Characterization of Human Gingival Fibroblasts on Zirconia Surfaces Containing Niobium Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Characterization of Titanium and Zirconia Discs
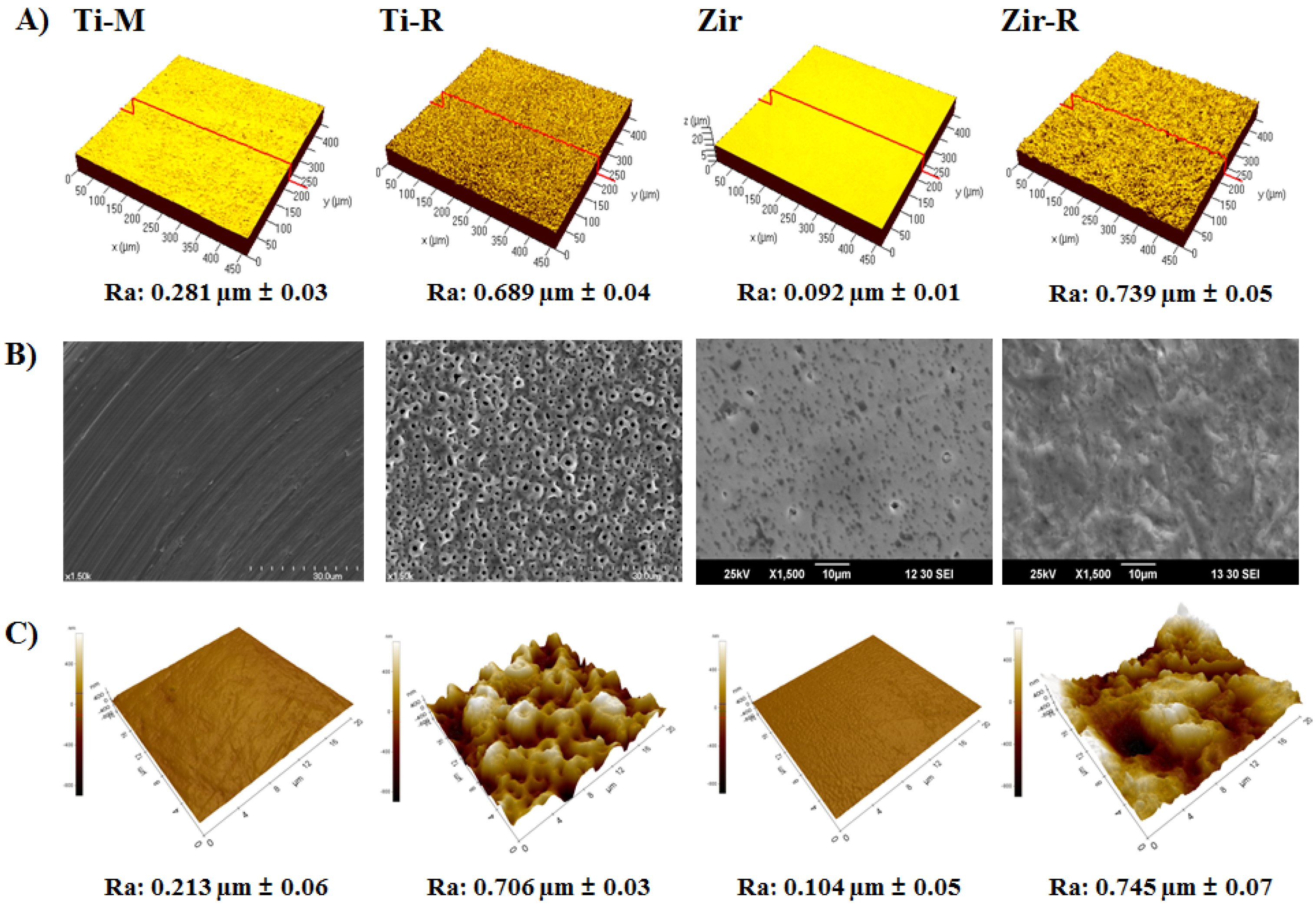
2.2. Cell Attachment and Morphology

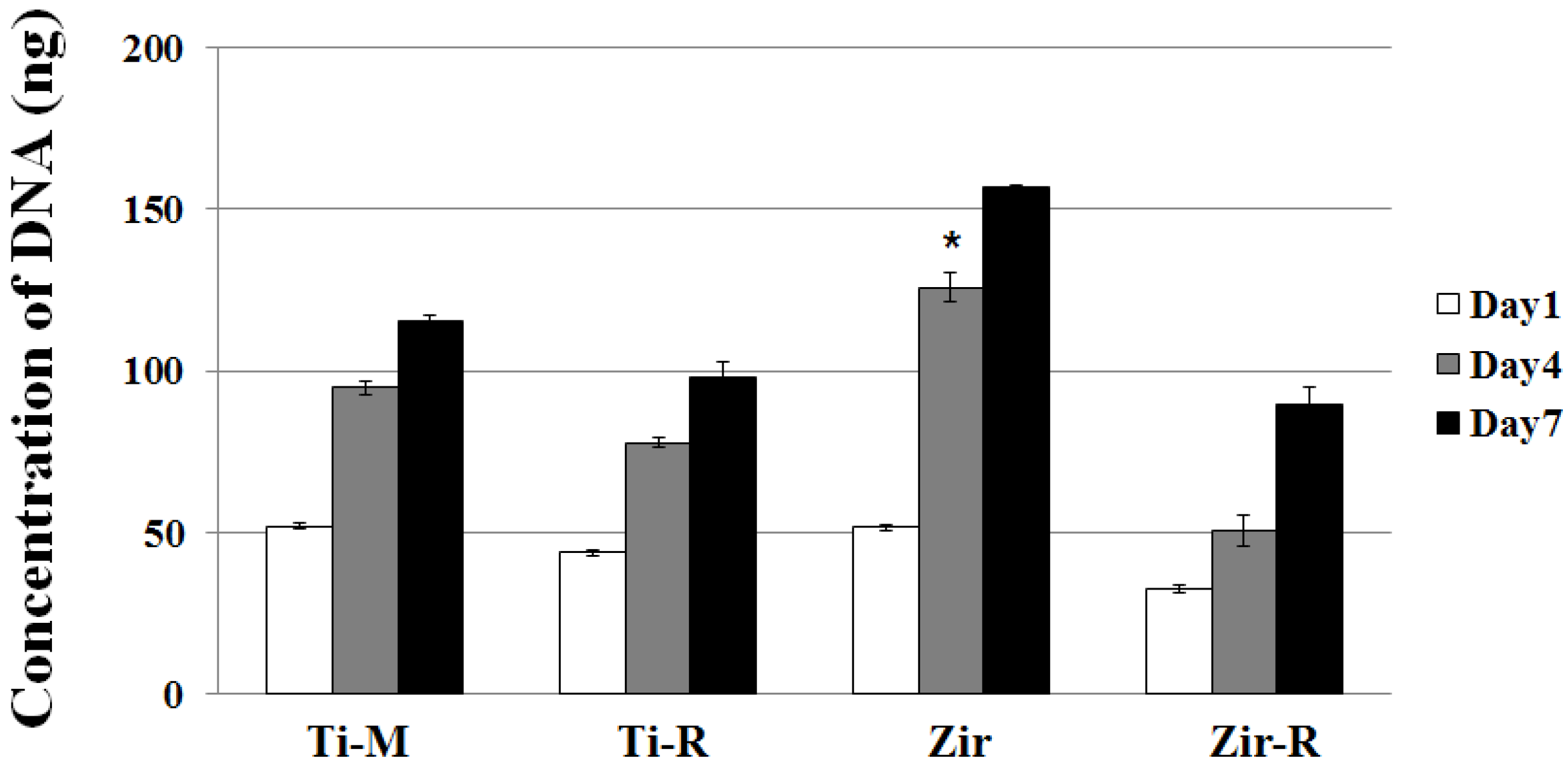
2.3. Cell Proliferation
2.4. Cell Differentiation

3. Materials and Methods
3.1. Specimen Preparation
3.2. Surface Roughness Assessment
3.3. Cell Culture
3.4. Cell Attachment Observation
3.5. Cell Proliferation Assay
3.6. Reverse-Transcription PCR and Quantitative Real-Time PCR
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Zembic, A.; Jung, R.E.; Hämmerle, C.H.; Mattiola, A. Single-tooth implant reconstructions: Esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur. J. Esthet. Dent. 2007, 2, 296–310. [Google Scholar]
- Lughi, V.; Sergo, V. Low temperature degradation-aging-of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Jung, H.J.; Jang, J.W.; Lee, H.L. Fracture toughness, ionic conductivity, and low-temperature phase stability of tetragonal zirconia codoped with yttria and niobium oxide. J. Am. Ceram. Soc. 1998, 81, 2309–2314. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, M.H.; Lee, D.Y.; Han, J.S. Mechanical properties, phase stability, and biocompatibility of (Y,Nb)-TZP/Al2O3 composite abutments for dental implant. J. Biomed. Mater. Res. 2000, 53, 438–443. [Google Scholar] [CrossRef]
- Kim, D.J. Effect of Ta2O5, Nb2O5, and HfO2 alloying on the transformability of Y2O3-stabilized tetragonal ZrO2. J. Am. Ceram. Soc. 1990, 73, 115–120. [Google Scholar] [CrossRef]
