Bone Augmentation in Rabbit Tibia Using Microfixed Cobalt-Chromium Membranes with Whole Blood and Platelet-Rich Plasma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results

2.1.1. Bone Measurement
| Days | Whole Blood | PRP | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| 30 | Partially covered | Not covered | Not covered | Partially covered | Totally covered | Totally covered |
| 45 | Partially covered | Starting of bone formation process in the borders of membrane | Not covered | Not covered | Not covered | Not covered |
| 60 | Partially covered | Not covered | Partially covered | Starting of bone formation process in the borders of membrane | Starting of bone formation process in the borders of membrane | Not covered |
| 110 | Totally covered | Partially covered | Totally covered | Partially covered | Partially covered | Totally covered |
| Rabbit Number | Membrane Dimensions [mm] (width-long-depth) | Membrane Interior Volume [mm3] | Bone Dimensions [mm] (high-long-depth) | Bone Volume [mm3] | % (BV/MV × 100) |
|---|---|---|---|---|---|
| Whole Blood | |||||
| 6 | 7.85 × 8.0 × 0.0 | 0.0 | 0.42 × 5.32 × 4.24 | 7.44 | - |
| 5 | 3.6 × 7.3 × 0.35 | 9.2 | 0.04 × 6.49 × 5.17 | 1.05 | 11.41 |
| 4 | 5.35 × 7.8 × 1.2 | 50.08 | 0.94 × 8.07 × 5.58 | 33.24 | 66.37 |
| 3 | 5.3 × 7.6 × 1.1 | 44.31 | 1.09 × 8.95 × 6.13 | 46.97 | 106.00 |
| 2 | 5.25 × 7.6 × 1.2 | 47.88 | 1.15 × 8.48 × 5.49 | 53.54 | 111.82 |
| 1 | - | - | - | - | - |
| PRP | |||||
| 6 | 5.85 × 10.0 × 1.75 | 102.37 | 0.0 × 5.89 × 3.97 | - | - |
| 5 | 3.95 × 5.75 × 0.55 | 12.49 | 0.92 × 0.75 × 0.44 | 0.24 | 1.92 |
| 4 | 4.5 × 5.85 × 0.95 | 25.01 | 0.52 × 4.53 × 3.07 | 5.68 | 22.71 |
| 3 | 5.5 × 7.8 × 1.1 | 47.19 | 0.97 × 7.95 × 5.61 | 33.98 | 72.01 |
| 2 | 5.35 × 7.55 × 1.15 | 46.45 | 1.02 × 7.8 × 5.7 | 45.35 | 97.63 |
| 1 | 4.2 × 5.9 × 0.75 | 18.59 | 0.57 × 7.45 × 5.4 | 22.93 | 123.35 |
2.1.2. Densitometric Measurement
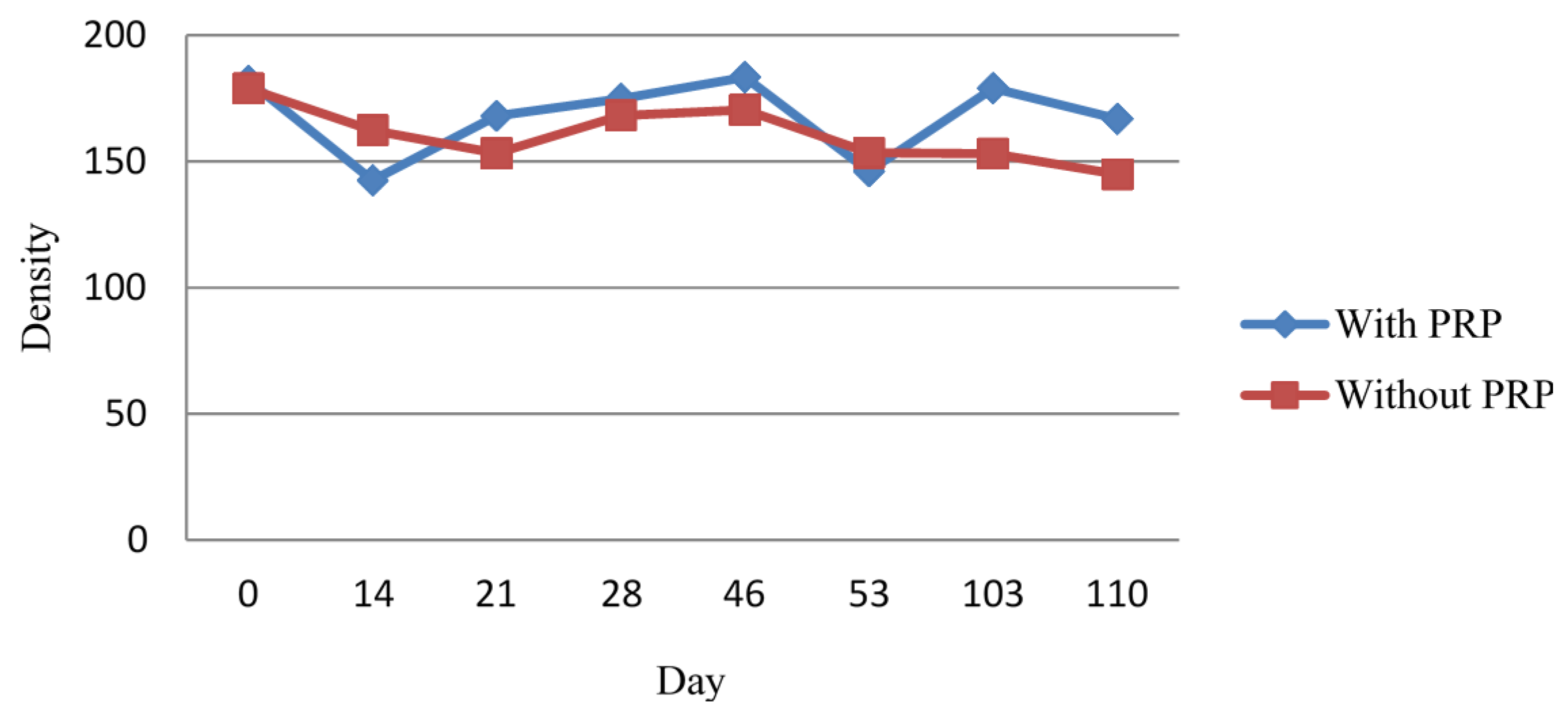
2.1.3. Histological Examination
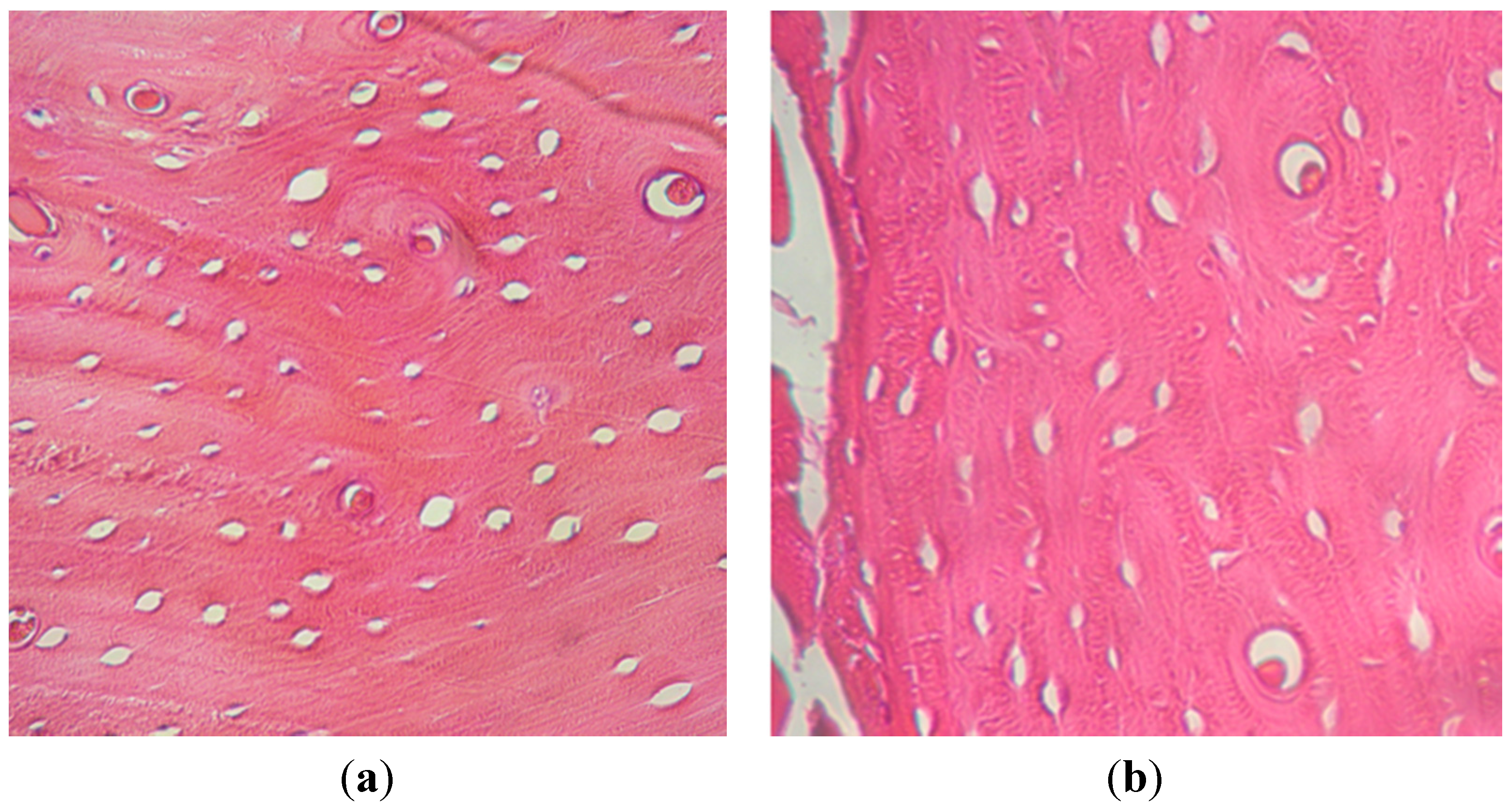
2.2. Discussion
3. Experimental Section
3.1. Surgical Procedure
3.2. PRP Preparation Technique
3.3. Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Varkey, M.; Gittens, S.A.; Uludag, H. Growth factor delivery for bone tissue repair: An update. Expert Opin. Drug Deliv. 2004, 1, 19–36. [Google Scholar]
