Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterisation of Pt and TCO Free CEs for DSSCs
2.2. Raman Spectroscopy Studies
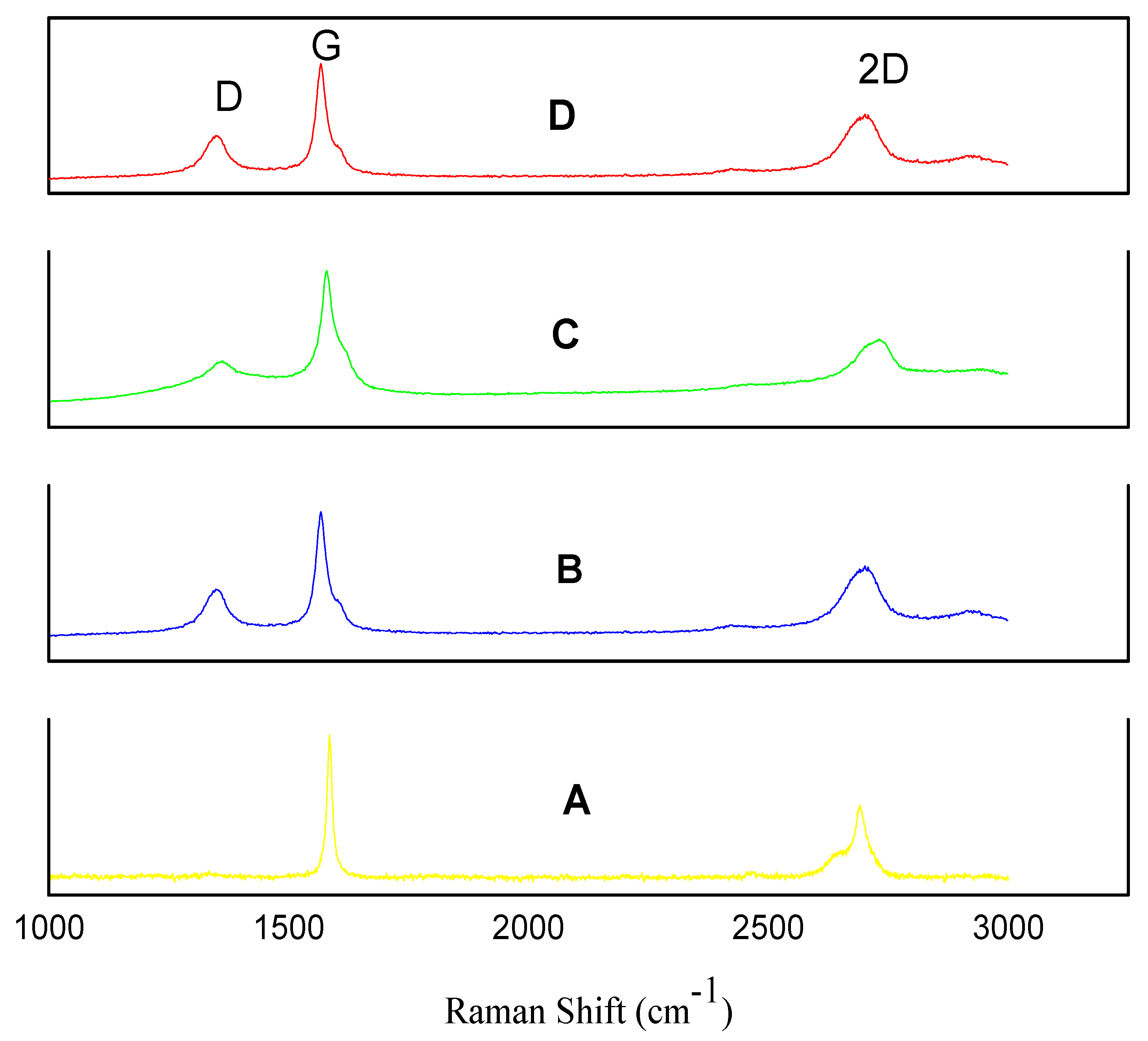
| Powder Type | ID/IG |
|---|---|
| Graphite powder (Reference) | 0.02 |
| GNPs type 1 | 0.40 |
| GNPs type 2 | 0.34 |
| GNPs type 3 | 0.40 |
2.3. Scanning Electron Microscopy (SEM) Studies
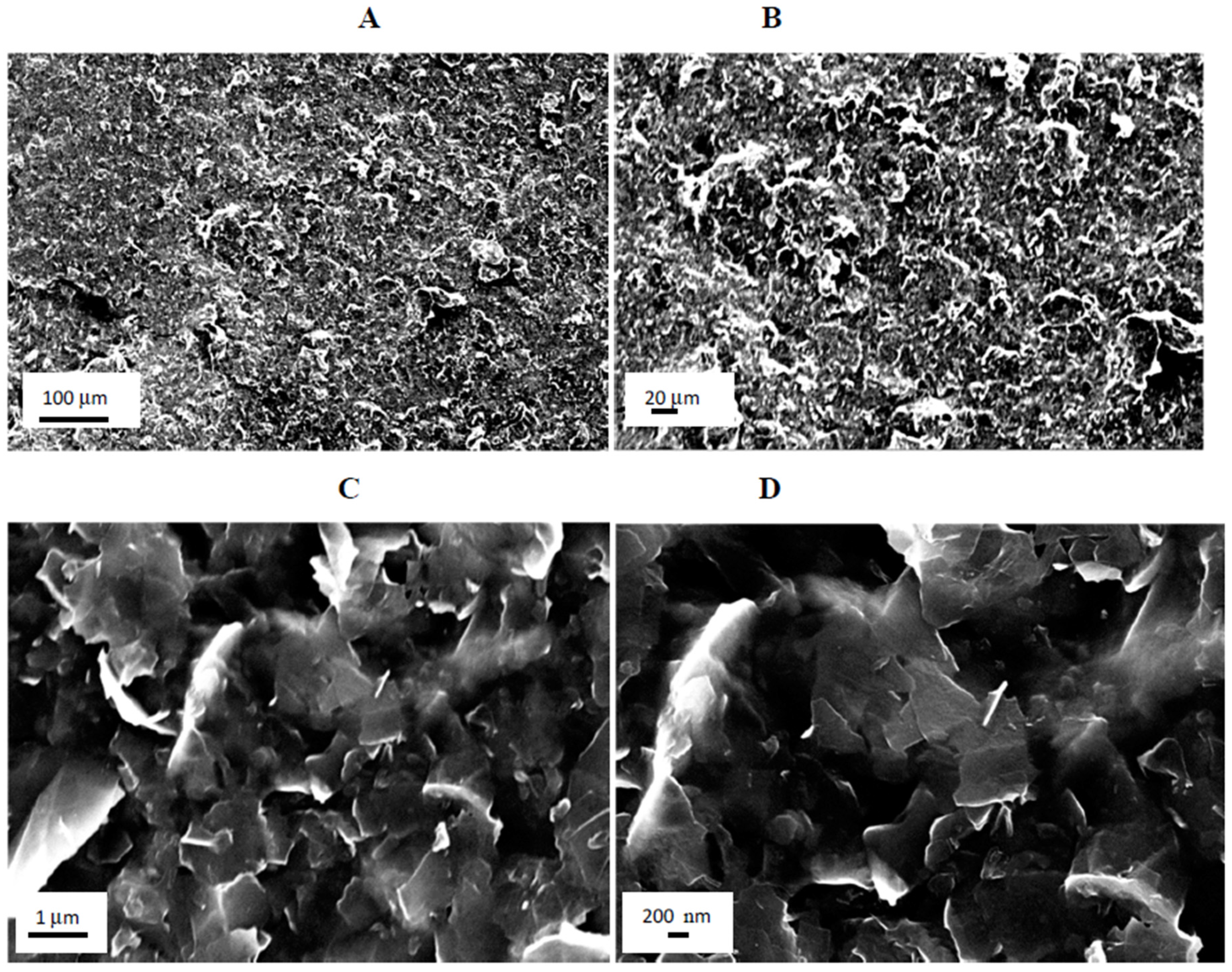
2.4. Thermogravimetric Analysis (TGA) Studies

2.5. Electrical Conductivity Measurement
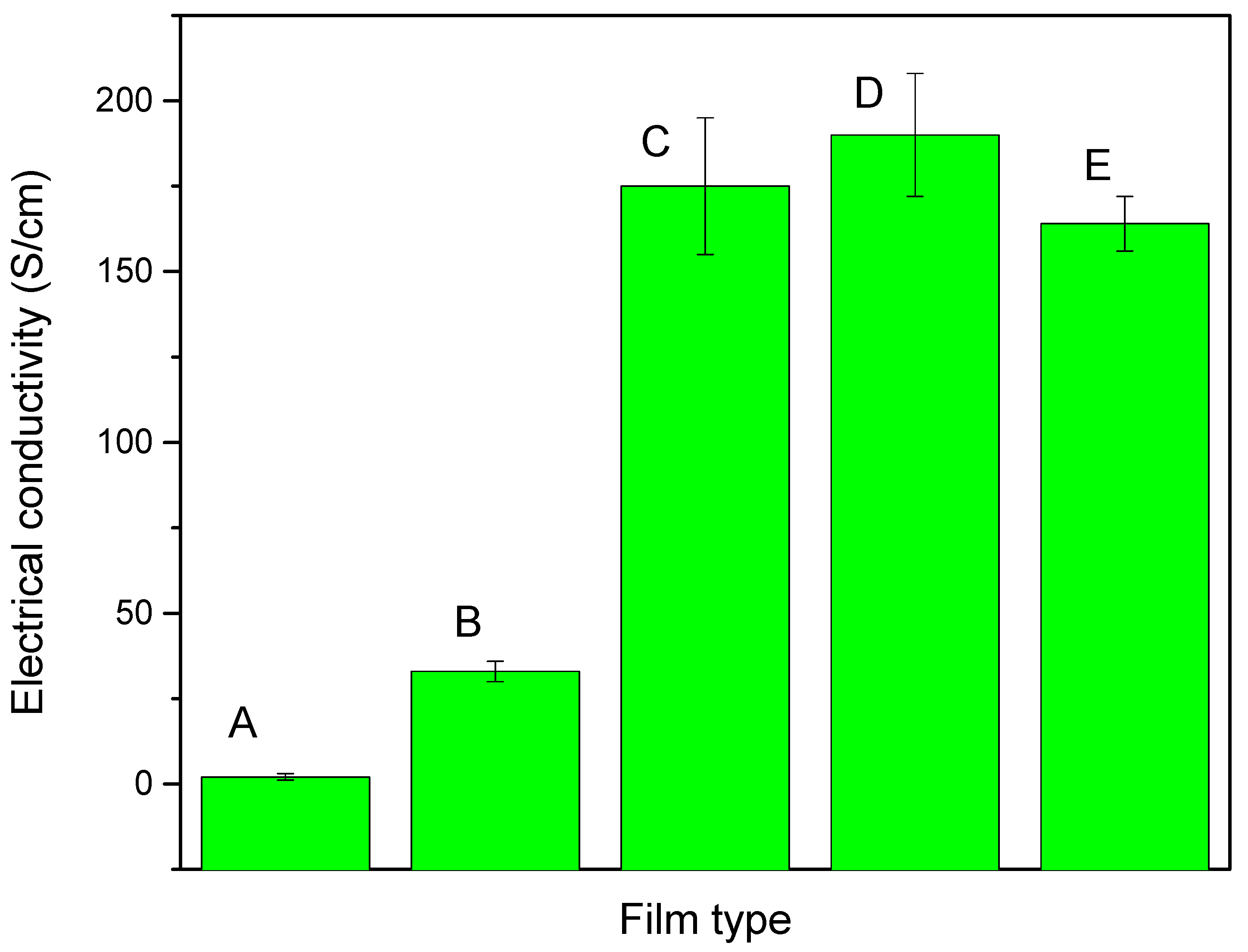
2.6. Investigation of the Photovoltaic Performance of DSSCs
| CE Type | Voc (V) | Jsc (mA/cm2) | FF | Efficiency (%) |
|---|---|---|---|---|
| Pt/FTO/glass | 0.730 | 13.12 | 0.49 | 4.72 ± 0.10 |
| NMP doped PEDOT:PSS/glass/CE | 0.760 | 5.67 | 0.31 | 1.35 ± 0.05 |
| GNPs type 1 + NMP doped PEDOT:PSS/glass | 0.710 | 11.4 | 0.41 | 3.36 ± 0.06 |
| GNPs type 2+NMP doped PEDOT:PSS/glass | 0.690 | 12.45 | 0.43 | 3.70 ± 0.10 |
| GNPs type 3 + NMP doped PEDOT:PSS/glass | 0.690 | 12.80 | 0.31 | 2.78 ± 0.08 |
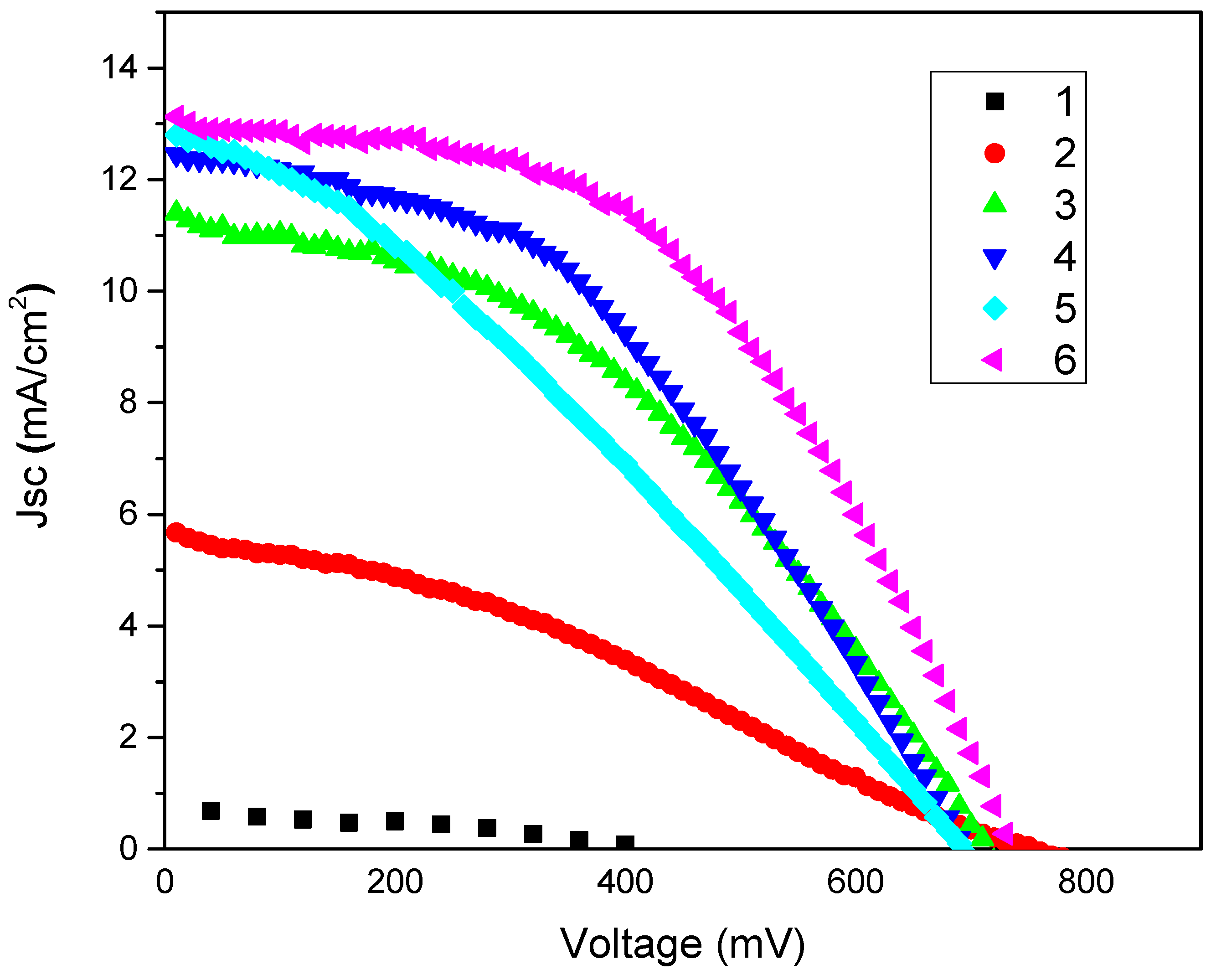
2.7. Investigation of Sintering on the Properties of the CEs and Performance of the Corresponding DSSCs
2.7.1. Effect of Sintering on the Electrical Conductivity of GNPs Type 2 with NMP Doped PEDOT:PSS Films/CEs
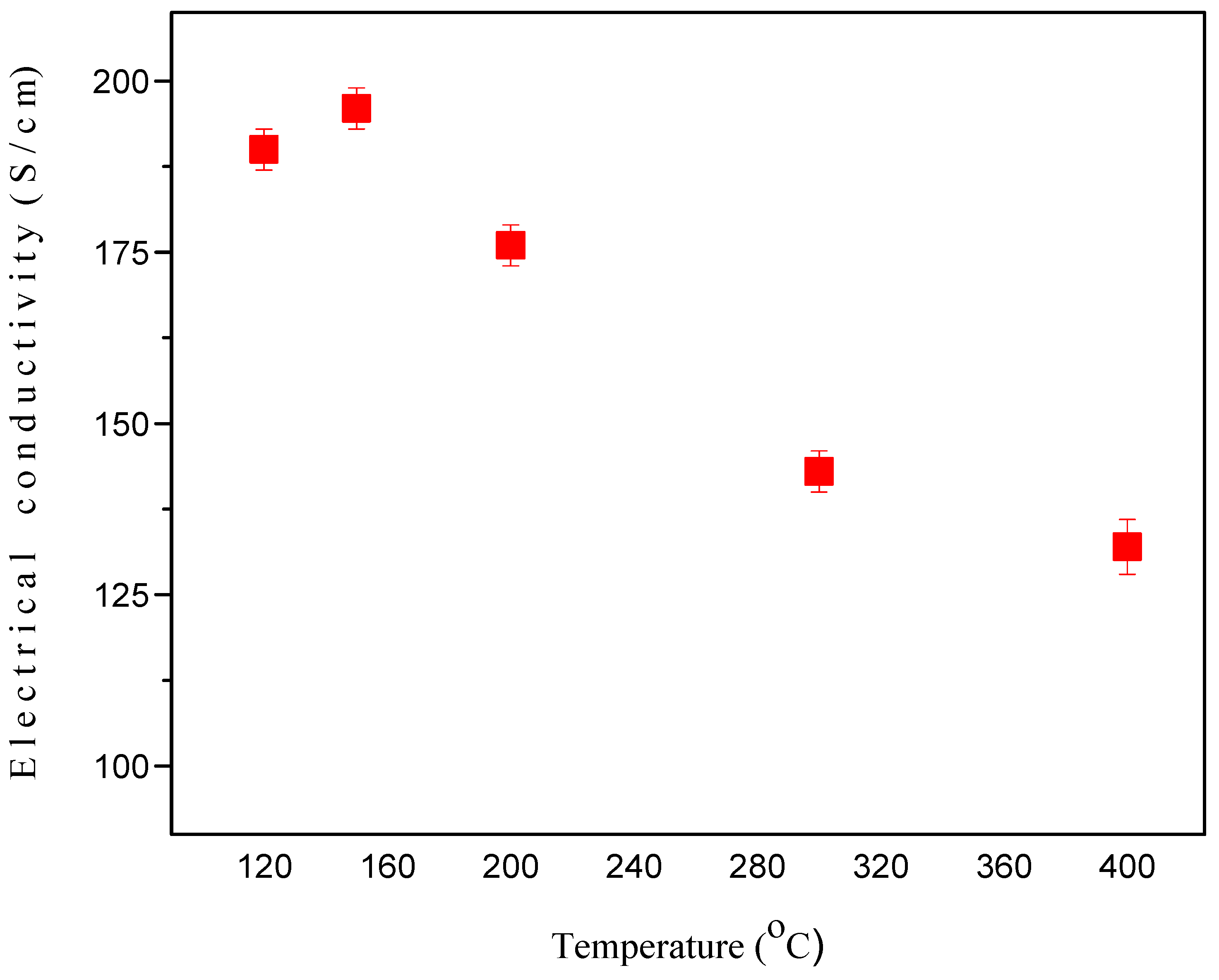
2.7.2. Raman Spectroscopy of GNPs Type 2 with NMP Doped PEDOT:PSS Films/CEs Sintered at Various Temperatures
2.7.3. Effect of Sintering Temperature on the Photovoltaic Performance of DSSCs with GNPs Type 2 with PEDOT:PSS Composite Films/CEs

| Sintering Temperature | ID/IG |
|---|---|
| GNPs 2 type powder | 0.34 |
| 120 °C | 0.13 |
| 150 °C | 0.13 |
| 200 °C | 0.13 |
| 300 °C | 0.02 |
| 400 °C | 0.06 |
| CE Type | Sintering Temp. (°C) | Voc (V) | Jsc (mA/cm) | FF | Efficiency (%) |
|---|---|---|---|---|---|
