Preparation of LuAG Powders with Single Phase and Good Dispersion for Transparent Ceramics Using Co-Precipitation Method
Abstract
:1. Introduction
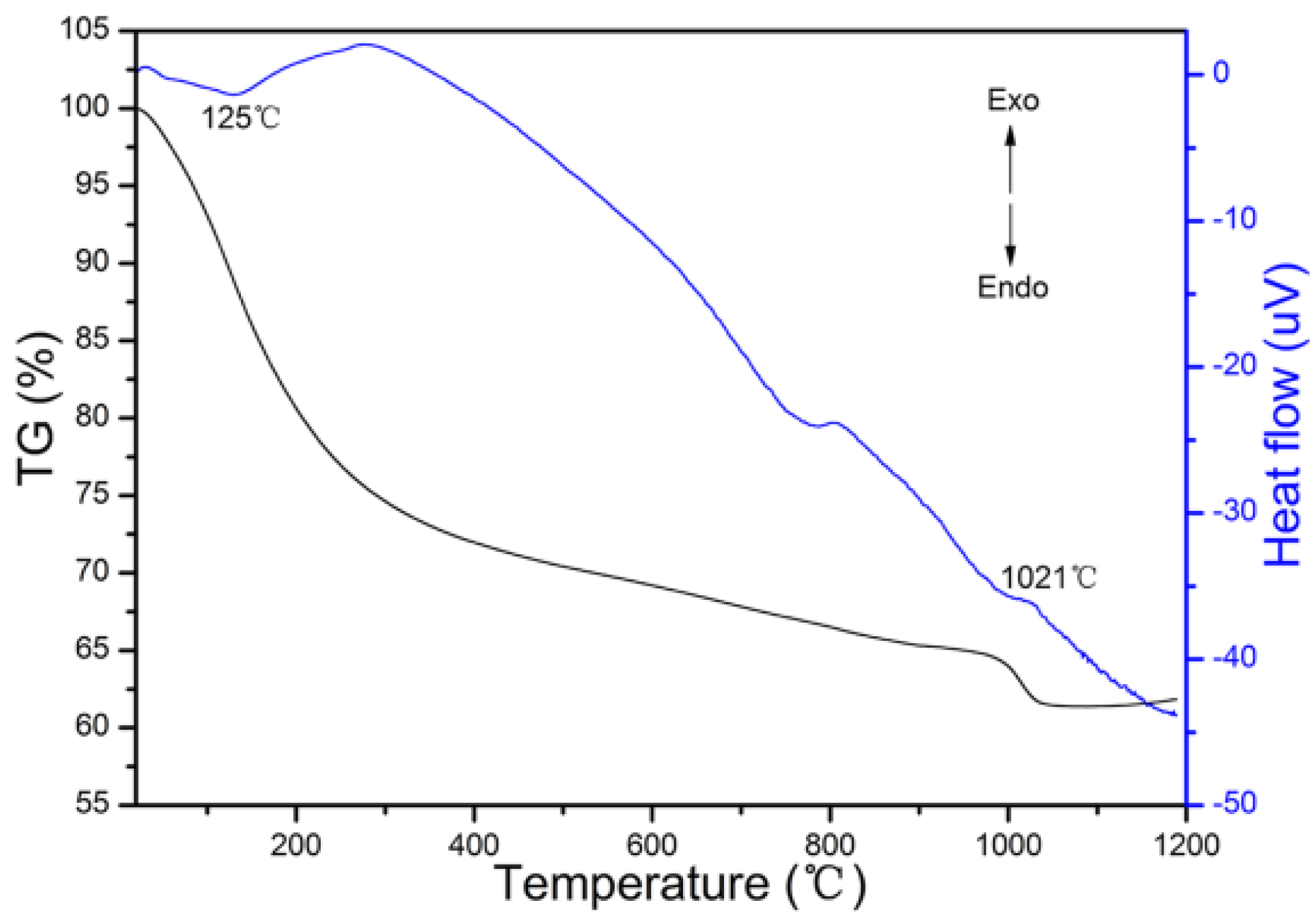
2. Results and Discussion
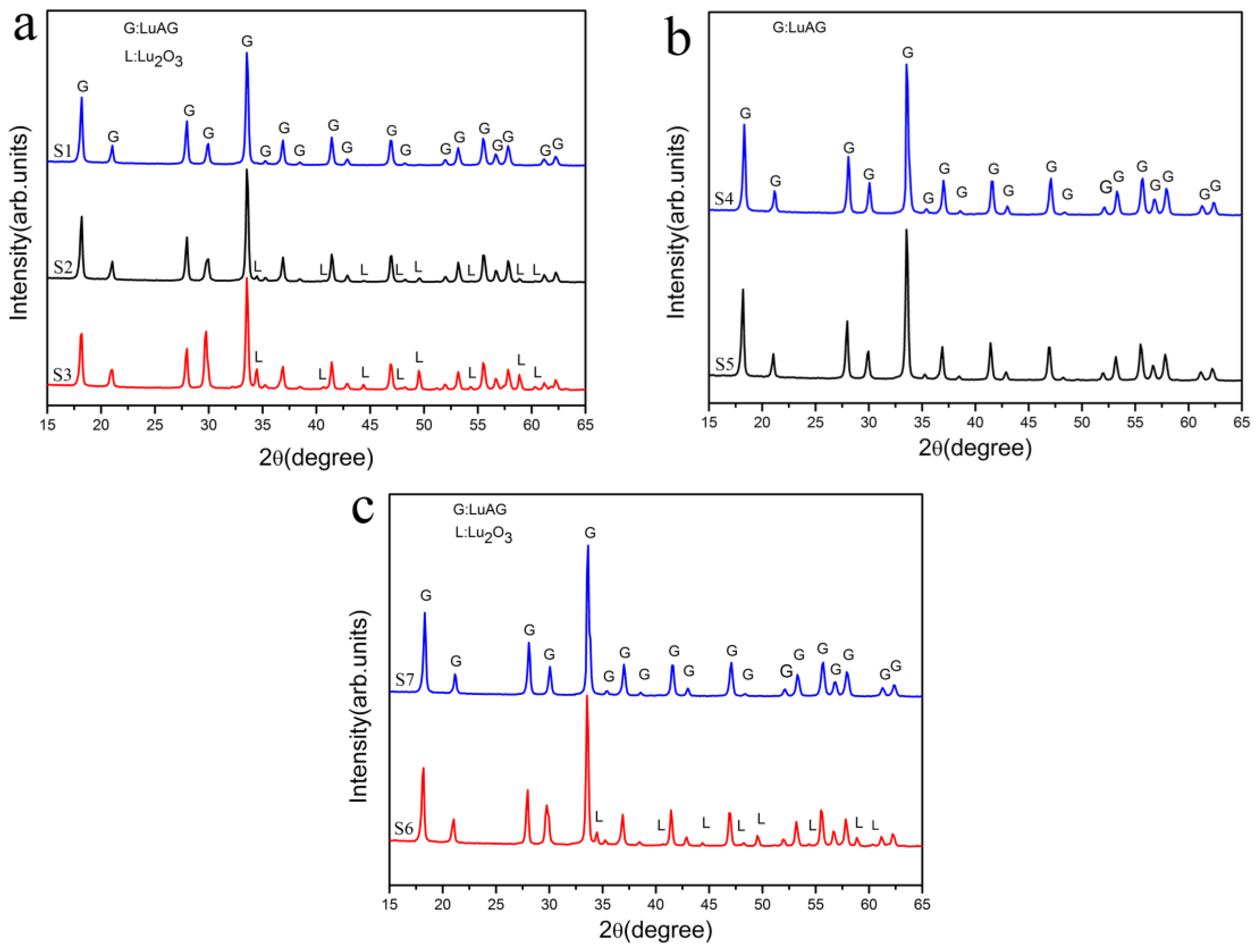
| Samples | Aging Time | Dripping Speed | pH Value of Initial Salt Solution | pH Value of Terminal Solution |
