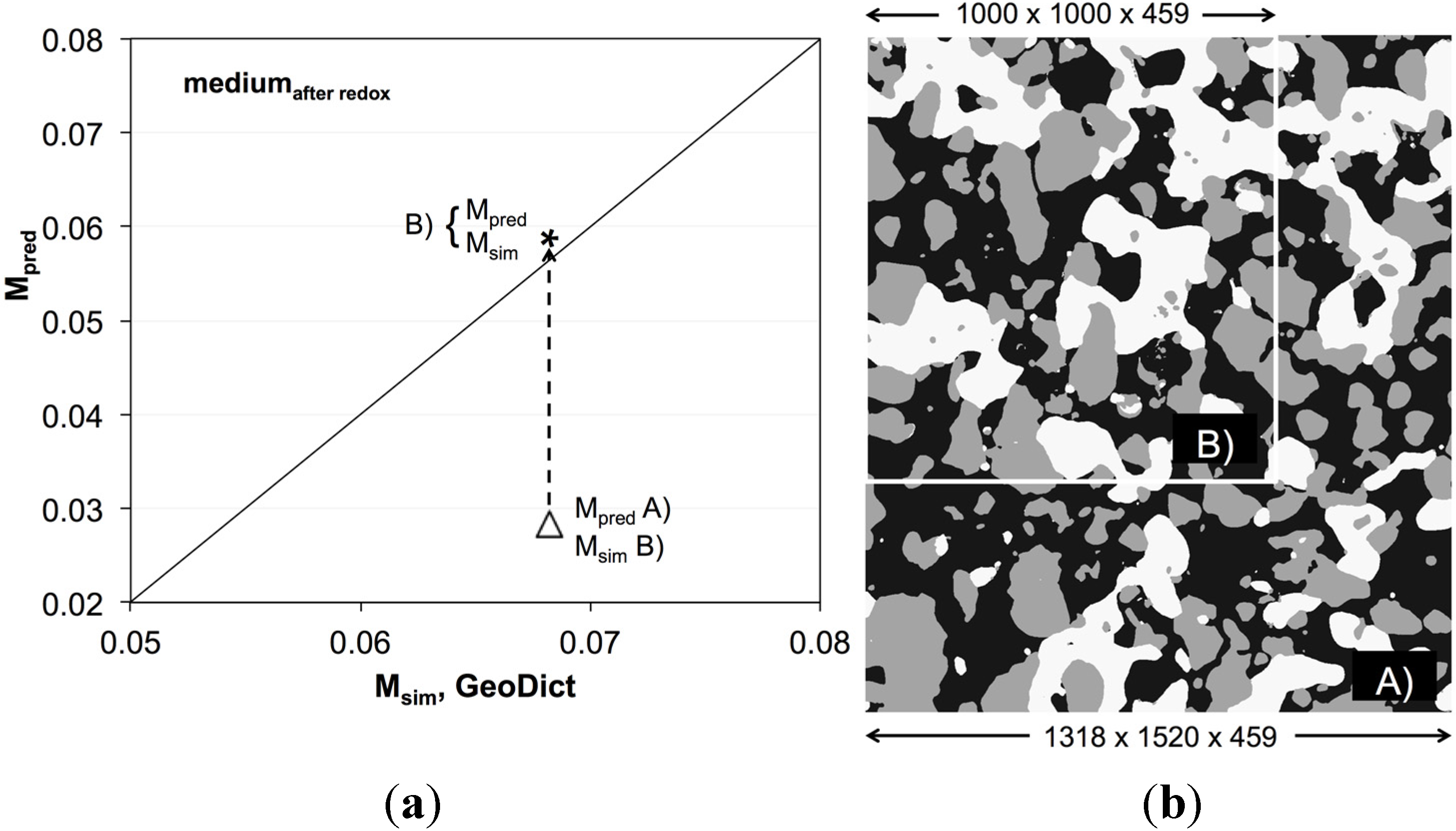
3.6. Simulation of Effective Transport Properties (σeff) and Microstructure Factors (Msim)

4. Discussion
4.1. Prediction of Effective Transport Properties


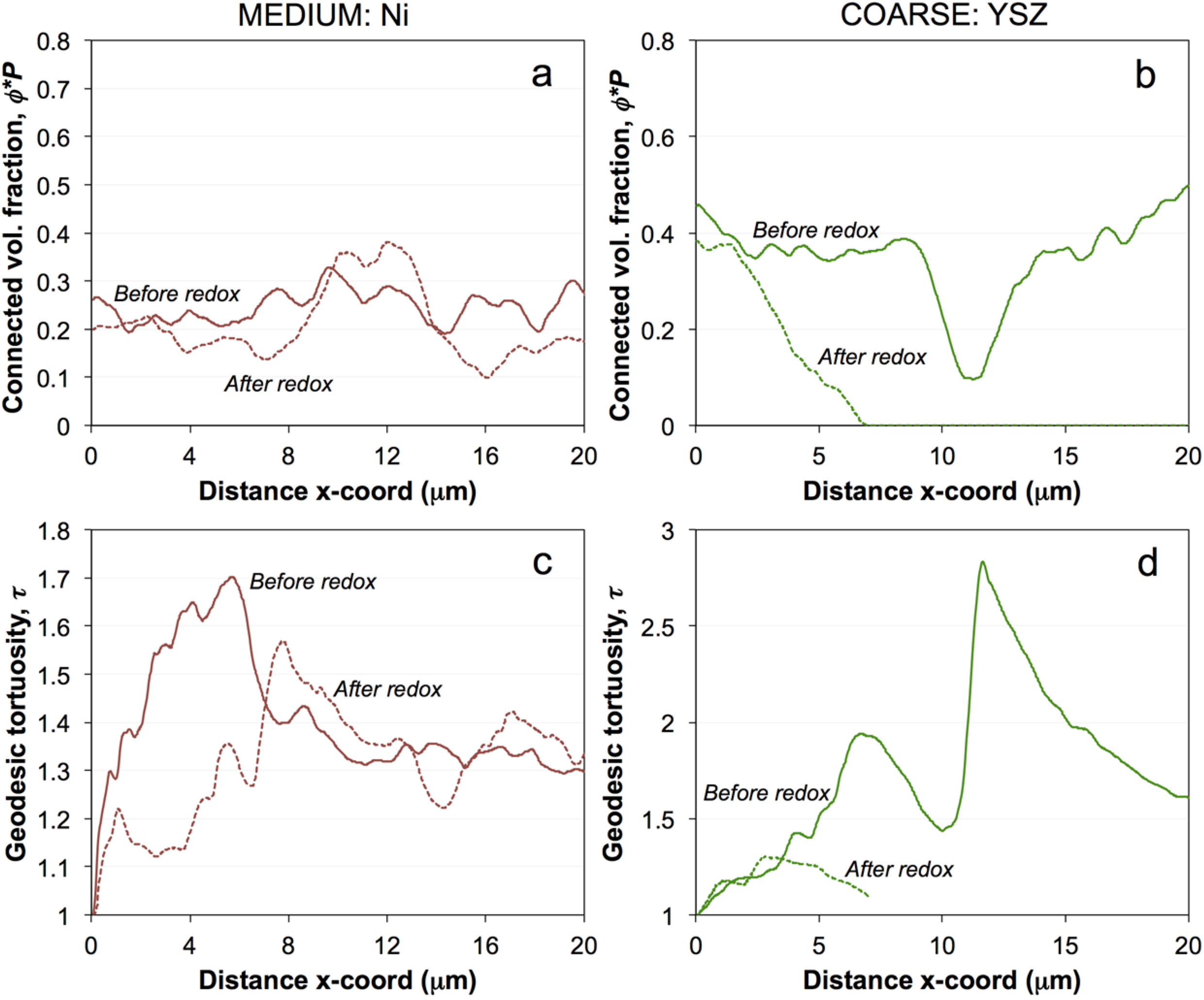
4.2. Degradation Mechanism
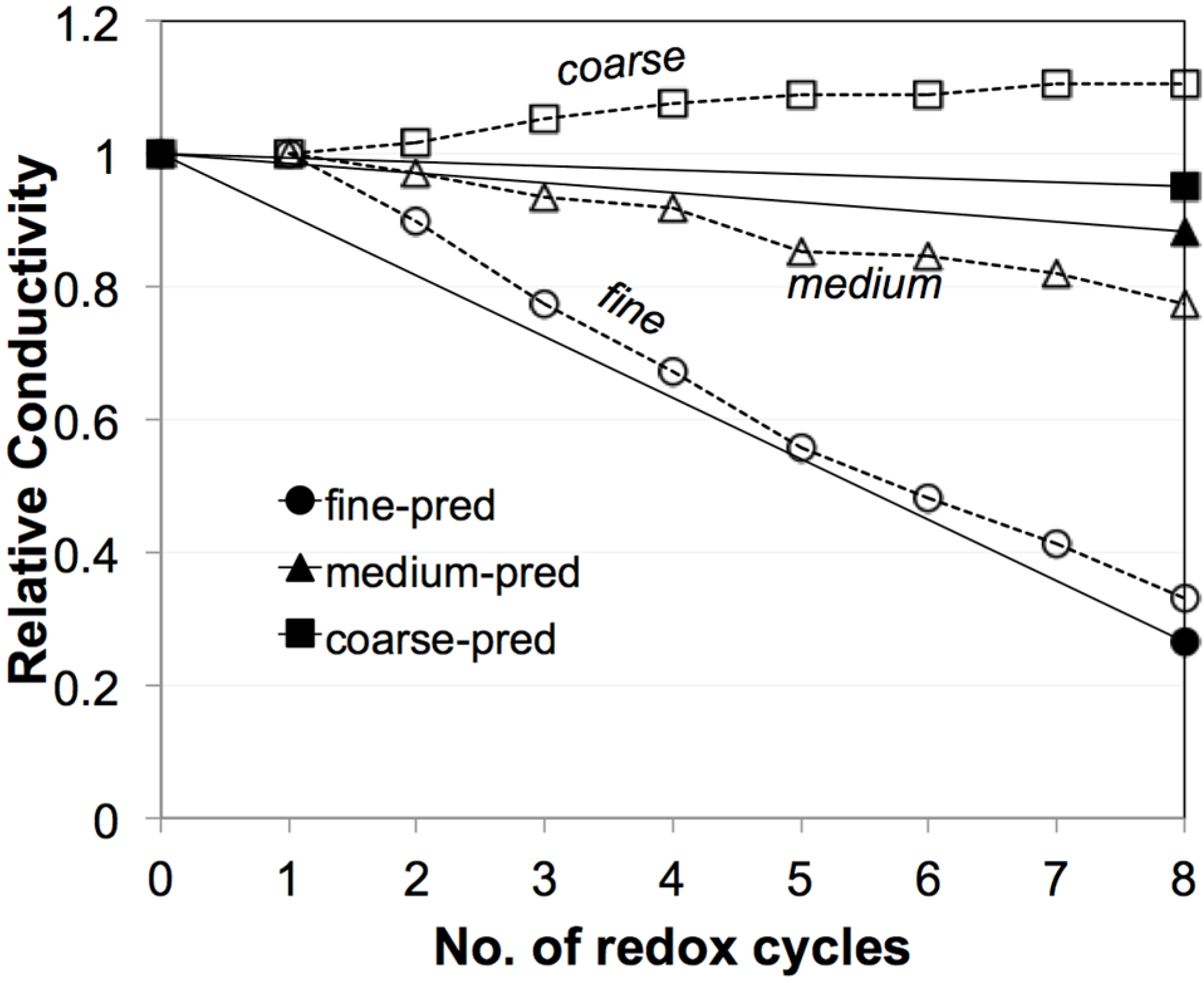
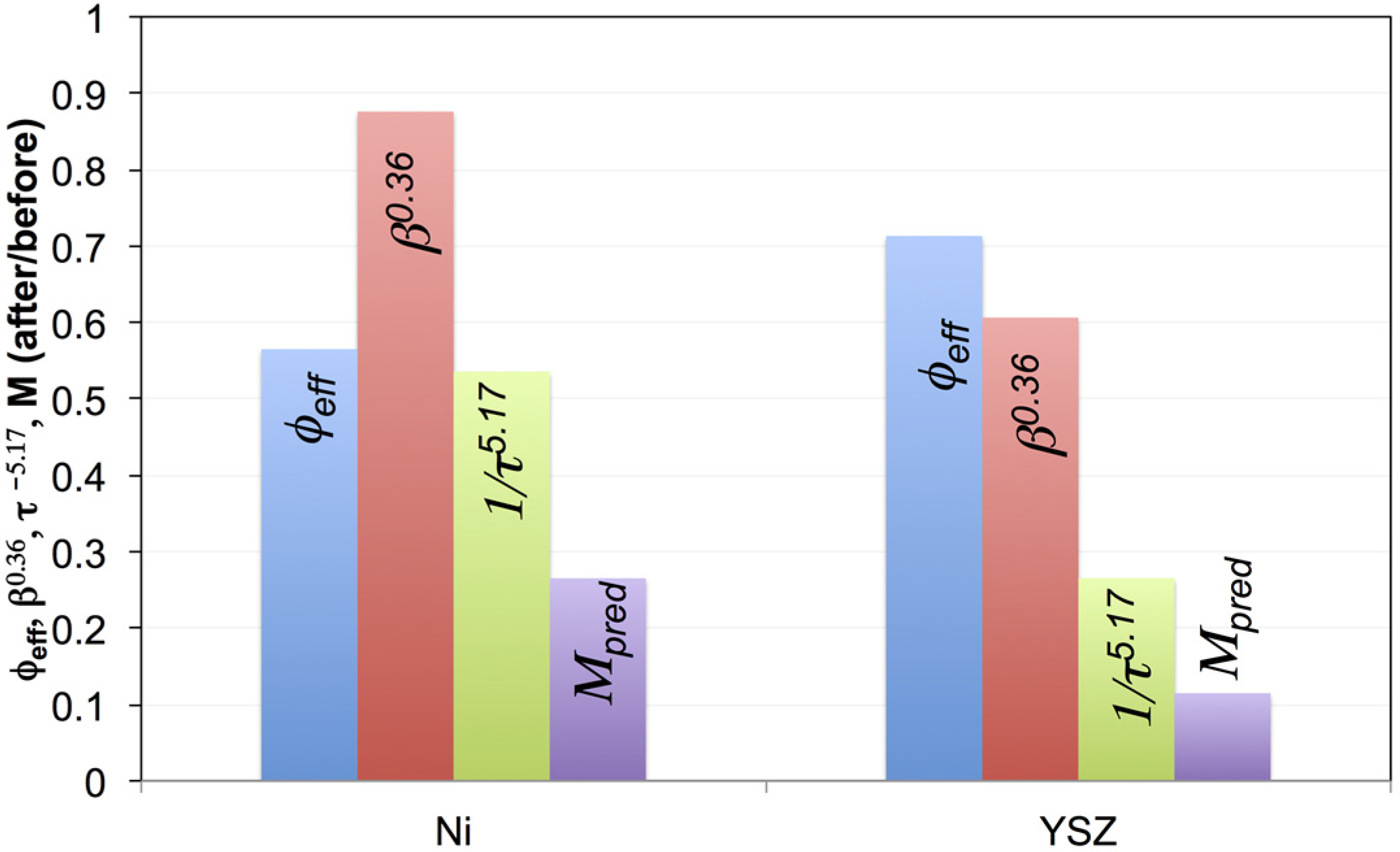
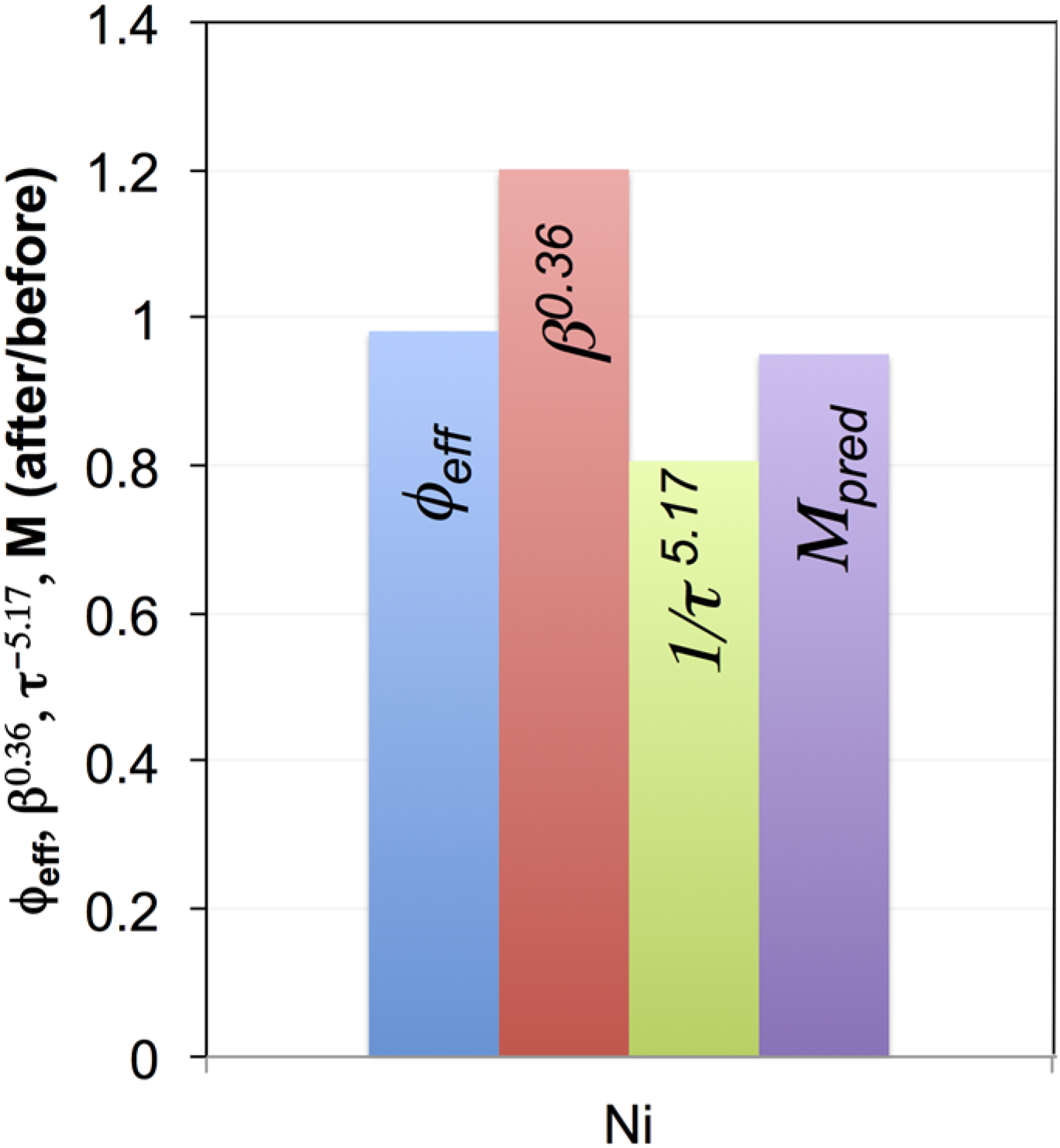


4.3. Mixing Different Powders
| State of Degradation | FINE | COARSE | ||
|---|---|---|---|---|
| Ni | YSZ | Ni | YSZ | |
| Before redox cycling | (+) High initial e− conductivity | (+) High initial ionic conductivity | (–) Low initial e− conductivity | (–) Low initial ionic conductivity |
| After redox cycling | (–) Strong coarsening, (–) Loss of percolation, (–) Increase of τ | (+) Strong bottlenecks due to high sinter activity, (+) Small increase of τ | (–) Significant coarsening but, (+) Low loss of percolation, and (+) Low change of β and τ | (–) Weak bottlenecks due to low sinter activity, (–) High loss of percolation, (−) Strong change of β and τ |

5. Conclusions
5.1. Comparison of Fine vs. Coarse Anodes: Before Redox Cycling
5.2. Comparison of Ni in Fine vs. Coarse Anodes: After Eight Redox Cycles
5.3. Comparison of YSZ in Fine vs. Coarse Anodes: After Eight Redox Cycles
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gauckler, L.J.; Beckel, D.; Buergler, B.E.; Jud, E.; Muecke, U.P.; Prestat, M.; Rupp, J.L.M.; Richter, J. Solid Oxide Fuel Cells: Systems and Materials. CHIMIA Int. J. Chem. 2004, 58, 837–850. [Google Scholar] [CrossRef]
- Mantzaras, J.; Freunberger, S.A.; Büchi, F.N.; Roos, M.; Brandstätter, W.; Prestat, M.; Gauckler, L.J.; Andreaus, B.; Hajbolouri, F.; Senn, S.M.; et al. Fuel cell modeling and simulations. CHIMIA Int. J. Chem. 2004, 58, 857–868. [Google Scholar] [CrossRef]