- Landro, M.E.; Francalaccia, V.; Douglas Price, A.L. Medicina regenerativa. Su aplicación en traumatología. Rev. Asoc. Argent. Ortop. Traumatol. 2010, 75, 398–403. [Google Scholar]
- Chen, F.M.; Jin, Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng. Part B 2010, 16, 19–55. [Google Scholar]
- McAllister, B.S.; Haghighat, K.J. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar]
- Jardini, M.A.; De Marco, A.C.; Lima, L.A. Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: A histomorphometric study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 666–673. [Google Scholar]
- Buser, D.; Hoffmann, B.; Bernard, J.P.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of filling materials in membrane-protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin. Oral Implant. Res. 1998, 9, 137–150. [Google Scholar]
- Decco, O.; Cura, A.; Beltrán, V.; Lezcano, F.; Engelke, W. Bone augmentation in rabbit tibia using microfixed cobalt-chromium membranes with whole blood, tricalcium phosphate and bone marrow cells. Int. J. Clin. Exp. Med. 2015, 8, 135–144. [Google Scholar]
- Engelke, W.; Deccó, O.; Cura, A.C.; Borie, E.; Beltrán, V. Rigid occlusive titanium barriers for alveolar bone augmentation: Two reports with 24-month follow-up. Int. J. Clin. Exp. Med. 2014, 7, 1160–1165. [Google Scholar]
- Beltrán, V.; Engelke, W.; Prieto, R.; Valdivia-Gandur, I.; Navarro, P.; Manzanares, M.C.; Borie, E.; Fuentes, R. Augmentation of intramembranous bone in rabbit calvaria using an occlusive barrier in combination with demineralized bone matrix (DBM): A pilot study. Int. J. Surg. 2014, 12, 378–383. [Google Scholar]
- Nikolidakis, D.; Jansen, J.A. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng. Part B Rev. 2008, 14, 249–258. [Google Scholar]
- Kim, S.-G.; Chung, C.-H.; Kim, Y.-K. Bone conditioning to enhance implant osseointegration: An experimental study in pigs. Int. J. Oral Maxillofac. Implant. 2002, 17, 86–94. [Google Scholar]
- Schlegel, K.A.; Kloss, F.R.; Kessler, P.; Schultze-Mosgau, S.; Nkenke, E.; Wiltfang, J. Bone conditioning to enhance implant osseointegration: an experimental study in pigs. Int. J. Oral Maxillofac. Implant. 2003, 18, 505–511. [Google Scholar]
- Zechner, W.; Tangl, S.; Tepper, G. Influence of platelet-rich plasma on osseous healing of dental implants: A histologic and histomorphometric study in mini pigs. Int. J. Oral Maxillofac. Implant. 2003, 18, 15–22. [Google Scholar]