|---|---|---|---|---|
| S1 | 1 h | 3 mL·min−1 | 3.10 | 7.77 |
| S2 | 15 h | 3 mL·min−1 | 3.10 | 7.86 |
| S3 | 24 h | 3 mL·min−1 | 3.10 | 8.04 |
| S4 * | 0.5 h | 3 mL·min−1 | 3.10 | 8.35 |
| S5 | 24 h | 3 mL·min−1 | 1.20 | 7.32 |
| S6 | 1 h | 1.5 mL·min−1 | 3.10 | 7.90 |
| S7 | 15 h | 6 mL·min−1 | 3.10 | 7.61 |
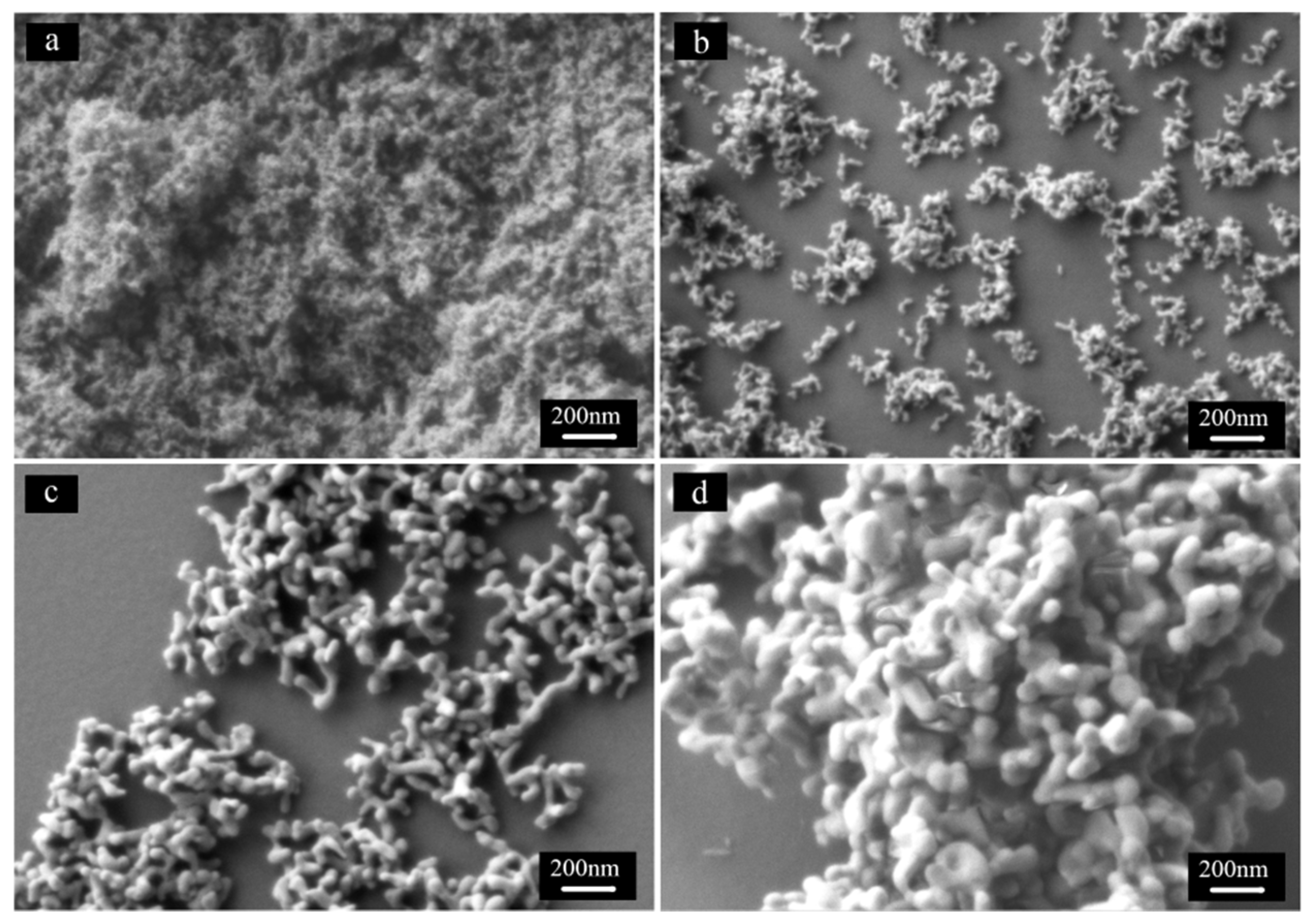
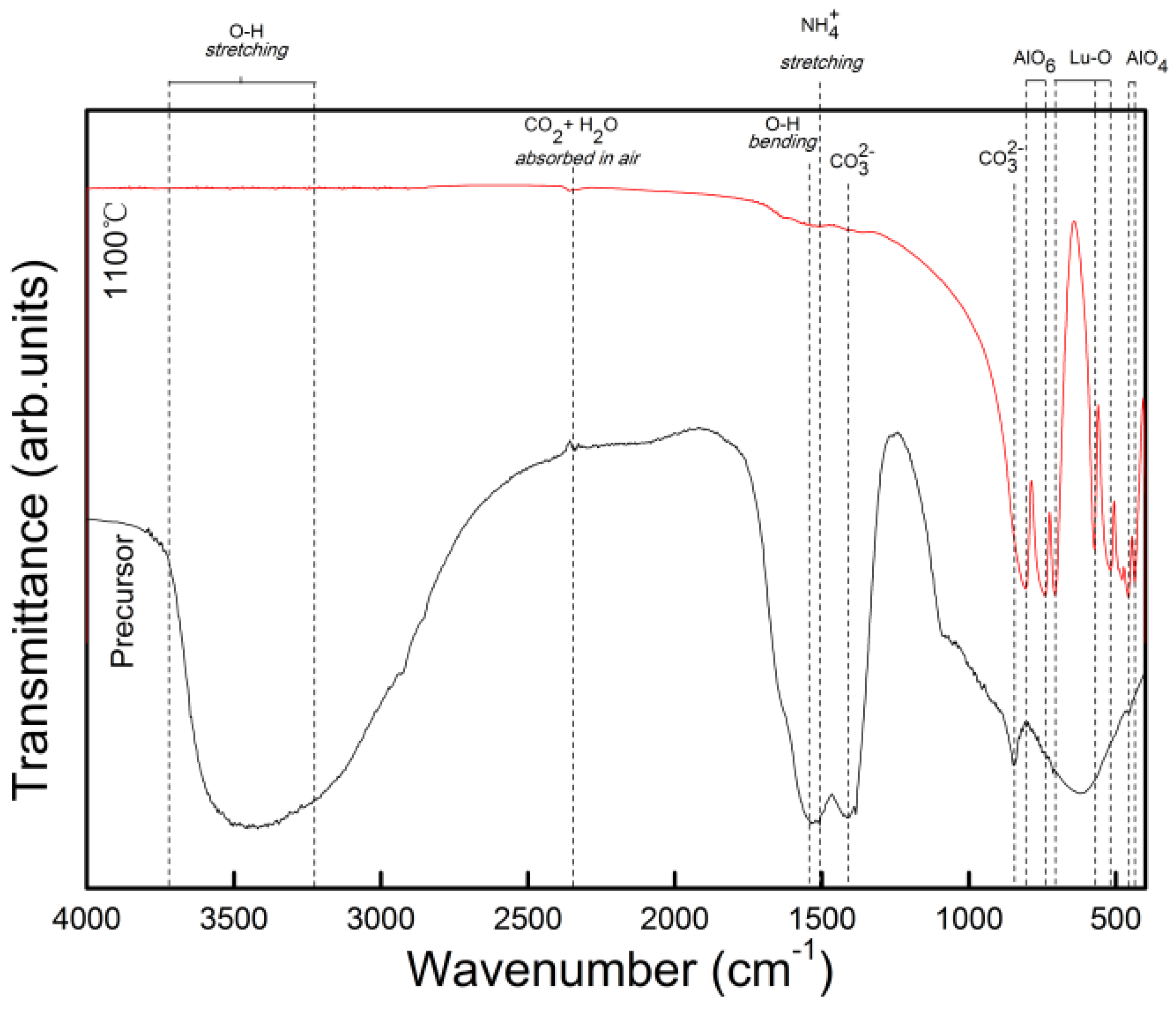