- Adler, S.B.; Bessler, W.G. Elementary kinetic modeling of solid oxide fuel cell electrode reactions. Handb. Fuel Cells 2010. [Google Scholar] [CrossRef]
- Pecho, O.; Holzer, L.; Yáng, Z.; Martynczuk, J.; Hocker, T.; Flatt, R.J.; Prestat, M. Influence of strontium-rich pore-filling phase on the performance of La0.6Sr0.4CoO3-δ thin-film cathodes. J. Power Sources 2015, 274, 295–303. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Atkinson, A. Redox cycling of Ni-based solid oxide fuel cell anodes: A review. Fuel Cells 2007, 7, 246–258. [Google Scholar] [CrossRef]
- Klemenso, T.; Mogensen, M. Ni-YSZ solid oxide fuel cell anode behavior upon reodx cycling based on electrical characterization. J. Am. Ceram. Soc. 2007, 90, 3582–3588. [Google Scholar] [CrossRef]
- Fouquet, D.; Muller, A.C.; Weber, A.; Ivers-Tiffee, E. Kinetics of oxidation and reduction of Ni/YSZ cermets. Ionics 2003, 9, 103–108. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Holzer, L.; Mai, A.; Schütze, M. Nickel agglomeration in solid oxide fuel cells under different operating conditions. In Proceedings of the 10th European SOFC Forum, Luzern, Switzerland, 26–29 June 2012; pp. 5–15.
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.J.; Grew, K.N.; Izzo, J.R., Jr.; Lombardo, J.J.; Harris, W.M.; Faes, A.; Hessler-Wyser, A.; van Herle, J.; Wang, S.; Chu, Y.S.; et al. Three-dimensional microstructural changes in the Ni-YSZ solid oxide fuel cell anode during operation. Acta Mater. 2012, 60, 3491–3500. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Takeuchi, T.; Nomura, K.; Kageyama, H.; Takeda, Y. Aging effect of 8 mol % YSZ ceramics with different microstructures. J. Electrochem. Soc. 2004, 151, A1286–A1291. [Google Scholar] [CrossRef]
- Hattori, M.; Takeda, Y.; Sakaki, Y.; Nakanishi, A.; Ohara, S.; Mukai, K.; Lee, J.-H.; Fukui, T. Effect of aging on conductivity of yttria stabilized zirconia. J. Power Sources 2004, 126, 23–27. [Google Scholar] [CrossRef]
