Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of Samples and Electrolytes
| Elements | Fe | Si | Cu | Mn | Mg | Zn | Al |
|---|---|---|---|---|---|---|---|
| Composition (wt.%) | 0.15 | 0.10 | 0.05 | 0.02 | 0.001 | 0.01 | Bal. |
2.2. Electrochemical Measurements
2.3. Metallographic Examination
3. Results and Discussion
3.1. Microstructure of Al-B4C Composites
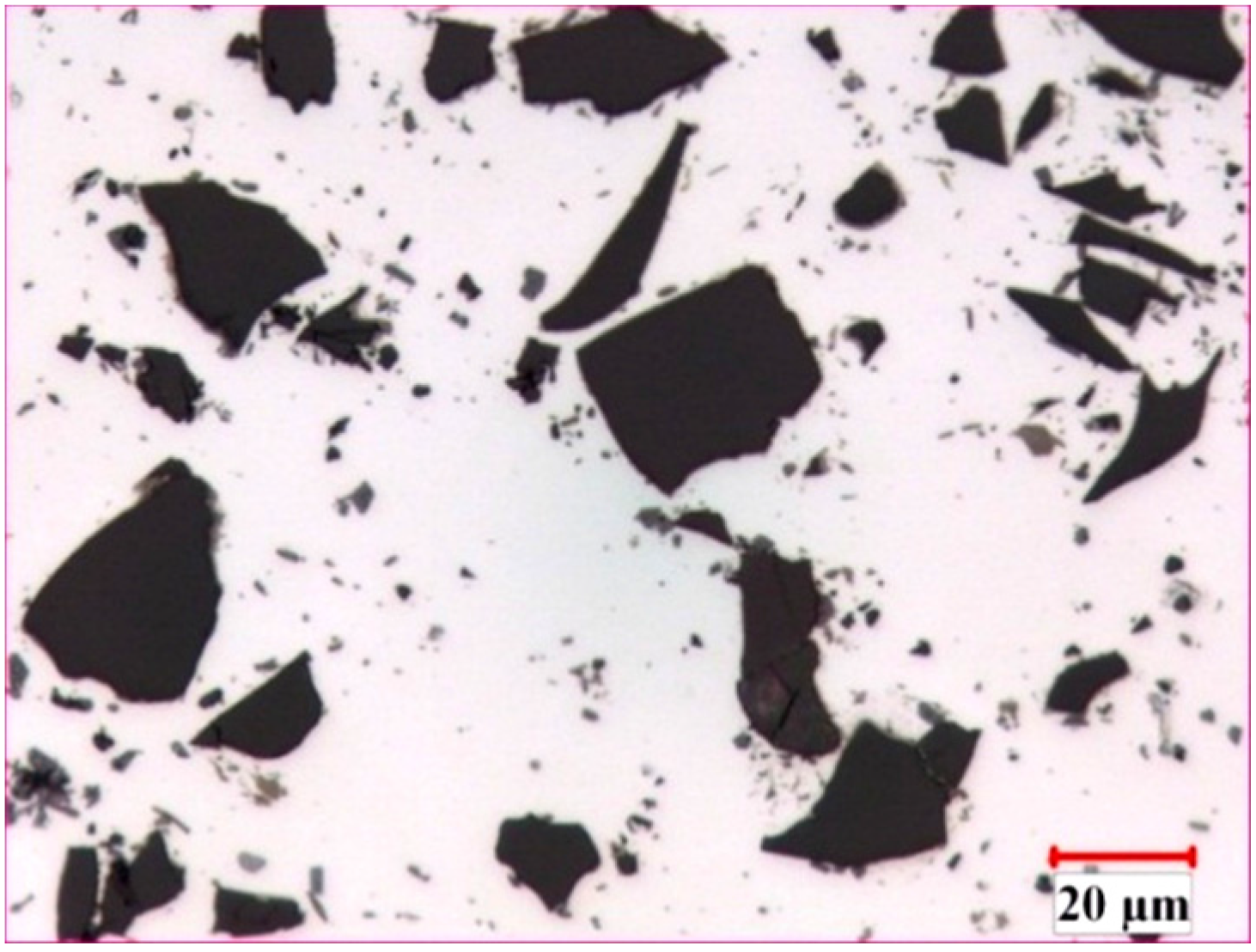
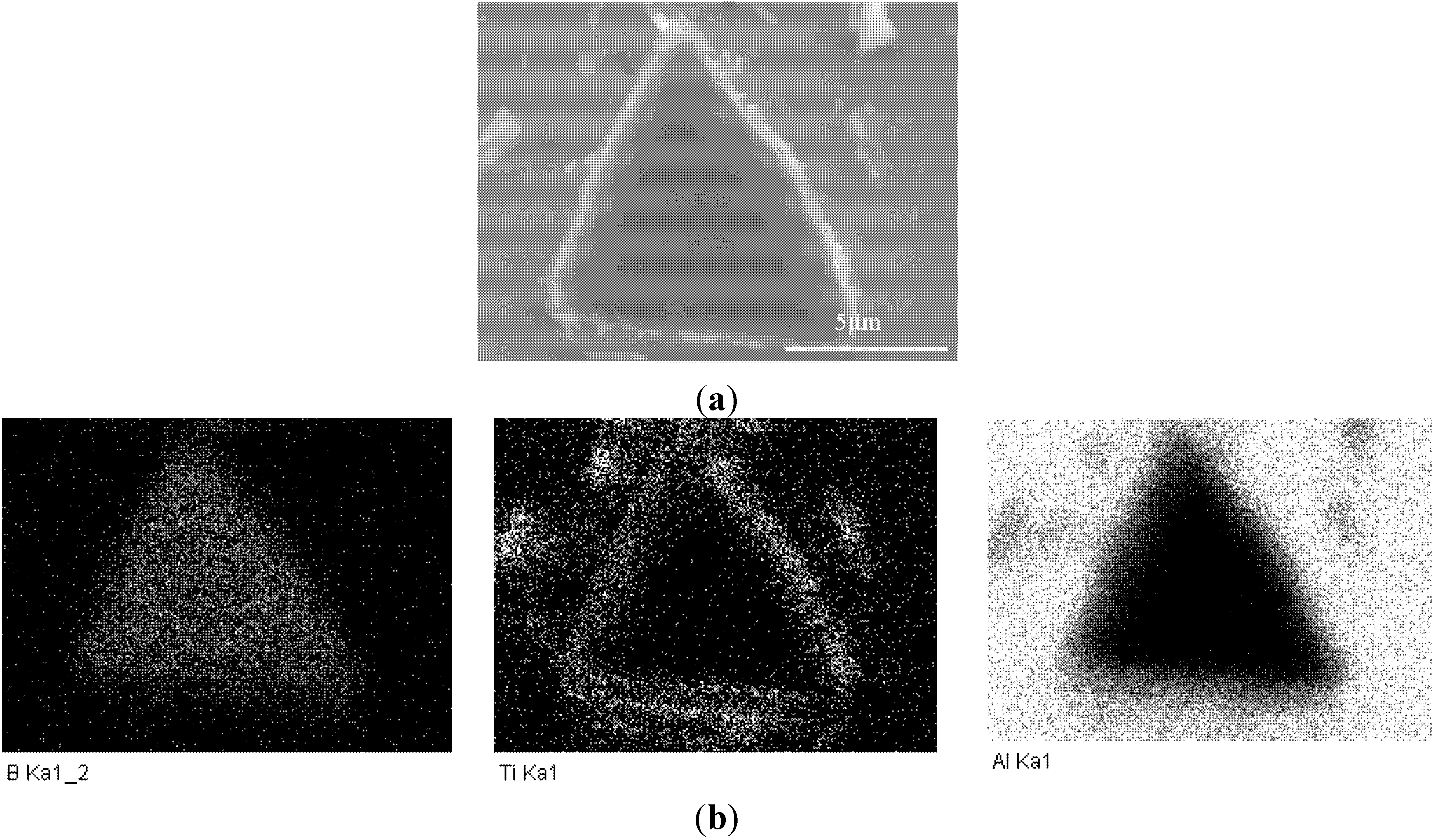
3.2. Corrosion Behavior of Al-B4C Composites
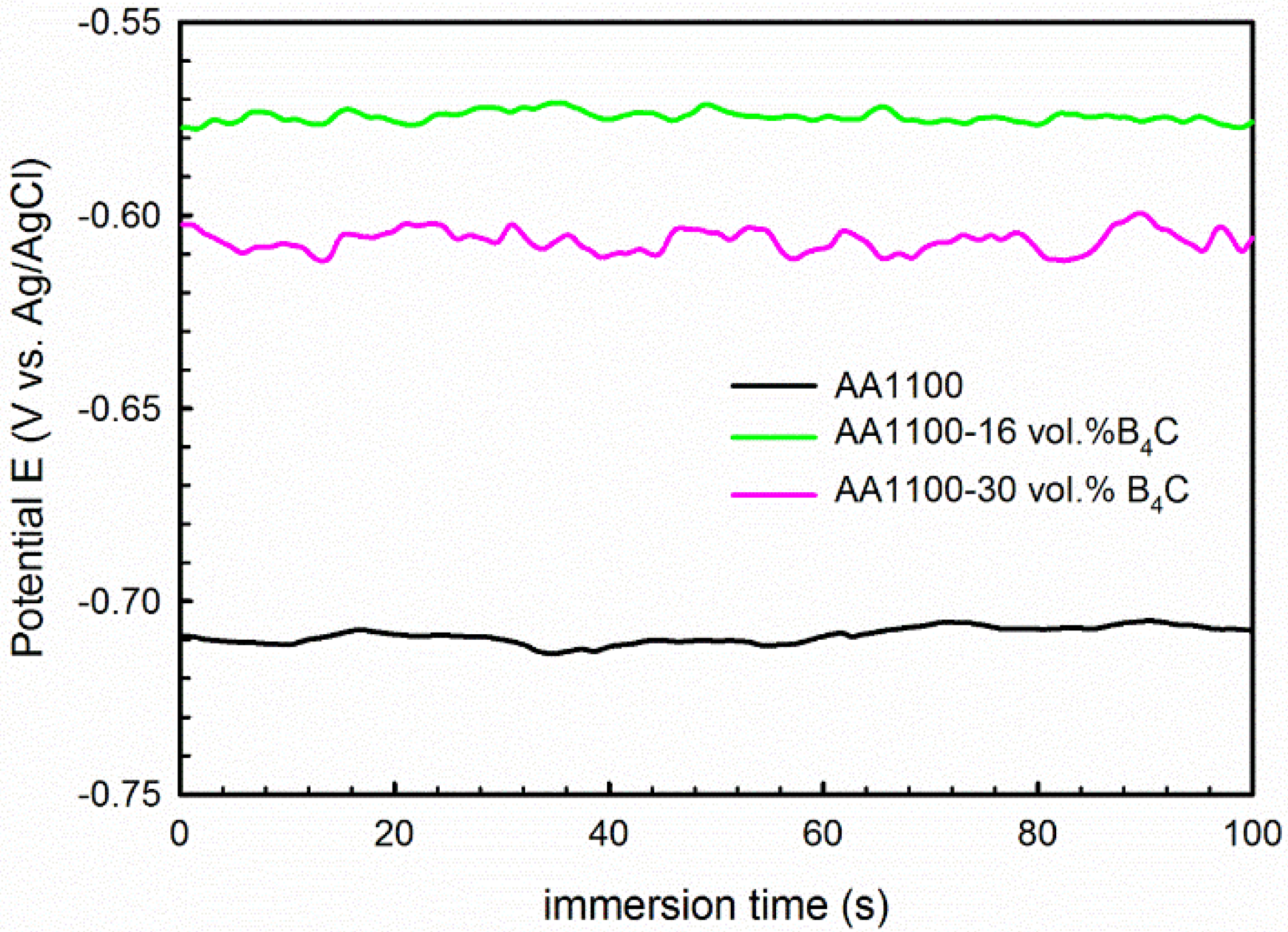
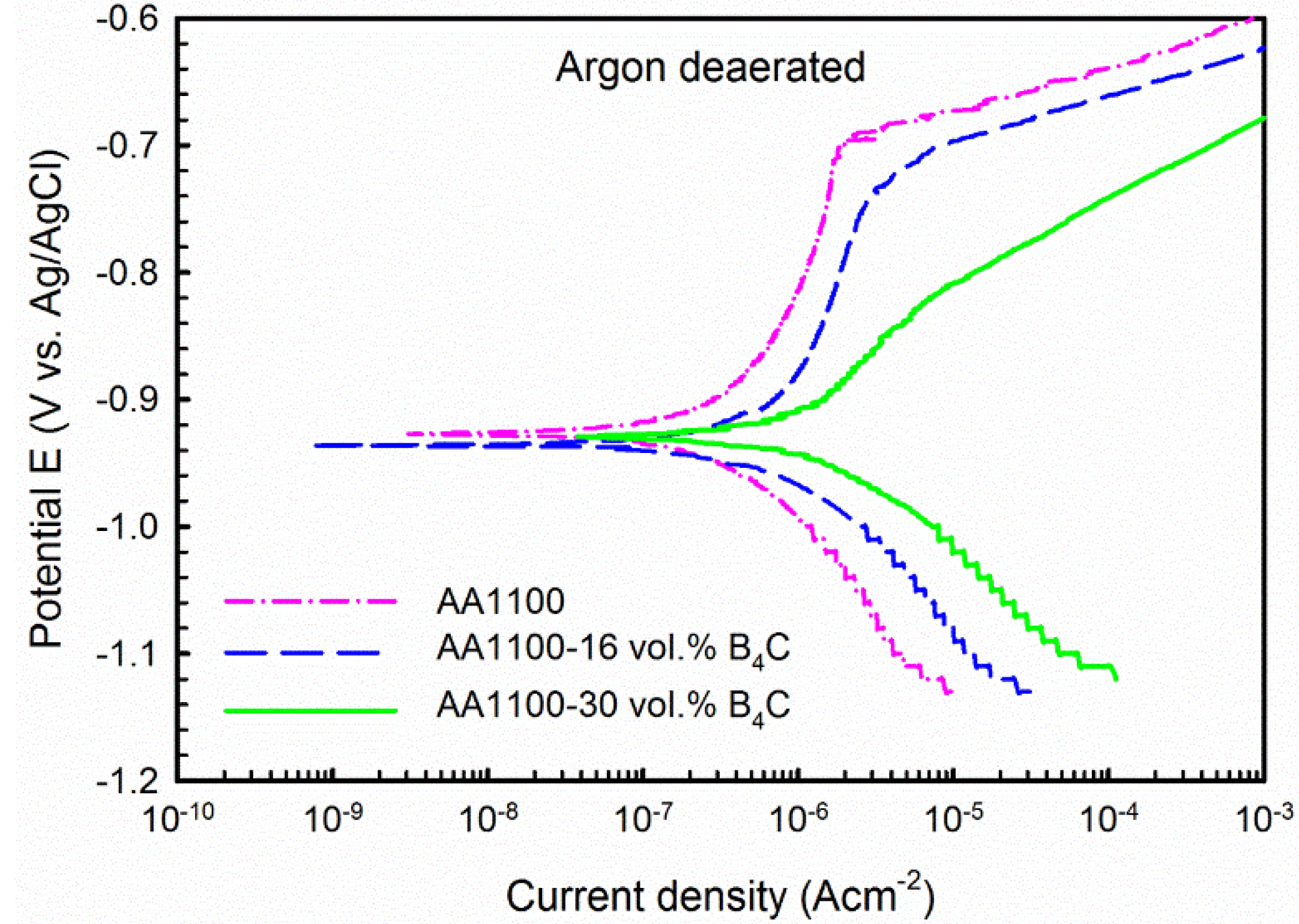
3.2.1. Potentiodynamic Polarization
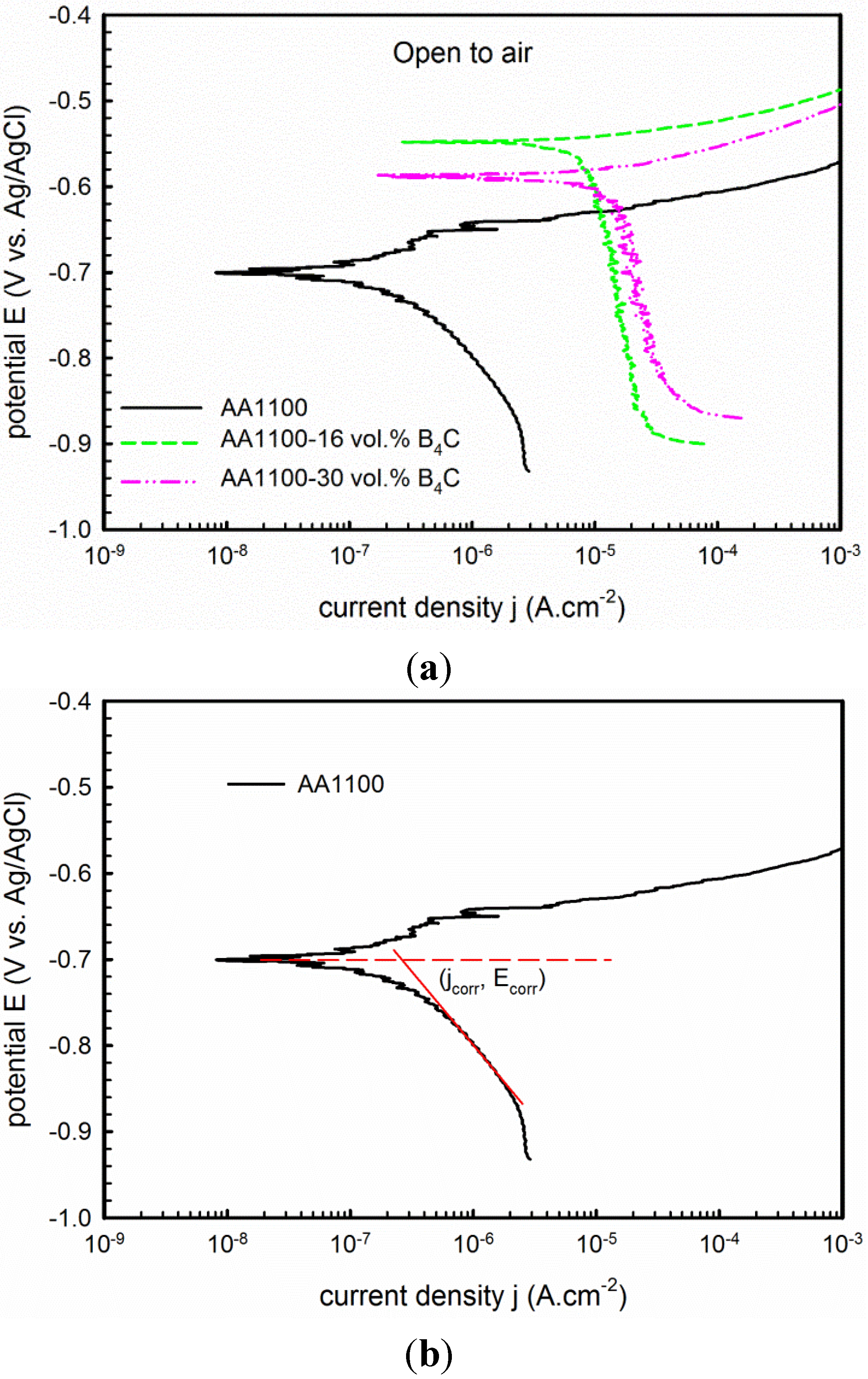
| Materials | Open to Air | Argon Deaerated | ||||
|---|---|---|---|---|---|---|