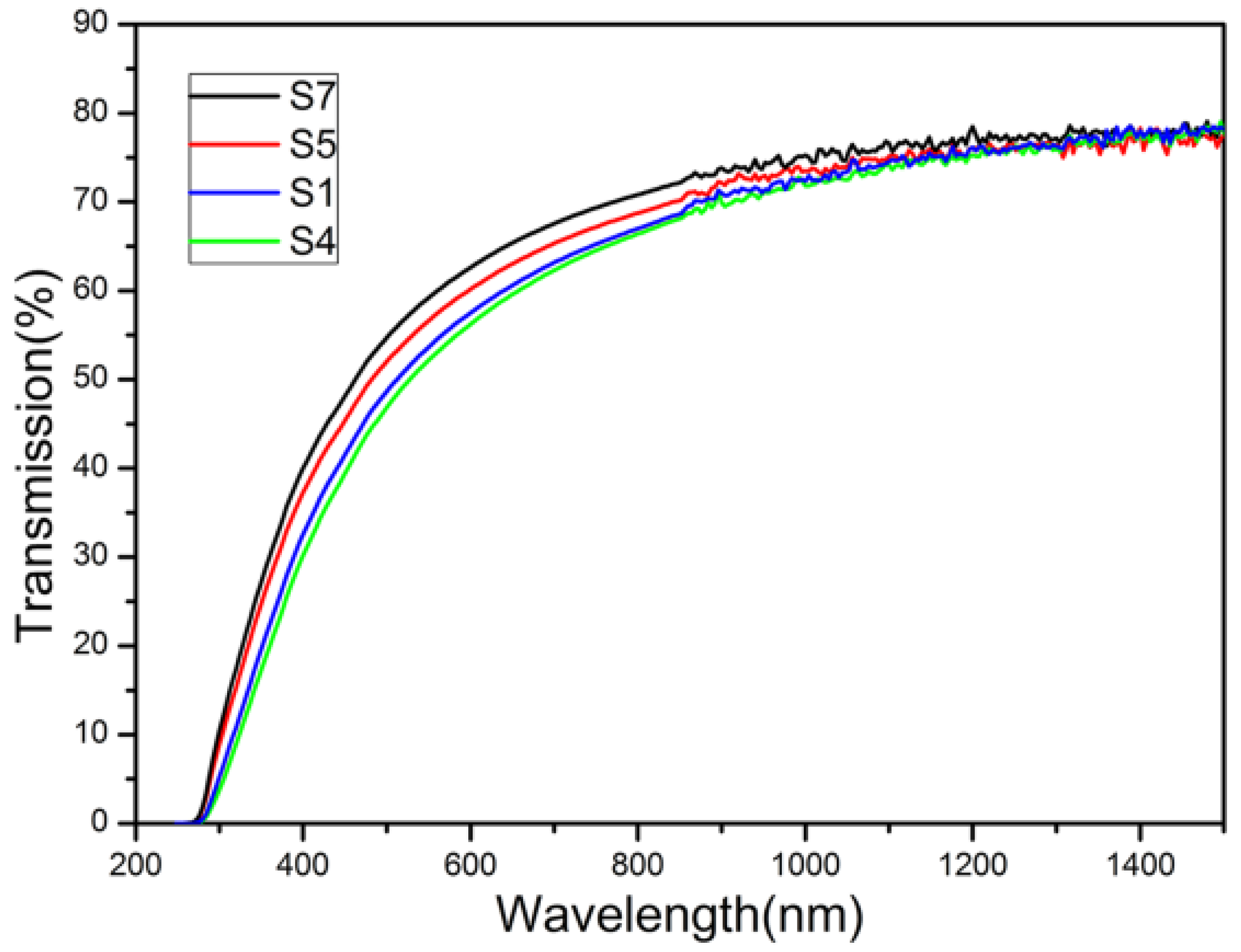
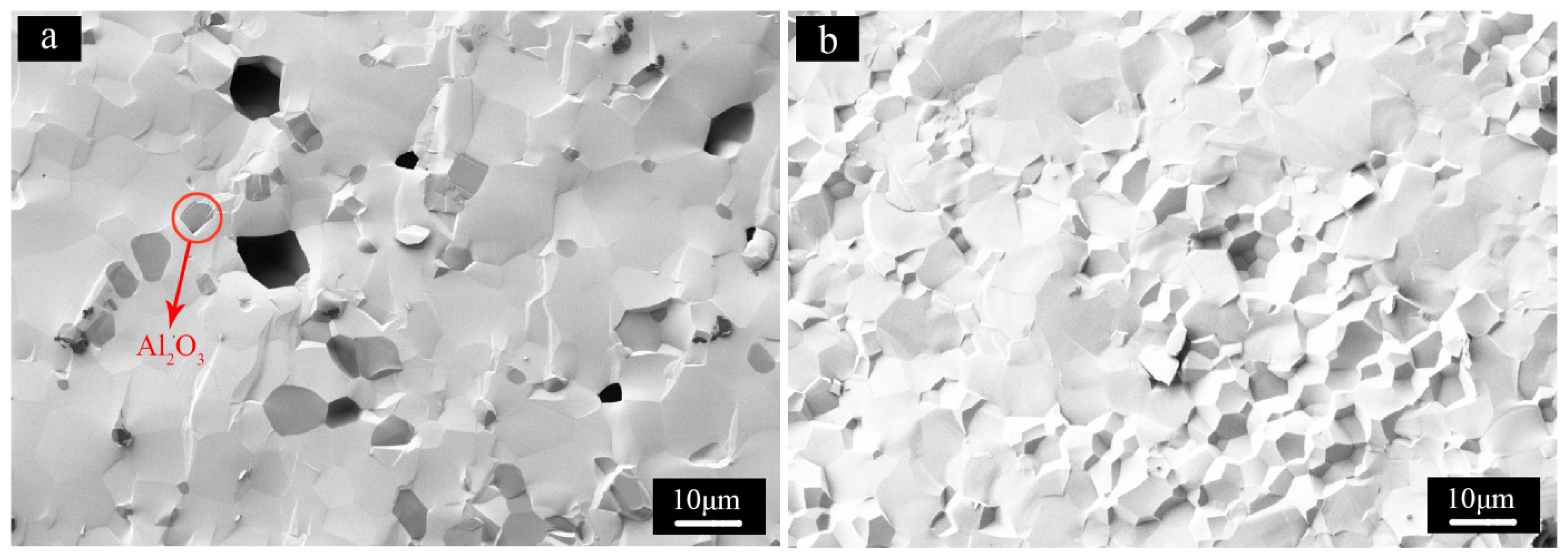
3. Experimental Section
4. Conclusions
- 1
- The Combined effect of pH and time. Higher pH value of terminal solution, longer titration time, and longer aging time resulted in the appearance of rhombus Lu precipitates.
- 2
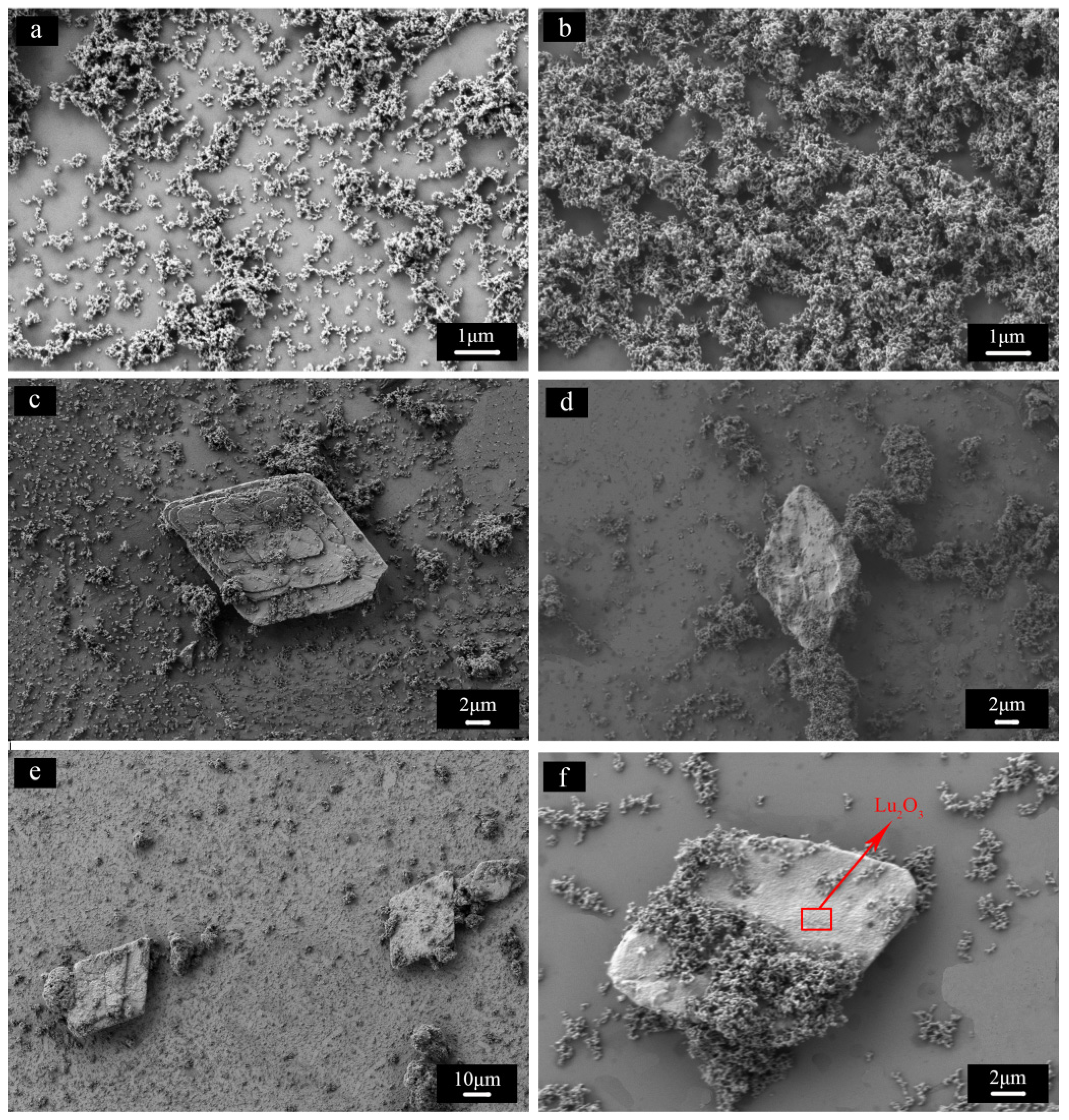