| GNPs 2 + NMP doped PEDOT:PSS/CE | 120 | 0.690 | 12.45 | 0.43 | 3.70 ± 0.10 |
| As above | 150 | 0.710 | 13.75 | 0.44 | 4.29 ± 0.12 |
| As above | 200 | 0.710 | 11.28 | 0.40 | 3.23 ± 0.09 |
| As above | 300 | 0.720 | 7.66 | 0.24 | 1.35 ± 0.08 |
| As above | 400 | 0.720 | 6.03 | 0.18 | 0.84 ± 0.05 |

3. Experimental Section
3.1. General Procedures
3.2. Preparation of Pt and TCO Free CEs for DSSCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Koerber, C.; Wachau, A.; Saeuberlich, F.; Gassenbauer, Y.; Harvey, S.P.; Proffit, D.E.; Mason, T.O. Transparent conducting oxides for photovoltaics: Manipulation of fermi level, work function and energy band alignment. Materials 2010, 3, 4892–4914. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Ko, J.; Im, S.S. Spherical polypyrrole nanoparticles as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 8146–8151. [Google Scholar] [CrossRef]
- Smestad, G.; Bignozzi, C.; Argazzi, R. Testing of dye sensitized TiO2 solar cells i: Experimental photocurrent output and conversion efficiencies. Sol. Energy Mater. Sol. Cells 1994, 32, 259–272. [Google Scholar] [CrossRef]
- Liu, C.T.; Wang, Y.C.; Dong, R.X.; Wang, C.C.; Huang, K.C.; Vittal, R.; Ho, K.C.; Lin, J.J. A dual-functional Pt/CNT TCO-free counter electrode for dye-sensitized solar cell. J. Mater. Chem. 2012, 22, 25311–25315. [Google Scholar] [CrossRef]
- Kwon, J.; Ganapathy, V.; Kim, Y.H.; Song, K.D.; Park, H.G.; Jun, Y.; Yoo, P.J.; Park, J.H. Nanopatterned conductive polymer films as a Pt, tco-free counter electrode for low-cost dye-sensitized solar cells. Nanoscale 2013, 5, 7838–7843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Li, X.D.; Li, H.B.; Chen, S.; Sun, Z.; Yin, X.J.; Huang, S.M. Graphene-based counter electrode for dye-sensitized solar cells. Carbon 2011, 49, 5382–5388. [Google Scholar] [CrossRef]