| Ecorr (VAg/AgCl) | jcorr (μA·cm−2) | βc (V/decade) | Ecorr (VAg/AgCl) | jcorr (μA·cm−2) | bc (V/decade) | |
| AA1100 | −0.70 | 0.35 | 0.18 | −0.93 | 0.33 | 0.16 |
| AA1100-16 vol.% B4C | −0.55 | 8.39 | 0.71 | −0.94 | 0.99 | 0.14 |
| AA1100-30 vol.% B4C | −0.59 | 11.21 | 0.51 | −0.93 | 2.00 | 0.12 |
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
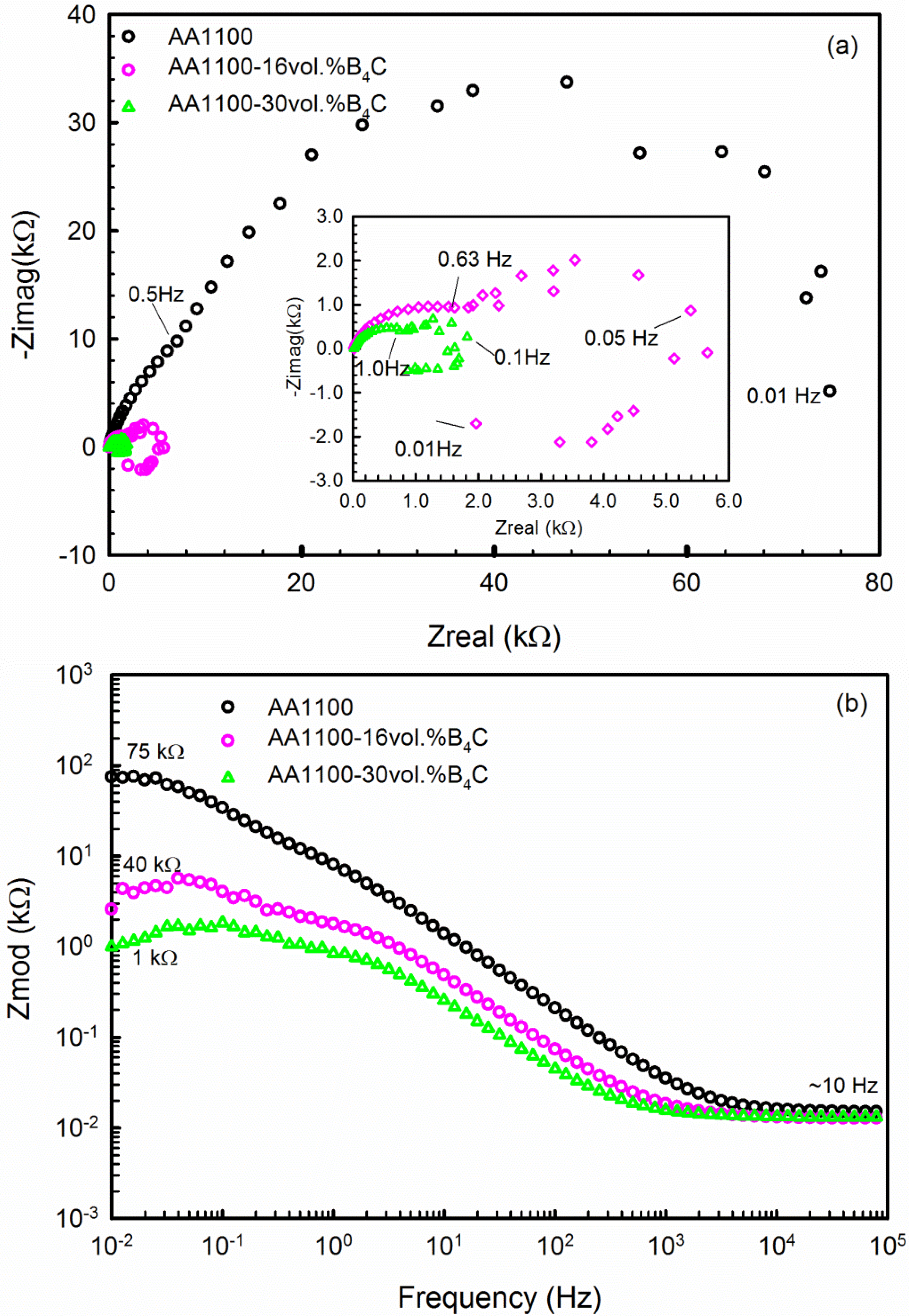
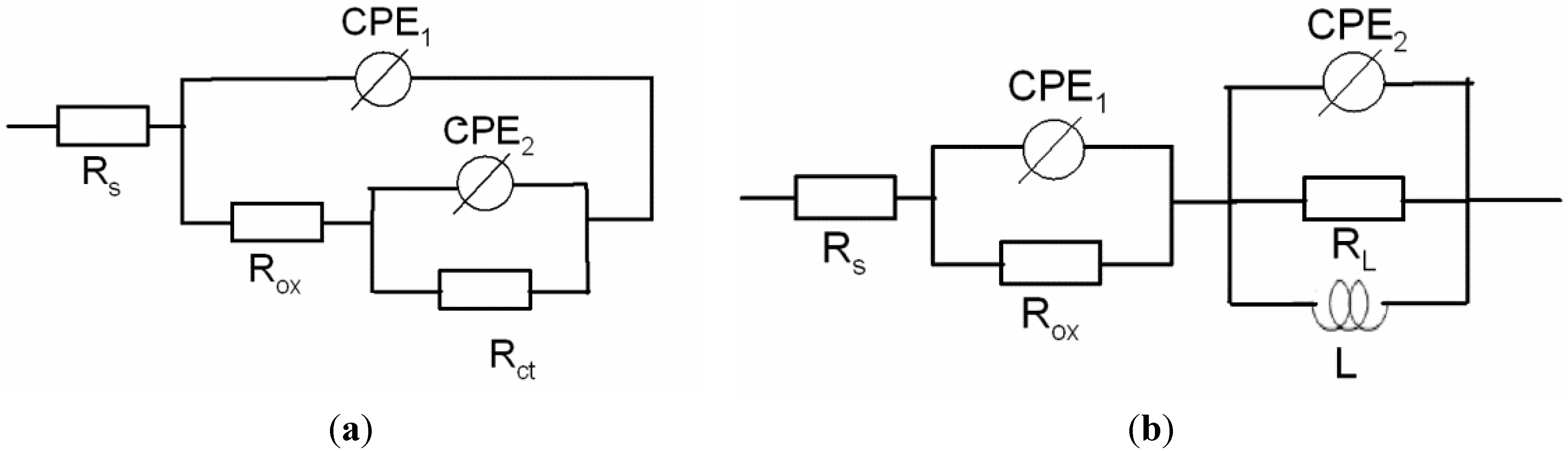
| Materials | Rs (Ω cm2) | CPE1 (μF cm−2) | α1 | Rox (kΩ cm2) | CPE2 (μF cm−2) | α2 | Rct or RL (kΩ cm2) | L (H) |
|---|---|---|---|---|---|---|---|---|
| AA1100 | 14.79 | 20.83 | 0.85 | 23.74 | 30.93 | 0.96 | 54.26 | - |
| Al-16 vol.% B4C | 12.1 | 1.0 | 0.90 | 1.850 | 13.0 | 0.89 | 4.001 | 2200 |