- Cho, Y.D.; Shin, J.C.; Kim, H.L.; Gerelmaa, M.; Yoon, H.I.; Ryoo, H.M.; Kim, D.J.; Han, J.S. Comparison of the osteogenic potential of titanium- and modified zirconia-based bioceramics. Int. J. Mol. Sci. 2014, 15, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Hong, J.S.; Ryoo, H.M.; Kim, D.J.; Park, J.H.; Han, J.S. Osteogenic responses to zirconia with hydroxyapatite coating by aerosol deposition. J. Dent. Res. 2015, 94, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Meyle, J.; Nachtigall, W.; Breme, J. Influence of the surface structure of titanium materials on the adhesion of fibroblasts. Biomaterials 1996, 17, 1399–1403. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Heydenrijk, K.; Meijer, H.J.; van der Reijden, W.A.; Raghoebar, G.M.; Vissink, A.; Stegenga, B. Microbiota around root-form endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2002, 17, 829–838. [Google Scholar]
- Bruckmann, C.; Walboomers, X.F.; Matsuzaka, K.; Jansen, J.A. Periodontal ligament and gingival fibroblast adhesion to dentin-like textured surfaces. Biomaterials 2005, 26, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Palaiologou, A.A.; Yukna, R.A.; Moses, R.; Lallier, T.E. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J. Periodontol. 2001, 72, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implant. Res. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Koka, S. The implant-mucosal interface and its role in the long-term success of endosseous oral implants: A review of the literature. Int. J. Prosthodont. 1998, 11, 421–432. [Google Scholar] [PubMed]
- Tetè, S.; Mastrangelo, F.; Bianchi, A.; Zizzari, V.; Scarano, A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int. J. Oral Maxillofac. Implant. 2009, 24, 52–58. [Google Scholar]
- Oates, T.W.; Maller, S.C.; West, J.; Steffensen, B. Human gingival fibroblast integrin subunit expression on titanium implant surfaces. J. Periodontol. 2005, 76, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; de Angelis, E.; Caldara, G.; Corradi, A.; Cacchioli, A.; Gabbi, C. Adaptive response of osteoblasts grown on a titanium surface: Morphology, cell proliferation and stress protein synthesis. Vet. Res. Commun. 2005, 29, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 1998, 19, 2219–2232. [Google Scholar] [CrossRef]