- Li, P.J.; Wu, J.H.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Li, Q.G. High-performance and low platinum loading Pt/carbon black counter electrode for dye-sensitized solar cells. Sol. Energy 2009, 83, 845–849. [Google Scholar] [CrossRef]
- Cruz, R.; Tanaka, D.A.P.; Mendes, A. Reduced graphene oxide films as transparent counter-electrodes for dye-sensitized solar cells. Sol. Energy 2012, 86, 716–724. [Google Scholar] [CrossRef]
- Guai, G.H.; Song, Q.L.; Guo, C.X.; Lu, Z.S.; Chen, T.; Ng, C.M.; Li, C.M. Graphene-Pt\ITO counter electrode to significantly reduce pt loading and enhance charge transfer for high performance dye-sensitized solar cell. Sol. Energy 2012, 86, 2041–2048. [Google Scholar] [CrossRef]
- Xu, S.J.; Luo, Y.F.; Zhong, W. Investigation of catalytic activity of glassy carbon with controlled crystallinity for counter electrode in dye-sensitized solar cells. Sol. Energy 2011, 85, 2826–2832. [Google Scholar] [CrossRef]
- Murakami, T.N.; Wang, Q.; Nazeeruddin, M.K.; Bessho, T.; Liska, P.; Baker, R.H.; Pascal, C.; Péchy, P.; Graetzel, M. Highly efficient dye-sensitized solar cells based on carbon black counter electrodes. J. Electrochem. Soc. 2006, 153. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, Y.J.; Kim, B.S.; Lee, J.S. Enhancement of the efficiency of dye-sensitized solar cell by utilizing carbon nanotube counter electrode. Scr. Mater. 2010, 62, 148–150. [Google Scholar] [CrossRef]
- Ahmad, I.; McCarthy, J.E.; Bari, M.; Gun’ko, Y.K. Carbon nanomaterial based counter electrodes for dye sensitized solar cells. Sol. Energy 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/pedot–pss composite films as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Anwar, H.; George, A.E.; Hill, I.G. Vertically-aligned carbon nanotube counter electrodes for dye-sensitized solar cells. Sol. Energy 2013, 88, 129–136. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.H. Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8182–8188. [Google Scholar] [CrossRef]