- Wilson, J.R.; Kobsiriphat, W.; Mendoza, R.; Chen, H.-Y.; Hiller, J.M.; Miller, D.J.; Thornton, K.; Voorhees, P.W.; Adler, S.B.; Barnett, S.A. Three-dimensional reconstruction of a solid-oxide fuel-cell anode. Nat. Mater. 2006, 5, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Shearing, P.R.; Cai, Q.; Golbert, J.I.; Yufit, V.; Adjiman, C.S.; Brandon, N.P. Microstructural analysis of a solid oxide fuel cell anode using focused ion beam techniques coupled with electrochemical simulation. J. Power Sources 2010, 195, 4804–4810. [Google Scholar] [CrossRef]
- Vivet, N.; Chupin, S.; Estrade, E.; Piquero, T.; Pommier, P.L.; Rochais, D.; Bruneton, E. 3D Microstructural characterization of a solid oxide fuel cell anode reconstructed by focused ion beam tomography. J. Power Sources 2011, 196, 7541–7549. [Google Scholar] [CrossRef]
- Shikazono, N.; Kanno, D.; Matsuzaki, K.; Teshima, H.; Sumino, S.; Kasagi, N. Numerical assessment of SOFC anode polarization based on three-dimensional model microstructure reconstructed from FIB-SEM images. J. Electrochem. Soc. 2010, 157, B665–B672. [Google Scholar] [CrossRef]
- Holzer, L.; Muench, B.; Iwanschitz, B.; Cantoni, M.; Hocker, T.; Graule, T. Quantitative relationships between composition, particle size, triple phase boundary length and surface area in nickel-cermet anodes for Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 7076–7089. [Google Scholar] [CrossRef]
- Holzer, L.; Iwanschitz, B.; Hocker, Th.; Münch, B.; Prestat, M.; Wiedenmann, D.; Vogt, U.; Holtappels, P.; Sfeir, J.; Mai, A.; Graule, Th. Microstructure degradation of cermet anodes for solid oxide fuel cells: Quantification of nickel grain growth in dry and in humid atmospheres. J. Power Sources 2011, 196, 1279–1294. [Google Scholar] [CrossRef]