- The evolution of impurities. From the morphology of flake-like Lu2O3, it is obvious that rhombus Lu precipitate transformed into Lu2O3 upon calcinations at 1200 °C for 3 h. It is not easy for big sized rhombus Lu precipitate to react with fine Al precipitate to transform into LuAG phase because of long diffusion distance. As a consequence, there were two kind of impurity phases in the LuAG powder of S3, one was flake-like Lu2O3, the other was fine Al2O3.
- 3
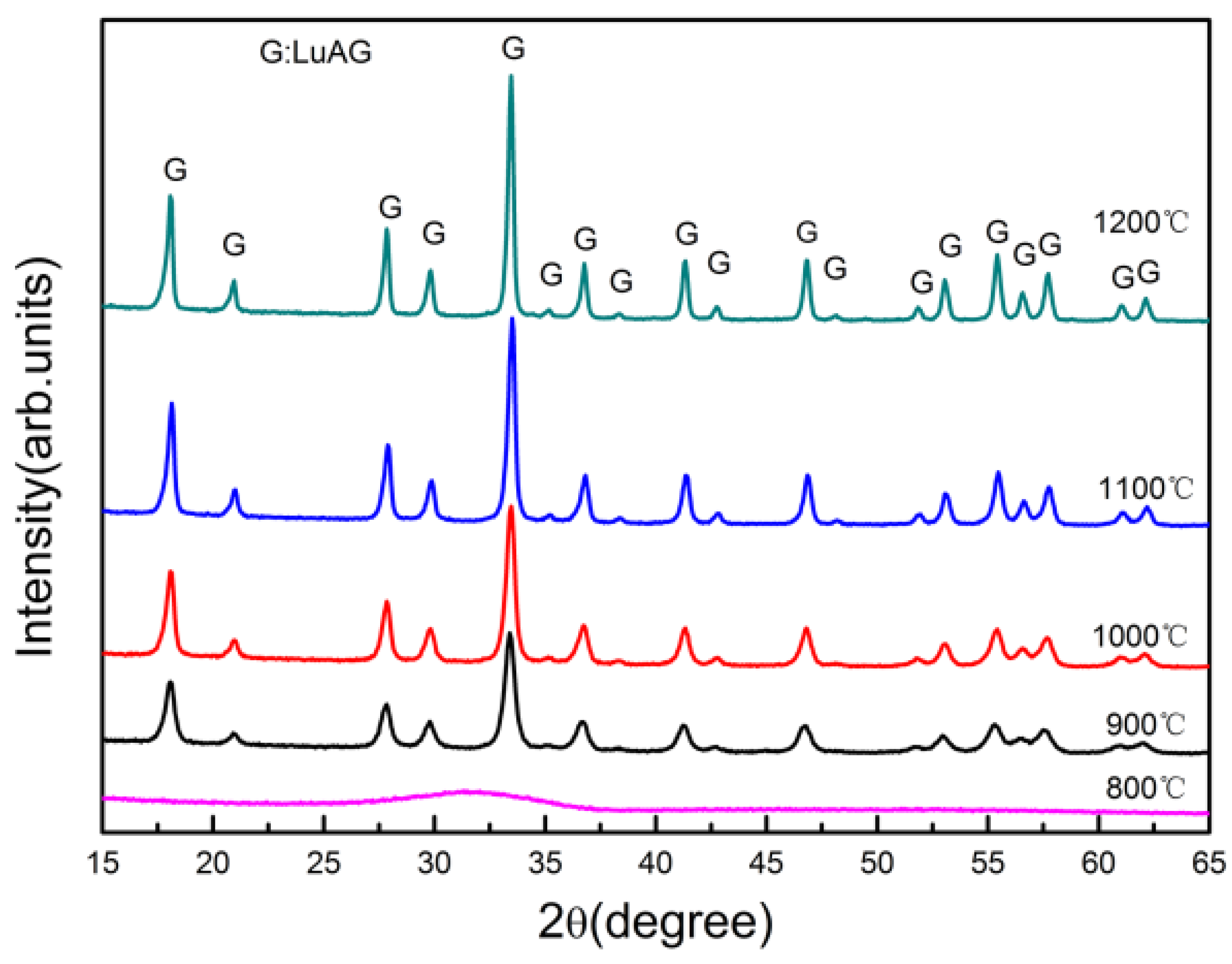
- The dispersity of powder. It is an easy way to obtain well dispersed LuAG powder by calcining at different temperatures. Higher calcination temperature resulted in drastic increase in crystallite size and severed agglomeration with decreasing sinterability. While the powders produced at lower temperature, smaller crystallite size, and high sintering activity caused to abnormal grain growth and intragranular porosity in the sintering process. The excellent dispersion and sintering properties of powder need to further change synthesis conditions, including species and dosage of dispersant, co-precipitation temperature and so on.