- Brennan, L.J.; Byrne, M.T.; Bari, M.; Gun’ko, Y.K. Carbon nanomaterials for dye-sensitized solar cell applications: A bright future. Adv. Energy Mater. 2011, 1, 472–485. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.; Hwang, S.; Choi, W.; Jeon, M. Dye-sensitized solar cells using graphene-based carbon nano composite as counter electrode. Sol. Energy Mater. Sol. Cells 2011, 95, 323–325. [Google Scholar] [CrossRef]
- Wang, G.; Zhuo, S.; Xing, W. Graphene/polyaniline nanocomposite as counter electrode of dye-sensitized solar cells. Mater. Lett. 2012, 69, 27–29. [Google Scholar] [CrossRef]
- Rong, Y.; Li, X.; Ku, Z.; Liu, G.; Wang, H.; Xu, M.; Liu, L.; Hu, M.; Xiang, P.; Zhou, Z.; et al. Monolithic all-solid-state dye-sensitized solar module based on mesoscopic carbon counter electrodes. Sol. Energy Mater. Sol. Cells 2012, 105, 148–152. [Google Scholar] [CrossRef]
- Lee, S.U.; Choi, W.S.; Hong, B. A comparative study of dye-sensitized solar cells added carbon nanotubes to electrolyte and counter electrodes. Sol. Energy Mater. Sol. Cells 2010, 94, 680–685. [Google Scholar] [CrossRef]
- Lee, W.J.; Ramasamy, E.; Lee, D.Y.; Song, J.S. Performance variation of carbon counter electrode based dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2008, 92, 814–818. [Google Scholar] [CrossRef]
- Imoto, K.; Takahashi, K.; Yamaguchi, T.; Komura, T.; Nakamura, J.; Murata, K. High-performance carbon counter electrode for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2003, 79, 459–469. [Google Scholar] [CrossRef]