- Anselme, K.; Linez, P.; Bigerelle, M.; Le Maguer, D.; Le Maguer, A.; Hardouin, P.; Hildebrand, H.F.; Iost, A.; Leroy, J.M. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 2000, 21, 1567–1577. [Google Scholar] [CrossRef]
- Cochran, D.L.; Simpson, J.; Weber, H.P.; Buser, D. Attachment and growth of periodontal cells on smooth and rough titanium. Int. J. Oral Maxillofac. Implant. 1994, 9, 289–297. [Google Scholar]
- Bächle, M.; Kohal, R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implant. Res. 2004, 15, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chehroudi, B.T.R.L.; Gould, T.R.L.; Brunette, D.M. Titanium-coated micromachined grooves of different dimensions affect epithelial and connective-tissue cells differently in vivo. J. Biomed. Mater. Res. 1990, 24, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Murakami, H.; Chehroudi, B.; Textor, M.; Brunette, D.M. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 354–365. [Google Scholar]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Odén, A.; Wennerberg, A.; Hultenby, K.; Arvidson, K. The influence of surface topography of ceramic abutments on the attachment and proliferation of human oral fibroblasts. Biomaterials 2005, 26, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Wroblewski, J.; Lopez, B.S.; Wennerberg, A.; Hultenby, K.; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implant. Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Borsani, E.; Salgarello, S.; Mensi, M.; Boninsegna, R.; Stacchiotti, A.; Rezzani, R.; Sapelli, P.; Bianchi, R.; Rodella, L.F. Histochemical and immunohistochemical evaluation of gingival collagen and metalloproteinases in peri-implantitis. Acta Histochem. 