| Al-30 vol.% B4C | 11.0 | 7.6 | 0.87 | 0.898 | 65.0 | 0.96 | 0.960 | 3200 |
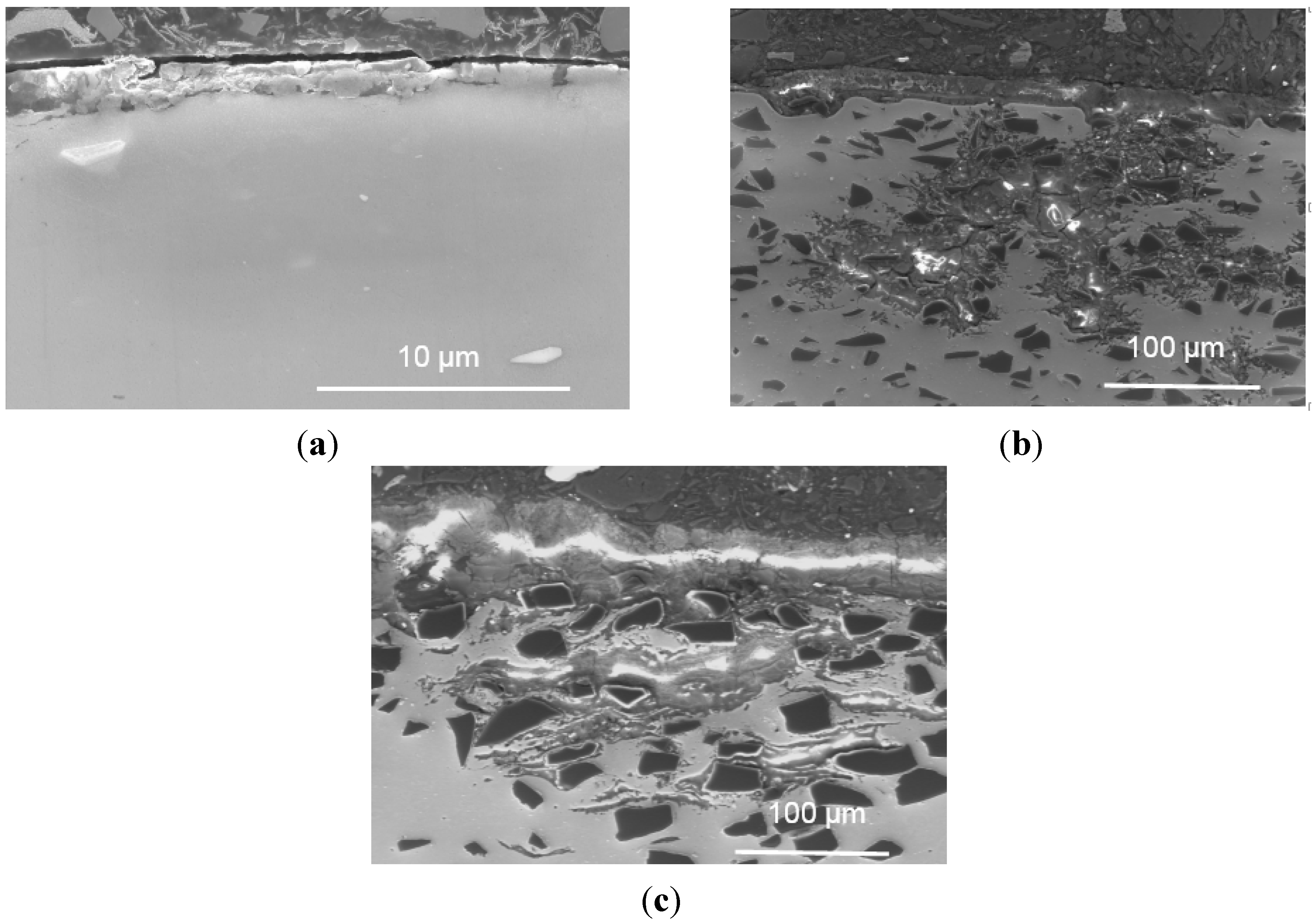
3.3. Corrosion Mechanism Investigation
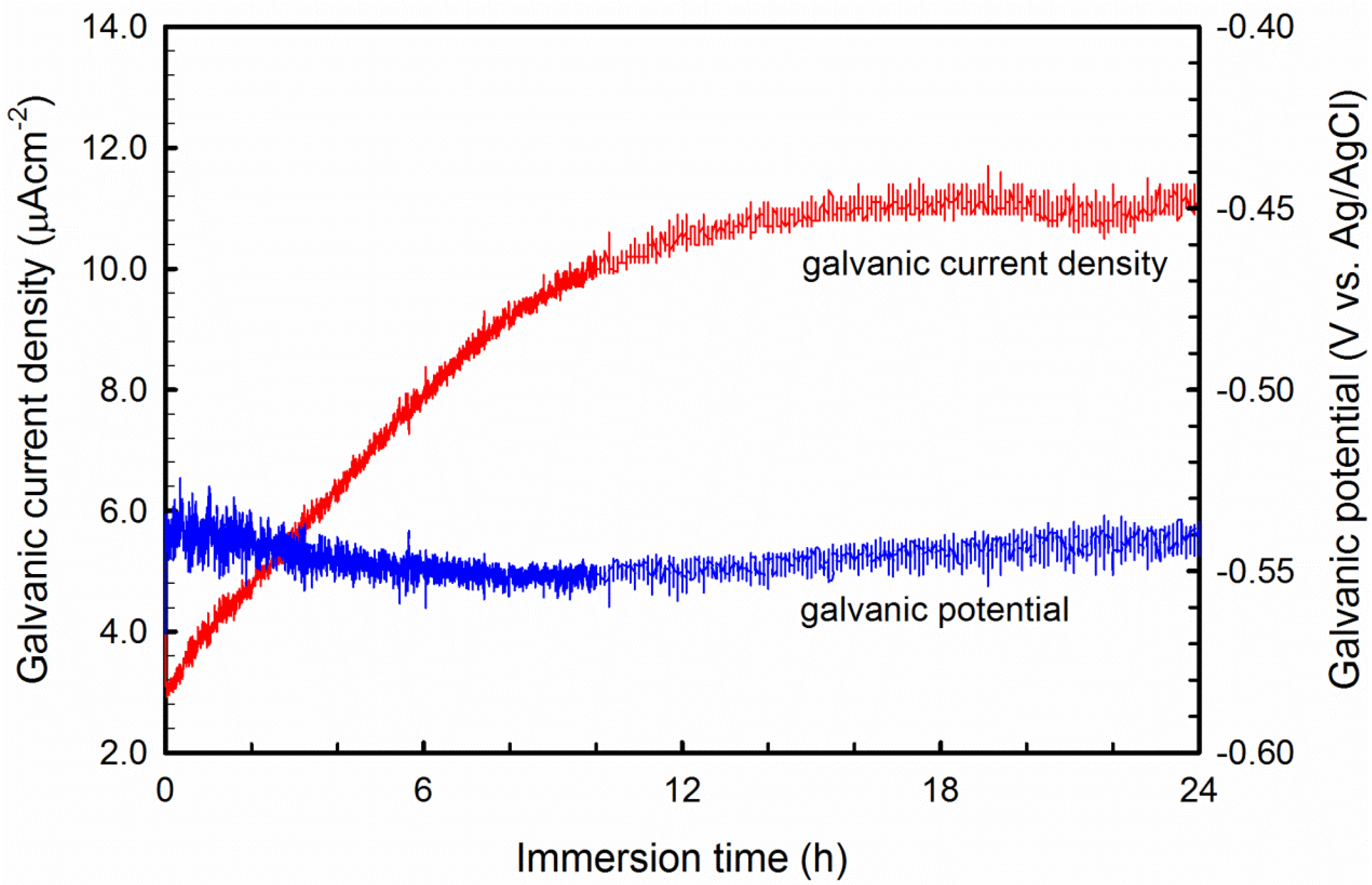
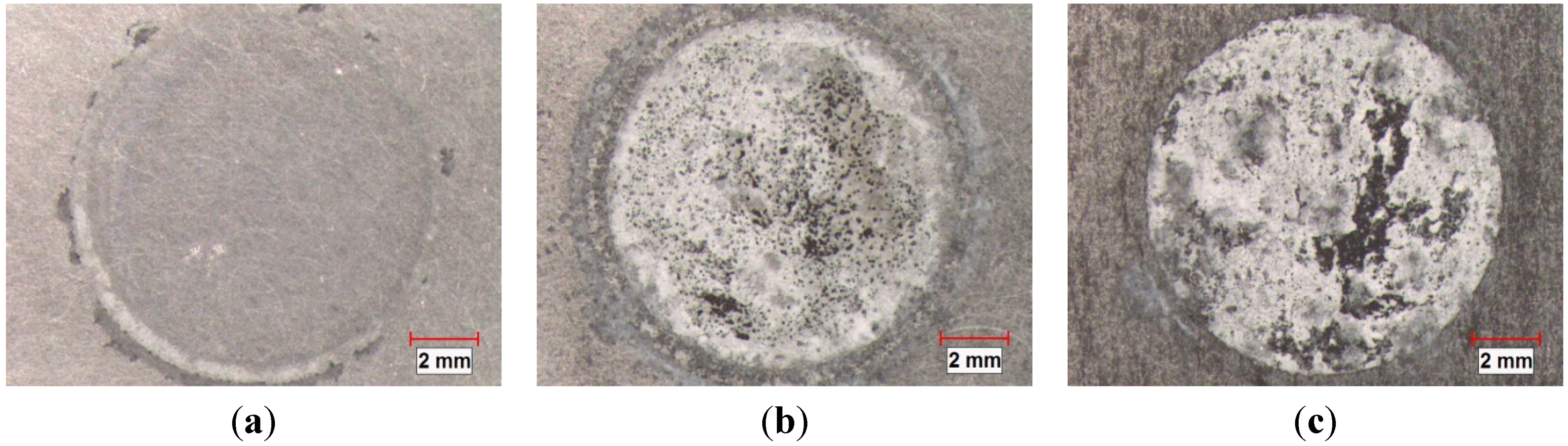
3.4. Galvanic Current Measurement
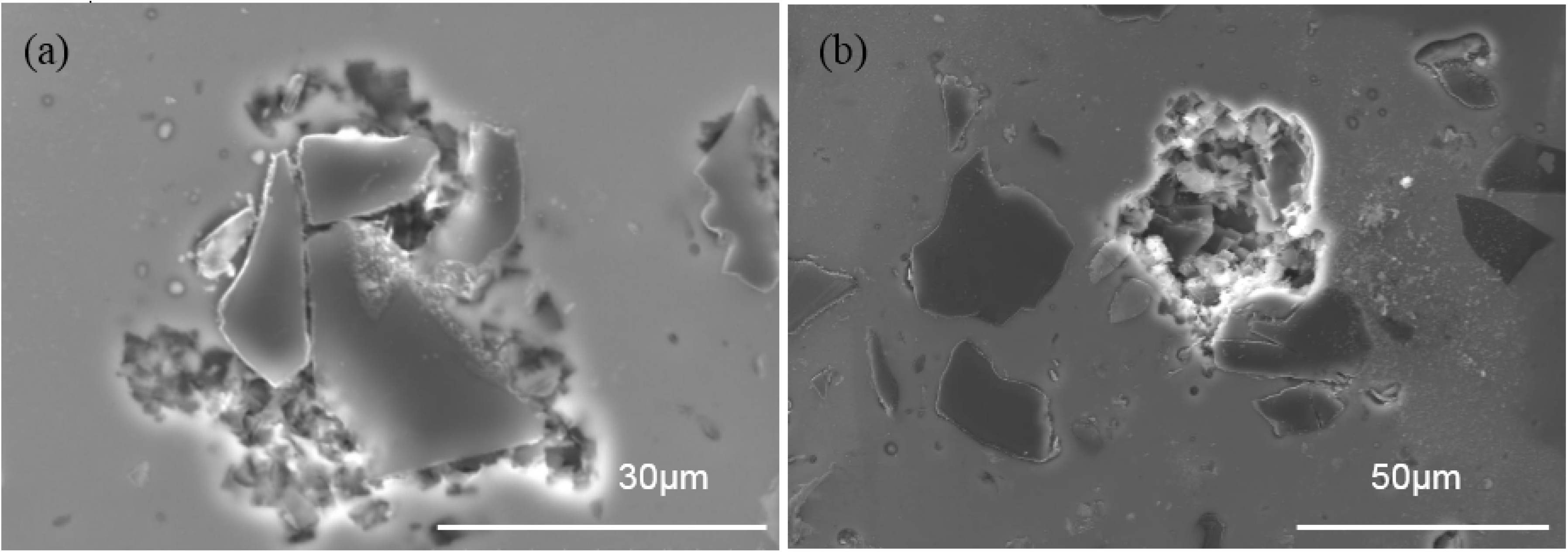
3.5. Pitting Morphology
4. Conclusions
- (1)
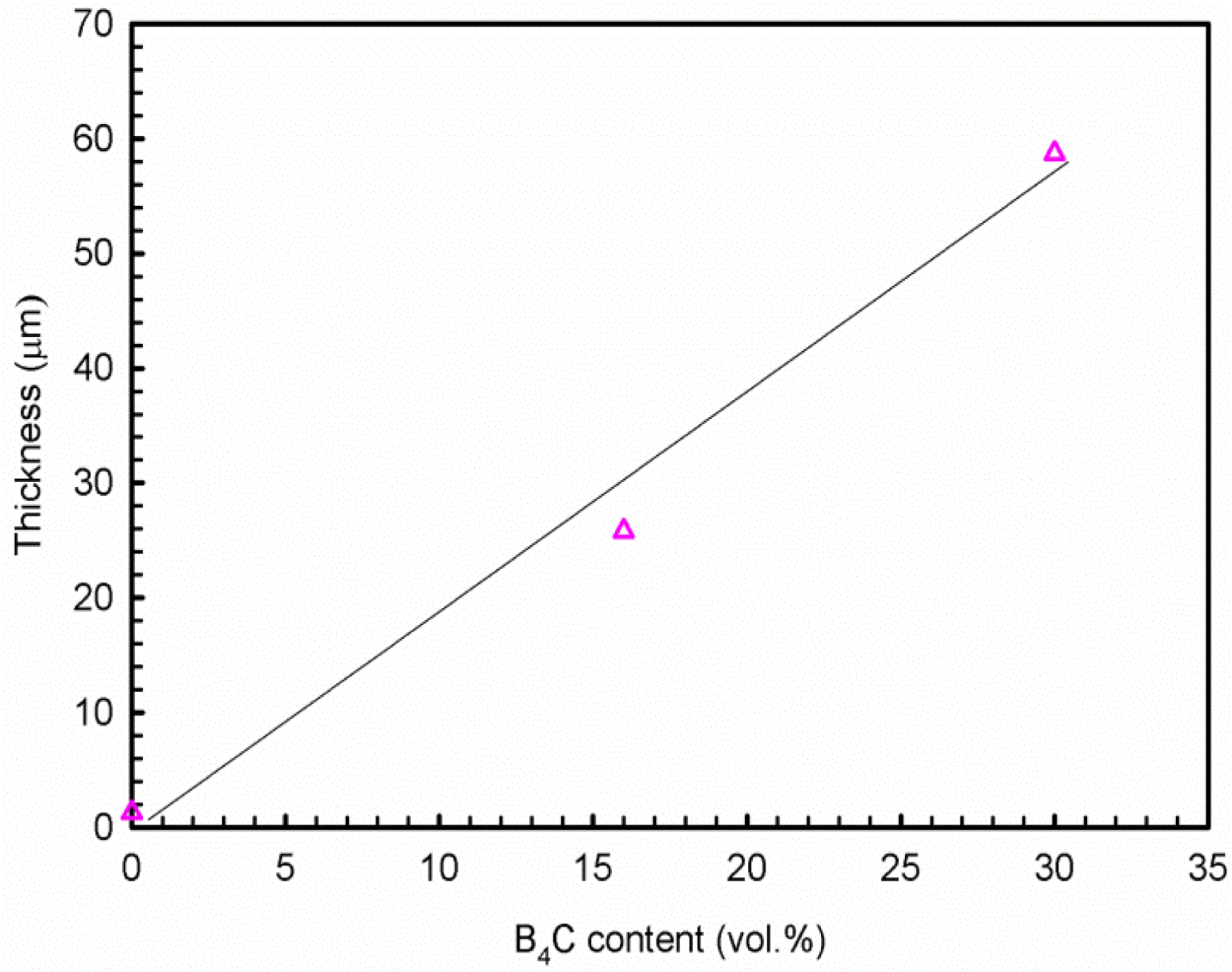
- The polarization and impedance results show that the Al-B4C composites are less corrosion-resistant than the base Al alloy and that the corrosion resistance of the composites decreases when increasing the B4C particle volume fraction. The cross-sectional images demonstrate that the thickness of corrosion products increases linearly with the B4C volume fraction.