- Chen, F.M.; Zhang, M.; Wu, Z.F. Review Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Schupbach, P.; Cooper, L. The implant surface and biological response. In Osseointegration and Dental Implants; Jokstad, A., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 213–223. [Google Scholar]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implant. Res. 2003, 14, 63–71. [Google Scholar]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar]
- Knothe Tate, M.L.; Niederer, P.; Knothe, U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 1998, 22, 107–117. [Google Scholar]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M. Reciprocal actions of platelet secreted TGF-beta1 on the production of VEGF and HGF by human tendoncells. Plast. Reconstr. Surg. 2007, 119, 950–959. [Google Scholar]
- Aspenberg, P.; Virchenko, O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop. Scand 2004, 75, 93–99. [Google Scholar]
- Kitoh, H.; Kitakoji, T.; Tsuchiya, H.; Katoh, M.; Ishiguro, N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrowcells and platelet-rich plasma. J. Pediatr. 2007, 27, 629–634. [Google Scholar]
- Gandhi, A.; Bibbo, C.; Pinzur, M.; Lin, S.S. The role of platelet-rich plasma in foot and ankle surgery. Foot Ankle Clin. 2005, 10, 621–637. [Google Scholar]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar]
- Yokota, K.; Ishida, O.; Sunagawa, T.; Suzuki, O.; Nakamae, A.; Ochi, M. Platelet-rich plasma accelerated surgicalangio-genesis in vascular-implanted necrotic bone: An experimental study in rabbits. Acta Orthop. 2008, 79, 106–110. [Google Scholar]
- Casati, L.; Celotti, F.; Negri-Cesi, P.; Sacchi, M.C.; Castano, P.; Colciago, A. Platelet derived growth factor (PDGF) contained in Platelet Rich Plasma (PRP) stimulates migration of osteoblasts by reorganizing actin cytoskeleton. Cell Adh. Migr. 2014, 8, 16–20. [Google Scholar]
- Zhang, N.; Wu, Y.P.; Qian, S.J.; Teng, C.; Chen, S.; Li, H. Research progress in the mechanism of effect of prp in bone deficiency healing. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Albanese, A.; Licata, M.E.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 23, 1–10. [Google Scholar]
- Malhotra, A.; Pelletier, M.H.; Yu, Y.; Walsh, W.R. Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch. Orthop. Trauma Surg. 2013, 2, 153–165. [Google Scholar]