- Jiao, Z.; Shikazono, N. Simulation of nickel morphological and crystal structures evolution in solid oxide fuel cell anode using phase field method. J. Electrochem. Soc. 2014, 161, F577–F582. [Google Scholar] [CrossRef]
- Wilson, J.R.; Cronin, J.S.; Barnett, S.A. Linking the microstructure, performance and durability of Ni-yttria-stabilized zirconia solid oxide fuel cell anodes using three-dimensional focused ion beam-scanning electron microscopy imaging. Scr. Mater. 2011, 65, 67–72. [Google Scholar] [CrossRef]
- Shearing, P.R.; Golbert, J.; Chater, R.J.; Brandon, N.P. 3D reconstruction of SOFC anodes using a focused ion beam lift-out technique. Chem. Eng. Sci. 2009, 64, 3928–3933. [Google Scholar] [CrossRef]
- Izzo, J.R.; Joshi, A.S.; Grew, K.N.; Chiu, W.K.S.; Tkachuk, A.; Wang, S.H.; Yun, W. Nondestructive reconstruction and analysis of SOFC anodes using X-ray computed tomography at sub-50 nm resolution. J. Electrochem. Soc. 2008, 155, B504–B508. [Google Scholar] [CrossRef]
- Grew, K.N.; Chu, Y.S.; Yi, J.; Peracchio, A.A.; Izzo, J.R.; Hwu, Y.; de Carlo, F.; Chiu, W.K.S. Nondestructive nanoscale 3D elemental mapping and analysis of a solid oxide fuel cell anode. J. Electrochem. Soc. 2010, 157, B783–B792. [Google Scholar] [CrossRef]
- Usseglio-Viretta, F.; Laurencin, J.; Delette, G.; Villanova, J.; Cloetens, P.; Leguillon, D. Quantitative microstructure characterization of a Ni/YSZ bi-layer coupled with simulated electrode polarization. J. Power Sources 2014, 256, 394–403. [Google Scholar] [CrossRef]
- Cronin, J.S.; Chen-Wiegart, Y.K.; Wang, J.; Barnett, S.A. Three-dimensional reconstruction and analysis of an entire solid oxide fuel cell by full-field transmission X-ray microscopy. J. Power Sources 2013, 233, 174–179. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.J.; Gong, Y.H.; Liu, G.; Zhang, X.B.; Chen, J.; Gelb, J.; Yun, W.B.; Xiong, Y.; Tian, Y.C.; Wang, H.Q. Analysis of the three-dimensional microstructure of a solid-oxide fuel cell anode using nano X-ray tomography. J. Power Sources 2011, 196, 1915–1919. [Google Scholar] [CrossRef]