- 4
- The transmittance of ceramics. It is true that our samples were not as transparent as solid-state reactive sintered LuAG laser ceramics. However, there is a strong segregation of rare earth ions at grain boundaries in solid-state reactive sintered ceramics, which affects the performance in laser applications. Using synthesized nano-powders via AHC co-precipitation could solve this problem. Besides, the transmittance of our samples is superior or comparable to LuAG ceramics using the synthesized LuAG nano powders via AHC co-precipitation method recently reported results. Decreasing the agglomeration of the synthesized LuAG nano-powders, refining forming, and optimizing the sintering process can improve the transmittance of transparent LuAG ceramics. Research on these subjects are underway in our laboratory.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Norvig, P.; Relman, D.A.; Goldstein, D.B.; Kammen, D.M.; Weinberger, D.R.; Aiello, L.C.; Church, G.; Hennessy, J.L.; Sachs, J.; Burrows, A.; et al. 2020 Visions. Nature 2010, 463, 26–32. [Google Scholar]
- Bayramian, A.J.; Bullington, A.L.; Beach, R.J.; Boley, C.D.; Caird, J.A.; Deri, R.J.; Dunne, A.M.; Flowers, D.L.; Henesian, M.A.; Manes, K.R.; et al. Comparison of Nd: Phosphate glass, Yb: YAG and Yb: S-FAP laser beamlines for laser inertial fusion energy. Opt. Mater. Express 2011, 1, 1341–1352. [Google Scholar]
- Saiki, T.; Imasaki, K.; Motokoshi, S.; Yamanaka, C.; Fujita, H.; Nakatsuka, M.; Izawa, Y. Disk-type Nd/Cr: YAG ceramic lasers pumped by arc-metal-halide-lamp. Opt. Commun. 2006, 268, 155–159. [Google Scholar] [CrossRef]
- Yamanakaa, C. High repetition rate laser pulses amplified by Nd/Cr: YAG ceramic amplifier under CW arc-lamp-light pumping. Opt. Commun. 2009, 282, 2556–2559. [Google Scholar]
- Sanghera, J.; Frantz, J.; Kim, W.; Villalobos, G.; Baker, C.; Shaw, B.; Sadowski, B.; Hunt, M.; Miklos, F.; Lutz, A.; Aggarwal, I. 10% Yb3+-Lu2O3 ceramic laser with 74% efficiency. Opt. Lett. 2011, 36, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Baker, C.; Villalobos, G.; Frantz, J.; Shaw, B.; Lutz, A.; Sadowski, B.; Kung, F.; Hunt, M.; Sanghera, J.; et al. Synthesis of high purity Yb3+-doped Lu2O3 powder for high power solid-state lasers. J. Am. Ceram. Soc. 2011, 94, 3001–3005. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.B.; Zeng, Y.P.; Liu, W.B.; Jiang, B.X.; Guo, J.K. The history, development, and future prospects for laser ceramics: A review. Int. J. Refract. Met. Hard Mater. 2013, 39, 44–52. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Luo, D.W.; Lin, H.; Shen, D.Y.; Tang, D.Y. Novel transparent ceramics for solid-state lasers. High Power Laser Sci. Eng. 2013, 1, 138–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, M.; Jiang, B.X.; Fan, J.T.; Zhou, C.L.; Mao, X.J.; Zhang, L. Micro-structure of grain boundary in post-annealed Sinter plus HIPed Nd: Lu3Al5O12 ceramics. Opt. Mater. Express 2014, 4, 2182–2189. [Google Scholar] [CrossRef]
- Yagi, H.; Yanagitani, T.; Takaichi, K.; Ueda, K.I.; Kaminskii, A.A. Characterizations and laser performances of highly transparent Nd3+: Y3Al5O12 laser ceramics. Opt. Mater. 2007, 29, 1258–1262. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Lee, J.H.; Mori, T.; Yajima, Y. Co-precipitation synthesis and sintering of yttrium aluminum garnet (YAG) powders: The effect of precipitant. J. Eur. Ceram. Soc. 2000, 20, 2395–2405. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, T.C.; Wei, N.; Shi, Y.L.; Ma, B.Y.; Luo, H.; Zhang, Z.B.; Deng, J.; Guan, Z.G.; Zhang, H.R.; et al. Co-precipitation synthesis and vacuum sintering of Nd: YAG powders for transparent ceramics. Mater. Res. Bull. 2015, 70, 365–372. [Google Scholar] [CrossRef]
- Tong, S.H.; Lu, T.C.; Guo, W. Synthesis of YAG powder by alcohol-water co-precipitation method. Mater. Lett. 2007, 61, 4287–4289. [Google Scholar] [CrossRef]