- Choi, B.H.; Zhu, S.J.; Kim, B.Y.; Huh, J.Y.; Lee, S.H.; Jung, J.H. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: An in vitro study. Int. J. Oral Maxillofac. Surg. 2005, 4, 420–424. [Google Scholar]
- Miloro, M.; Haralson, D.J.; Desa, V. Bone healing in a rabbit mandibular defect using platelet-rich plasma. J. Oral. Maxillofac. Surg. 2010, 6, 1225–1230. [Google Scholar]
- Daif, E.T. Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent Traumatol. 2013, 5, 399–403. [Google Scholar]
- Anitua, E.; Sánchez, M.; Orive, G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv. Drug Deliv. Rev. 2010. [Google Scholar] [CrossRef]
- Roffi, A.; Filardo, G.; Kon, E.; Marcacci, M. Does PRP enhance bone integration with grafts, graft substitutes, or implants? A systematic review. BMC Musculoskelet. Disord. 2013, 21, 314–330. [Google Scholar]
- Perut, F.; Filardo, G.; Mariani, E.; Cenacchi, A.; Pratelli, L.; Devescovi, V.; Kon, E.; Marcacci, M.; Facchini, A.; Baldini, N.; et al. Preparation method and growth factor content of platelet concentrate influence the osteogenic differentiation of bone marrow stromal cells. Cytotherapy 2013, 7, 830–839. [Google Scholar]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S.; Weinstein, R.L. Effect of autologous growth factors in maxillary sinus augmentation: A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 2, 205–216. [Google Scholar]
- Chiapasco, M.; Abati, S.; Romeo, E.; Vogel, G. Clinical outcomes of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin. Oral Implant. Res. 1999, 4, 278–288. [Google Scholar]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar]
- Linde, F.; Sorensen, H.C. The effect of different storage methods on the mechanical properties of trabecular bone. J. Biomech. 1993, 10, 1249–1252. [Google Scholar]
- Zellin, G.; Linde, A. Importance of delivery systems for growth-stimulatory factors in combination with osteopromotive membranes. An experimental study using rhBMP-2 in rat mandibular defects. J. Biomed. Mater. Res. 1997, 35, 181–190. [Google Scholar]
- Antoun, H.; Sitbon, J.M.; Martinez, H.; Missika, P. A prospective randomized study comparing two techniques of bone augmentation: Onlay graft alone or associated with a membrane. Clin. Oral Implant. Res. 2001, 12, 632–639. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat Res. 1986, 205, 299–308. [Google Scholar]