- Jang, J.H.; Ryu, J.H.; Oh, S.M. Microstructure of Ni/YSZ cermets according to particle size of precursor powders and their anodic performances in SOFC. Ionics 2000, 6, 86–91. [Google Scholar] [CrossRef]
- Ribeiro, N.F.P.; Souza, M.M.V.M.; Macedo Neto, O.R.; Vasconcelos, S.M.R.; Schmal, M. Investigating the microstructure and catalytic properties of Ni/YSZ cermets as anodes for SOFC applications. Appl. Catal. A 2009, 353, 305–309. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J. The effect of nickel oxide microstructure on the performance of Ni/YSZ anode-supported SOFCs. Solid State Ionics 2008, 179, 1516–1520. [Google Scholar] [CrossRef]
- Kawashima, T.; Miyoshi, S.; Shibuta, Y.; Yamaguchi, S. Particle size dependence of polarization of Ni/YSZ cermet anodes for solid oxide fuel cells. J. Power Sources 2013, 234, 147–153. [Google Scholar] [CrossRef]
- Brown, M.; Primdahl, S.; Mogensen, M. Structure/Performance relations for Ni/Yttria-Stabilized zirconia anodes for solid oxide fuel cells. J. Electrochem. Soc. 2000, 147, 475–485. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, C.; Lü, Z.; Su, W. Effects of initial reduction temperature on the microstructures and performances of Ni/YSZ anodes for solid oxide fuel cells. J. Electrochem. Soc. 2013, 160, F1170–F1174. [Google Scholar] [CrossRef]
- Grew, K.N.; Peracchio, A.A.; Joshi, A.S.; Izzo, J.R., Jr.; Chiu, W.K.S. Characterization and analysis methods for the examination of the heterogeneous solid oxide fuel cell electrode microstructure. Part 1: Volumetric measurements of the heterogeneous structure. J. Power Sources 2010, 195, 7930–7942. [Google Scholar] [CrossRef]