- (2)
- Al-B4C composites are susceptible to pitting corrosion in the NaCl solution. Two types of pits are observed on the composite surface after polarization in the NaCl solution: (1) pits with an irregular shape that are preferentially initiated at Al/B4C interfaces and (2) hemispheric pits that initiate in the Al matrix where the intermetallic particles appeared.
- (3)
- The corrosion of Al-B4C composites in 3.5 wt.% NaCl solution is mainly controlled by oxygen reduction in the solution. Moreover, the galvanic couples formed between B4C particles and Al matrix is also responsible for the low corrosion resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pardo, A.; Merino, M.C.; Merino, S.; Viejo, F.; Carboneras, M.; Arrabal, R. Influence of reinforcement proportion and matrix composition on pitting corrosion behaviour of cast aluminium matrix composites (A3xx.x/SiCp). Corros. Sci. 2005, 47, 1750–1764. [Google Scholar] [CrossRef]
- Miracle, D.B. Metal matrix composites—From science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Chen, X.-G. Application of Al-B4C Metal Matrix Composites in the Nuclear Industry for Neutron Absorber Materials. In Proceedings of the Symposium on Solidification Processing of Metal Matrix Composites, San Antonio, TX, USA, 12–26 March 2006.
- Mohanty, R.M.; Balasubramanian, K.; Seshadri, S.K. Boron carbide-reinforced alumnium 1100 matrix composites: Fabrication and properties. Mater. Sci. Eng. A 2008, 498, 42–52. [Google Scholar] [CrossRef]