- Ishidou, Y.; Kitajima, I.; Obama, H.; Maruyama, I.; Murata, F.; Imamura, T.; Yamada, N.; Ten Dijke, P.; Miyazono, K.; Sakou, T. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J. Bone Miner. Res. 1995, 10, 1651–1659. [Google Scholar]
- Ogawa, M.; Tohma, Y.; Ohgushi, H.; Takakura, Y.; Tanaka, Y. Early Fixation of cobalt-chromium based alloy surgical implants to bone using a tissue-engineering approach. Int. J. Mol. Sci. 2012, 13, 5528–5541. [Google Scholar]
- Kransdorf, M.J.; Stull, M.A.; Gilkey, F.W.; Moser, R.P., Jr. Osteoid osteoma. Radiographics 1991, 11, 671–696. [Google Scholar]
- Gerard, D.; Carlson, E.R.; Gotcher, J.E.; Jacobs, M. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J. Oral Maxillofac. Surg. 2006, 3, 443–451. [Google Scholar]
- Gerard, D.; Carlson, E.R.; Gotcher, J.E.; Jacobs, M. Effects of platelet-rich plasma at the cellular level on healing of autologous bone-grafted mandibular defects in dogs. J. Oral Maxillofac. Surg. 2007, 4, 721–727. [Google Scholar]
- Aghaloo, T.L.; Moy, P.K.; Freymiller, E.G. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J. Oral. Maxillofac. Surg. 2002, 10, 1176–1181. [Google Scholar]
- Chaves Netto, H.D.M.; Olate, S.; Chaves, M.M.G.A.; Barbosa, A.J.R.; Mazzonetto, R. Análisis histológico del proceso de reparación en defectos óseos. Reconocimiento de defectos críticos. Int. J. Morphol. 2009, 4, 1121–1127. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decco, O.A.; Beltrán, V.; Zuchuat, J.I.; Cura, A.C.; Lezcano, M.F.; Engelke, W. Bone Augmentation in Rabbit Tibia Using Microfixed Cobalt-Chromium Membranes with Whole Blood and Platelet-Rich Plasma. Materials 2015, 8, 4843-4856. https://doi.org/10.3390/ma8084843
Decco OA, Beltrán V, Zuchuat JI, Cura AC, Lezcano MF, Engelke W. Bone Augmentation in Rabbit Tibia Using Microfixed Cobalt-Chromium Membranes with Whole Blood and Platelet-Rich Plasma. Materials. 2015; 8(8):4843-4856. https://doi.org/10.3390/ma8084843
Chicago/Turabian StyleDecco, Oscar A., Víctor Beltrán, Jésica I. Zuchuat, Andrea C. Cura, María F. Lezcano, and Wilfried Engelke. 2015. "Bone Augmentation in Rabbit Tibia Using Microfixed Cobalt-Chromium Membranes with Whole Blood and Platelet-Rich Plasma" Materials 8, no. 8: 4843-4856. https://doi.org/10.3390/ma8084843