- Gaiselmann, G.; Neumann, M.; Schmidt, V.; Pecho, O.; Hocker, T.; Holzer, L. Quantitative relationships between microstructure and effective transport properties based on virtual materials testing. AIChE J. 2014, 60, 1983–1999. [Google Scholar] [CrossRef]
- Wiedenmann, D.; Keller, L.; Holzer, L.; Stojadinović, J.; Münch, B.; Suarez, L.; Fumey, B.; Hagendorfer, H.; Brönnimann, R.; Modregger, P.; et al. Three-dimensional pore structure and ion conductivity of porous ceramic diaphragms. AIChE J. 2013, 59, 1446–1457. [Google Scholar] [CrossRef]
- Petersen, E.E. Diffusion in a pore of varying cross section. AIChE J. 1958, 4, 343–345. [Google Scholar] [CrossRef]
- Holzer, L.; Wiedenmann, D.; Muench, B.; Keller, L.; Prestat, M.; Gasser, P.; Robertson, I.; Grobéty, B. The influence of constrictivity on the effective transport properties of porous layers in electrolysis and fuel cells. J. Mater. Sci. 2013, 48, 2934–2952. [Google Scholar] [CrossRef]
- Grew, K.N.; Peracchio, A.A.; Chiu, W.K.S. Characterization and analysis methods for the examination of the heterogeneous solid oxide fuel cell electrode microstructure: Part 2. Quantitative measurement of the microstructure and contributions to transport losses. J. Power Sources 2010, 195, 7943–7958. [Google Scholar] [CrossRef]
- Nelson, G.J.; Peracchio, A.A.; Chiu, W.K.S. Analytical investigations of varying cross section microstructures on charge transfer in solid oxide fuel cell electrodes. J. Power Sources 2011, 196, 4695–4704. [Google Scholar] [CrossRef]
- Nelson, G.J.; Cassenti, B.N.; Peracchio, A.A.; Chiu, W.K.S. Two-dimensional charge transfer and space charge effects in extended surface solid oxide fuel cell electrodes. J. Power Sources 2010, 205, 48–56. [Google Scholar] [CrossRef]