- Hemanth, J. Tribological behavior of cryogenically treated B4Cp/Al-12% Si composites. Wear 2005, 258, 1732–1744. [Google Scholar] [CrossRef]
- Thévenot, F. Boron carbide—A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Lillo, T.M. Enhancing ductility of AL6061 + 10 wt.% B4C through equal-channel angular extrusion processing. Mater. Sci. Eng. A 2005, 410–411, 443–446. [Google Scholar]
- Abenojar, J.; Velasco, F.; Martínez, M.A. Optimization of processing parameters for the Al + 10% B4C system obtained by mechanical alloying. J. Mater. Process. Technol. 2007, 184, 441–446. [Google Scholar] [CrossRef]
- Kennedy, A.R. The microstructure and mechanical properties of Al-Si-B4C metal matrix composites. J. Mater. Sci. 2002, 37, 317–323. [Google Scholar] [CrossRef]
- Canakci, A.; Arslan, F.; Varol, T. Effect of volume fraction and size of B4C particles on production and microstructure properties of B4C reinforced aluminium alloy composites. Mater. Sci. Technol. 2013, 29, 954–960. [Google Scholar] [CrossRef]
- Abenojar, J.; Martinez, M.A.; Velasco, F. Effect of the boron content in the aluminium/boron composite. J. Alloys Compd. 2006, 422, 67–72. [Google Scholar] [CrossRef]
- Chen, X.-G.; Hark, R. Development of Al-30%B4C Metal Matrix Composites for Neutron Absorber Material. In Proceedings of the Aluminium Alloys: Fabrication, Characerization and Applications, New Orleans, LA, USA, 13–19 March 2008.