- Chang, L.Y.; Li, Y.Y.; Li, C.T.; Lee, C.P.; Fan, M.S.; Vittal, R.; Ho, K.C.; Lin, J.J. A composite catalytic film of Ni-nps/pedot:PSS for the counter electrodes in dye-sensitized solar cells. Electrochimica Acta 2014, 146, 697–705. [Google Scholar] [CrossRef]
- Hsu, S.H.; Li, C.T.; Chien, H.T.; Salunkhe, R.R.; Suzuki, N.; Yamauchi, Y.; Ho, K.C.; Wu, K.C.W. Platinum-free counter electrode comprised of metal-organic-framework (MOF)-derived cobalt sulfide nanoparticles for efficient dye-sensitized solar cells (DSSCs). Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Baek, J.M.; Chen, H.; Qu, J.; Dai, L. N-doped graphene nanoribbons as efficient metal-free counter electrodes for disulfide/thiolate redox mediated dsscs. Nanoscale 2015, 7, 7078–7083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, W.; Ma, Y.; Cao, W.; Wang, C.; Guo, L. Facet-dependent NiS2 polyhedrons on counter electrodes for dye-sensitized solar cells. Chem. Commun. 2015, 51, 12863–12866. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, H.K.; Wang, D.H.; Park, N.G.; Lee, J.Y.; Park, O.O.; Park, J.H. Dye-sensitized solar cells with pt- and tco-free counter electrodes. Chem. Commun. 2010, 46, 4505–4507. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, G.; Bojan, K.; Rhee, S.W. Sub-micrometer-sized graphite as a conducting and catalytic counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2011, 3, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.L.; Chen, H.Y.; Su, C.Y.; Kuang, D.B. A novel TCO- and Pt-free counter electrode for high efficiency dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 1724–1730. [Google Scholar] [CrossRef]
- Yun, D.J.; Jeong, Y.J.; Ra, H.; Kim, J.M.; An, T.K.; Seol, M.; Jang, J.; Park, C.E.; Rhee, S.W.; Chung, D.S. Fabrication of high-performance composite electrodes composed of multiwalled carbon nanotubes and glycerol-doped poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate for use in organic devices. J. Mater. Chem. C 2015, 3, 7325–7335. [Google Scholar] [CrossRef]