- Clennell, M.B. Tortuosity: A guide through the maze. Geol. Soc. 1997, 122, 299–344. [Google Scholar] [CrossRef]
- Stenzel, O.; Pecho, O.; Holzer, L.; Neumann, M.; Schmidt, V. Predicting effective conductivities based on geometric microstructure characteristics. AIChE J. 2015. submitted for publication. [Google Scholar]
- Holzer, L.; Iwanschitz, B.; Hocker, Th.; Keller, L.; Pecho, O.; Sartoris, G.; Gasser, Ph.; Muench, B. Redox cycling of Ni/YSZ anodes for solid oxide fuel cells: Influence of tortuosity, constriction and percolation factors on the effective transport properties. J. Power Sources 2013, 242, 179–194. [Google Scholar] [CrossRef]
- Münch, B.; Holzer, L. Contradicting geometrical concepts in pore size analysis attained with electron microscopy and mercury intrusion. J. Am. Ceram. Soc. 2008, 91, 4059–4067. [Google Scholar] [CrossRef]
- Iwanschitz, B. Degradation von Ni-Cermet-Anoden in Keramischen Hochtemperatur Brennstoffzellen. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2012. [Google Scholar]
- Iwanschitz, B.; Sfeir, J.; Mai, A.; Schütze, M. Degradation of SOFC anodes upon redox cycling: A comparison between Ni/YSZ and Ni/CGO. J. Electrochem. Soc. 2010, 157, B269–B278. [Google Scholar] [CrossRef]
- Fiji. Available online: http://fiji.sc/Fiji (accessed on 22 August 2015).
- Avizo, Version 8.1.1; Software for Scientific and Industrial Data. FEI: Hillsboro, OR, USA. Available online: http://www.fei.com/software/avizo3d/ (accessed on 22 August 2015).
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Thulasiraman, K.; Swamy, M.N.S. Graphs: Theory and Algorithms; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- GeoDict, Version 2014 Rev; Software for Scientific and Industrial Data. Math 2 Market: Kaiserslautern, Germany, March 2015. Available online: http://www.geodict.com/ (accessed on 22 August 2015).