- Bonnet, G.; Rohr, V.; Chen, X.G.; Bernier, J.L.; Chiocca, R.; Issard, H. Use of alcan’s Al-B4C metal matrix composites as neutron absorber material in TN international’s transportation and storage casks. Packag. Transp. Storage Secur. Radioact. Mater. 2009, 20, 98–102. [Google Scholar] [CrossRef]
- Han, Y.-M.; Gallant, D.; Chen, X.G. Galvanic corrosion associated with Al-B4C composites/SS304 and Al-B4C composites/AA6061 couples in NaCl and H3BO3 solutions. Electrochim. Acta 2013, 94, 134–142. [Google Scholar] [CrossRef]
- Han, Y.-M.; Gallant, D.; Chen, X.G. Corrosion inhibition of Al-B4C metal matrix composites in a NaCl solution by benzotriazole. Mater. Chem. Phys. 2013, 139, 187–195. [Google Scholar] [CrossRef]
- Abenojar, J.; Bautista, A.; Guzmán, S.; Velasco, F. Microstructural influence on corrosion properties of aluminium composites reinforced with amorphous iron borides. Mater. Corros. 2014, 65, 678–684. [Google Scholar] [CrossRef]
- Abenojar, J.; Bautista, A.; Guzmán, S.; Velasco, F.; Martinez, M.A. Study through potentiodynamic techniques of the corrosion resistance of different aluminium base MMC’s with boron additions. Mater. Sci. Forum 2010, 660–661, 203–208. [Google Scholar] [CrossRef]
- Reena Kumari, P.D.; Nayak, J.; Shetty, A.N. Corrosion behavior of 6061/Al-15 vol. pct. SiC (p) composite and the base alloy in sodium hydroxide solution. Arabian J. Chem. 2011. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Trowsdale, A.J.; Noble, B.; Harris, S.J.; Gibbins, I.S.R.; Thompson, G.E.; Wood, G.C. The influence of silicon carbide reinforcement on the pitting behaviour of aluminium. Corros. Sci. 1996, 38, 177–191. [Google Scholar] [CrossRef]
- Aziz, I.; Qi, Z.; Min, X. Corrosion inhibition of SiCp/5A06 aluminum metal matrix composite by cerium conversion treatment. Chin. J. Aeronaut. 2009, 22, 670–676. [Google Scholar] [CrossRef]
- Singh, I.B.; Mandal, D.P.; Singh, M.; Das, S. Influence of SiC particles addition on the corrosion behavior of 2014 Al-Cu alloy in 3.5% NaCl solution. Corros. Sci. 2009, 51, 234–241. [Google Scholar] [CrossRef]
- Zhu, J.; Hihara, L.H. Corrosion of continuous alumina-fibre reinforced Al-2 wt.% Cu-T6 metal-matrix composite in 3.15 wt.% NaCl solution. Corros. Sci. 2010, 52, 406–415. [Google Scholar] [CrossRef]
- Bhat, M.S.N.; Surappa, M.K.; Nayak, H.V.S. Corrosion behaviour of silicon carbide particle reinforced 6061/Al alloy composites. J. Mater. Sci. 1991, 26, 4991–4996. [Google Scholar] [CrossRef]
- Sun, H.; Koo, E.Y.; Wheat, H.G. Corrosion behavior of SiCp/6061 Al metal matrix composites. Corrosion 1991, 47, 741–753. [Google Scholar] [CrossRef]
- Roepstorff, S.; Maahn, E. Corrosion Resistance of Aluminum-Silicon Carbide Composite Materials. In Proceeding of the 12th Scandinavian Corrosion Congress and Eurocorr’92, Espoo, Finland, 31 May–4 June 1992.
- Ding, H.; Hihara, L. Localized corrosion currents and pH profile over B4C, SiC, and Al2O3 reinforced 6092 aluminum composites: I. in 0.5 M Na2SO4 solution. J. Electrochem. Soc. 2005, 152, B161–B167. [Google Scholar] [CrossRef]
- Katkar, V.A.; Gunasekaran, G.; Rao, A.G.; Koli, P.M. Effect of the reinforced boron carbide particulate content of AA6061 alloy on formation of the passive film in seawater. Corros. Sci. 2011, 53, 2700–2712. [Google Scholar] [CrossRef]
- Han, Y.; Gallant, D.; Chen, X.-G. Corrosion Behavior of Al-B4C Metal Matrix Composites in H3BO3, K2SO4 and NaCl Solutions. In Light Metal; MetSoc: Montreal, QC, Canada, 2011. [Google Scholar]
- Han, Y.; Gallant, D.; Chen, X.-G. Investigation on corrosion behavior of the Al-B4C metal matrix composite in a mildly oxidizing aqueous environment. Corrosion 2011, 67. [Google Scholar] [CrossRef]
- Zhang, Z.; Fortin, K.; Charette, A. Effect of titanium on microstructure and fluidity of Al-B4C composites. J. Mater. Sci. 2011, 46, 3176–3185. [Google Scholar] [CrossRef]
- Viala, J.C.; Bouix, J.; Gonzalez, G.; Esnouf, C. Chemical reactivity of aluminium with boron carbide. J. Mater. Sci. 1997, 32, 4559–4573. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Gamry Instrument. Basics of Electrochemical Impedance Spectroscopy; Gamry Instrument: Warminster, PA, USA, 2007. [Google Scholar]
- Mansfeld, F. An Introduction to Electrochemical Impedance Measurement. In Technique Report; Solartron Ltd.: Shildon, UK, 1999. [Google Scholar]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Kremmer, K.; Lämmel, C.; Sempf, K.; Herrmann, M. Galvanic corrosion of metal/ceramic coupling. Corros. Sci. 2014, 80, 191–196. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Influence of the cathodic intermetallics distribution on the reproducibility of the electrochemical measurements on AA5083 alloy in NaCl solutions. Corros. Sci. 2003, 45, 161–180. [Google Scholar]
- Monticelli, C.; Frignani, A.; Bellosi, A.; Brunoro, G.; Trabanelli, G. The corrosion behaviour of titanium diboride in neutral chloride solution. Corros. Sci. 2001, 43, 979–992. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Alfosail, F.; Gasem, Z. Electrochemical behavior of a discontinuously A6092/SiC/17.5p metal matrix composite in chloride containing solution. Electrochim. Acta 2013, 88, 129–134. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.-M.; Chen, X.-G. Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution. Materials 2015, 8, 6455-6470. https://doi.org/10.3390/ma8095314
Han Y-M, Chen X-G. Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution. Materials. 2015; 8(9):6455-6470. https://doi.org/10.3390/ma8095314
Chicago/Turabian StyleHan, Yu-Mei, and X.-Grant Chen. 2015. "Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution" Materials 8, no. 9: 6455-6470. https://doi.org/10.3390/ma8095314