- Bi, H.; Chen, J.; Zhao, W.; Sun, S.; Tang, Y.; Lin, T.; Huang, F.; Zhou, X.; Xie, X.; Jiang, M. Highly conductive, free-standing and flexible graphene papers for energy conversion and storage devices. RSC Adv. 2013, 3, 8454–8460. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Gerosa, M.; Turri, S.; Bongiovanni, R. Performance and stability improvements for dye-sensitized solar cells in the presence of luminescent coatings. J. Power Sources 2015, 283, 195–203. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, D.J.; Chi, W.S.; Kim, J.H. Hierarchical double-shell nanostructures of TiO2 nanosheets on SnO2 hollow spheres for high-efficiency, solid-state, dye-sensitized solar cells. Adv. Funct. Mater. 2014, 24, 5037–5044. [Google Scholar] [CrossRef]
- Nair, J.R.; Porcarelli, L.; Bella, F.; Gerbaldi, C. Newly elaborated multipurpose polymer electrolyte encompassing rtils for smart energy-efficient devices. ACS Appl. Mater. Interfaces 2015, 7, 12961–12971. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yuan, C.; Guo, J.; Yan, F. A review of poly(ionic liquid)s based functional materials. Acta Chim. Sin. 2015, 73, 310–315. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (dsscs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Chen, H.W.; Chiang, Y.D.; Kung, C.W.; Sakai, N.; Ikegami, M.; Yamauchi, Y.; Wu, K.C.W.; Miyasaka, T.; Ho, K.C. Highly efficient plastic-based quasi-solid-state dye-sensitized solar cells with light-harvesting mesoporous silica nanoparticles gel-electrolyte. J. Power Sources 2014, 245, 411–417. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Ramachandran, R.; Mani, V.; Chen, S.M.; Kumar, G.P.G.; Gajendran, P.; Devi, N.B.; Devasenathipathy, R. Recent progress in electrode fabrication materials and various insights in solar cells: Review. Int. J. Electrochem. Sci. 2015, 10, 3301–3318. [Google Scholar]