- Palmero, P.; Esnouf, C.; Montanaro, L.; Fantozzi, G. Influence of the co-precipitation temperature on phase evolution in Yttrium-Aluminium oxide materials. J. Eur. Ceram. Soc. 2005, 25, 1565–1573. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Lee, J.H.; Mori, T. Characterization of yttrium aluminate garnet precursors synthesized via precipitation using ammonium bicarbonate as the precipitant. J. Mater. Res. 2000, 15, 2375–2386. [Google Scholar] [CrossRef]
- Sang, Y.H.; Lv, Y.H.; Qin, H.M.; Zhang, X.L.; Liu, H.; Wang, J.Y.; Sun, X.D.; Boughton, R.I. Chemical composition evolution of YAG co-precipitate determined by pH during aging period and its effect on precursor properties. Ceram. Int. 2012, 38, 1635–1641. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Sang, Y.; Liu, H.; Wang, J.Y. Effects of aging on the characteristics of Nd: YAG nano-powders. J. Alloys. Compd. 2010, 502, 206–210. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhang, W.X.; Li, J.; Kou, H.M.; Shen, Y.Q.; Wang, L.; Shi, Y.; Zhang, D.; Pan, Y.B. Influence of pH values on (Nd + Y): Al molar ratio of Nd: YAG nanopowders and preparation of transparent ceramics. J. Alloys. Compd. 2010, 503, 525–528. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.Y.; Cui, H.M.; Zhang, X.D.; Han, F. Preparation of YAG: Nd nano-sized powder by co-precipitation method. Mat. Sci. Eng. A Struct. 2004, 379, 347–350. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhang, W.X.; Li, J.; Kou, H.M.; Zhang, D.; Pan, Y.B. Synthesis of Nd: YAG powders leading to transparent ceramics: The effect of MgO dopant. J. Eur. Ceram. Soc. 2011, 31, 653–657. [Google Scholar] [CrossRef]
- Liao, Y.K.; Jiang, D.Y.; Shi, J.L. Transparent lutetium aluminum garnet sintered from carbonate coprecipitated powders. Mater. Lett. 2005, 59, 3724–3727. [Google Scholar] [CrossRef]
- Sang, Y.H.; Liu, H.; Sun, X.; Zhang, X.L.; Qin, H.M.; Lv, Y.H.; Huo, D.; Liu, D.; Wang, J.Y.; Boughton, R.I. Formation and calcination temperature-dependent sintering activity of YAG precursor synthesized via reverse titration method. J. Alloys. Compd. 2011, 509, 2407–2413. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, X.D.; Li, J.G.; Xiu, Z.M.; Hi, D.; Liu, Y. Synthesis of nano-sized Lu2O3 powder for transparent ceramics fabrication using carbonate derived precursors. In Ceramic Materials and Components for Energy and Environmental Applications; Jiang, D.L., Zeng, Y.P., Singh, M., Heinrich, J., Eds.; Wiley: New York, NY, USA, 2010; pp. 597–603. [Google Scholar]
- Dulina, N.A.; Baumer, V.N.; Danylenko, M.I.; Mateychenko, P.V.; Tolmachev, A.V.; Vovk, O.M.; Yavetskiy, R.P. Effects of phase and chemical composition of precursor on structural and morphological properties of (Lu0.95Eu0.05)2O3. Ceram. Int. 2013, 39, 2397–2404. [Google Scholar] [CrossRef]
- Xie, J.J.; Shi, Y.; Hu, Y.M.; Chen, Q.W.; Shi, J.L. Synthesis study of Lu3Al5O12(Ce) nanoscaled powder by co-precipitation. J. Inorg. Mater. 2009, 24, 79–82. [Google Scholar] [CrossRef]
- Marlot, C.; Barraud, E.; Gallet, S.; Eichhorn, M.; Bernard, F. Synthesis of YAG nanopowder by the co-precipitation method: Influence of pH and study of the reaction mechanisms. J. Solid State Chem. 2012, 191, 114–120. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Jiang, B.; Fan, J.; Yang, Q.; Zhou, C.; Zhang, P.; Mao, X.; Zhang, L. Preparation of LuAG Powders with Single Phase and Good Dispersion for Transparent Ceramics Using Co-Precipitation Method. Materials 2015, 8, 5363-5375. https://doi.org/10.3390/ma8085247
Pan L, Jiang B, Fan J, Yang Q, Zhou C, Zhang P, Mao X, Zhang L. Preparation of LuAG Powders with Single Phase and Good Dispersion for Transparent Ceramics Using Co-Precipitation Method. Materials. 2015; 8(8):5363-5375. https://doi.org/10.3390/ma8085247
Chicago/Turabian StylePan, Liangjie, Benxue Jiang, Jintai Fan, Qiuhong Yang, Chunlin Zhou, Pande Zhang, Xiaojian Mao, and Long Zhang. 2015. "Preparation of LuAG Powders with Single Phase and Good Dispersion for Transparent Ceramics Using Co-Precipitation Method" Materials 8, no. 8: 5363-5375. https://doi.org/10.3390/ma8085247