2005, 107, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Pivodova, V.; Frankova, J.; Ulrichova, J. Osteoblast and gingival fibroblast markers in dental implant studies. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2011, 155, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Humphries, M.J. Integrin structure. Biochem. Soc. Trans. 2000, 28, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Hormia, M.; Ylänne, J.; Virtanen, I. Expression of integrins in human gingiva. J. Dent. Res. 1990, 69, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Ma, A.K.; Shanti, R.M.; Kim, S.W.; Wada, K.; Sukotjo, C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int. J. Oral Maxillofac. Implant. 2011, 26, 1247–1255. [Google Scholar]
- Riikonen, T.; Westermarck, J.; Koivisto, L.; Broberg, A.; Kähäri, V.M.; Heino, J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1 (I) gene expression. J. Biol. Chem. 1995, 270, 13548–13552. [Google Scholar] [CrossRef] [PubMed]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-D.; Shin, J.-C.; Yoon, H.-I.; Ku, Y.; Ryoo, H.-M.; Kim, D.-J.; Kim, D.-G.; Han, J.-S. Characterization of Human Gingival Fibroblasts on Zirconia Surfaces Containing Niobium Oxide. Materials 2015, 8, 6018-6028. https://doi.org/10.3390/ma8095288
Cho Y-D, Shin J-C, Yoon H-I, Ku Y, Ryoo H-M, Kim D-J, Kim D-G, Han J-S. Characterization of Human Gingival Fibroblasts on Zirconia Surfaces Containing Niobium Oxide. Materials. 2015; 8(9):6018-6028. https://doi.org/10.3390/ma8095288
Chicago/Turabian StyleCho, Young-Dan, Ji-Cheol Shin, Hyung-In Yoon, Young Ku, Hyun-Mo Ryoo, Dae-Joon Kim, Do-Gyoon Kim, and Jung-Suk Han. 2015. "Characterization of Human Gingival Fibroblasts on Zirconia Surfaces Containing Niobium Oxide" Materials 8, no. 9: 6018-6028. https://doi.org/10.3390/ma8095288