- Wang, H.J.; Chen, C.P.; Jeng, R.J. Polythiophenes comprising conjugated pendants for polymer solar cells: A review. Materials 2014, 7, 2411–2439. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Donor-acceptor block copolymers: Synthesis and solar cell applications. Materials 2014, 7, 3274–3290. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Fréchet, J.M.J. Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight. Adv. Mater. 2003, 15, 1519–1522. [Google Scholar] [CrossRef]
- Lang, U.; Mueller, E.; Naujoks, N.; Dual, J. Microscopical investigations of PEDOT:PSS thin films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant different conductivities of the two grades of poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate), clevios P and clevios PH1000, arising from different molecular weights. ACS Appl. Mater. Interfaces 2012, 4, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.S.; Whang, W.T.; Chen, C.P.; Chen, Y.C. High-conductivity poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) film for use in ito-free polymer solar cells. J. Mater. Chem. 2008, 18, 5948–5955. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-conductivity poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Jonsson, S.K.M.; Birgerson, J.; Crispin, X.; Greczynski, G.; Osikowicz, W.; van der Gon, A.W.D.; Salaneck, W.R.; Fahlman, M. The effects of solvents on the morphology and sheet resistance in poly (3,4-ethylenedioxythiophene)-polystyrenesulfonic acid (pedot-pss) films. Synth. Met. 2003, 139, 1–10. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Vidano, R.P.; Fischbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-concentration solvent exfoliation of graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. Lond. Ser. A 2004, 362, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, T.; Shi, M.; Li, N.; Ye, M. Polyelectrolyte-assisted one-step hydrothermal synthesis of ag-reduced graphene oxide composite and its antibacterial properties. Mater. Sci. Eng. C 2012, 32, 2042–2047. [Google Scholar] [CrossRef]
- Cui, P.; Lee, J.; Hwang, E.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of layer thickness and annealing of PEDOT:PSS layers in organic photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef]
- Kim, J.R.; Jung, J.H.; Shin, W.S.; So, W.W.; Moon, S.J. Efficient tco-free organic solar cells with modified poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) anodes. J. Nanosci. Nanotechnol. 2011, 11, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.H.; Wang, H.W. A freeze-dried graphene counter electrode enhances the performance of dye-sensitized solar cells. Thin Solid Films 2014, 550, 515–520. [Google Scholar] [CrossRef]
- Hung, K.H.; Chan, C.H.; Wang, H.W. Flexible tco-free counter electrode for dye-sensitized solar cells using graphene nanosheets from a Ti-Ti(iii) acid solution. Renew. Energy 2014, 66, 150–158. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; McCarthy, J.E.; Baranov, A.; Gun'ko, Y.K. Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells. Materials 2015, 8, 5953-5973. https://doi.org/10.3390/ma8095284
Ahmad I, McCarthy JE, Baranov A, Gun'ko YK. Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells. Materials. 2015; 8(9):5953-5973. https://doi.org/10.3390/ma8095284
Chicago/Turabian StyleAhmad, Iftikhar, Joseph E. McCarthy, Alexander Baranov, and Yurii K. Gun'ko. 2015. "Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells" Materials 8, no. 9: 5953-5973. https://doi.org/10.3390/ma8095284