- Wiegmann, A.; Zemitis, A. EJ-HEAT: A Fast Explicit Jump Harmonic Averaging Solver for the Effective Heat Conductivity of Composite Materials; Fraunhofer ITWM: Kaiserslautern, Germany, 2006. [Google Scholar]
- Iwai, H.; Shikazono, N.; Matsui, T.; Teshima, H.; Kishimoto, M.; Kishida, R.; Hayashi, D.; Matsuzaki, K.; Kanno, D.; Saito, M.; et al. Quantification of SOFC anode microstructure based on dual beam FIB-SEM technique. J. Power Sources 2010, 195, 955–961. [Google Scholar] [CrossRef]
- Kishimoto, M.; Iwai, H.; Saito, M.; Yoshida, H. Quantitative evaluation of transport properties of SOFC porous anode by random walk process. ECS Trans. 2009, 25, 1887–1896. [Google Scholar]
- Ananyev, M.V.; Bronin, D.I.; Osinkin, D.A.; Eremin, V.A.; Steinberger-Wilckens, R.; de Haart, L.G.J.; Mertens, J. Characterization of Ni-cermet degradation phenomena I. Long term resistivity monitoring, image processing and X-ray fluorescence analysis. J. Power Sources 2015, 286, 414–426. [Google Scholar] [CrossRef]
- Laurencin, J.; Quey, R.; Delette, G.; Suhonen, H.; Cloetens, P.; Bleuet, P. Characterisation of Solid Oxide Fuel Cell Ni-8YSZ substrate by synchrotron X-ray nano-tomography: From 3D reconstruction to microstructure quantification. J. Power Sources 2012, 198, 182–189. [Google Scholar] [CrossRef]
- Boigues-Muñoz, C.; Pumiglia, D.; McPhail, S.J.; Santori, G.; Montinaro, D.; Comodi, G.; Carlini, M.; Polonara, F. More accurate macro-models of solid oxide fuel cells through electrochemical and microstructural parameter estimation e Part II: Parameter estimation. J. Power Sources 2015, 286, 321–329. [Google Scholar] [CrossRef]
- Brus, G.; Miyawaki, K.; Iwai, H.; Saito, M.; Yoshida, H. Tortuosity of an SOFC anode estimated from saturation currents and a mass transport model in comparison with a real micro-structure. Solid State Ionics 2014, 265, 13–21. [Google Scholar] [CrossRef]
- Kanno, D.; Shikazono, N.; Takagi, N.; Matsuzaki, K.; Kasagi, N. Evaluation of SOFC anode polarization simulation using three-dimensional microstructures reconstructed by FIB tomography. Electrochim. Acta 2011, 56, 4015–4021. [Google Scholar] [CrossRef]
- Kishimoto, M.; Miyawaki, K.; Iwai, H.; Saito, M.; Yoshida, H. Effect of composition ratio of Ni-YSZ anode on distribution of effective three-phase boundary and power generation performance. Fuel Cells 2013, 13, 476–486. [Google Scholar] [CrossRef]
- Brus, G.; Isomoto, T.; Iwai, H.; Saito, M.; Yoshida, H.; Komatsu, Y.; Nowak, R.; Szmyd, J.S. Operating characteristics of an anode-supported planar SOFC stack with post-operation three-dimensional reconstruction of the electrodes microstructure. In Proceedings of the 11th European SOFC and SOE Forum, Luzern, Switzerland, 26–29 June 2012; pp. 69–79.
- Berg, C.K. Re-examining Archie’s law: Conductance description by tortuosity and constriction. Phys. Rev. E 2012, 86. [Google Scholar] [CrossRef]
- Berg, C.K. Permeability description by Characteristic Length, Tortuosity, Constriction and Porosity. Transp. Porous Media 2014, 103, 381–400. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, W.; Li, M.; Wang, X.; Chi, B.; Pu, J.; Li, J. Improved microstructure and performance of Ni-based anode for intermediate temperature solid oxide fuel cells. J. Power Sources 2014, 247, 170–177. [Google Scholar] [CrossRef]
- Buyukaksoy, A.; Petrovsky, V.; Dogan, F. Optimization of redox stable Ni-YSZ anodes for SOFCs by two-step infiltration. J. Electrochem. Soc. 2012, 159, F841–F848. [Google Scholar] [CrossRef]
- Fukui, T.; Ohara, S.; Mukai, K. Long-term stability of Ni-YSZ anode with a new microstructure prepared from composite powder. Electrochem. Solid-State Lett. 1998, 1, 120–122. [Google Scholar] [CrossRef]
- Pihlatie, M.; Kaiser, A.; Larsen, P.H.; Mogensen, M. Dimensional behavior of Ni-YSZ composites during redox cycling. J. Electrochem. Soc. 2009, 156, B322–B329. [Google Scholar] [CrossRef]
- Hocker, T.; Iwanchitz, B.; Holzer, L. Assessing the effect of electrode microstructure on repeat unit performance and cell degradation. In Proceedings of the 7th Symposium on Fuel Cell Modeling and Experimental Validation (ModVal 7), Morges, Switzerland, 23–24 March 2010.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecho, O.M.; Stenzel, O.; Iwanschitz, B.; Gasser, P.; Neumann, M.; Schmidt, V.; Prestat, M.; Hocker, T.; Flatt, R.J.; Holzer, L. 3D Microstructure Effects in Ni-YSZ Anodes: Prediction of Effective Transport Properties and Optimization of Redox Stability. Materials 2015, 8, 5554-5585. https://doi.org/10.3390/ma8095265
Pecho OM, Stenzel O, Iwanschitz B, Gasser P, Neumann M, Schmidt V, Prestat M, Hocker T, Flatt RJ, Holzer L. 3D Microstructure Effects in Ni-YSZ Anodes: Prediction of Effective Transport Properties and Optimization of Redox Stability. Materials. 2015; 8(9):5554-5585. https://doi.org/10.3390/ma8095265
Chicago/Turabian StylePecho, Omar M., Ole Stenzel, Boris Iwanschitz, Philippe Gasser, Matthias Neumann, Volker Schmidt, Michel Prestat, Thomas Hocker, Robert J. Flatt, and Lorenz Holzer. 2015. "3D Microstructure Effects in Ni-YSZ Anodes: Prediction of Effective Transport Properties and Optimization of Redox Stability" Materials 8, no. 9: 5554-5585. https://doi.org/10.3390/ma8095265






