Mineralized Collagen: Rationale, Current Status, and Clinical Applications
Abstract
:1. Introduction
2. Rationale for Mineralized Collagen

3. Studies on Biomimetic Mineralized Collagen
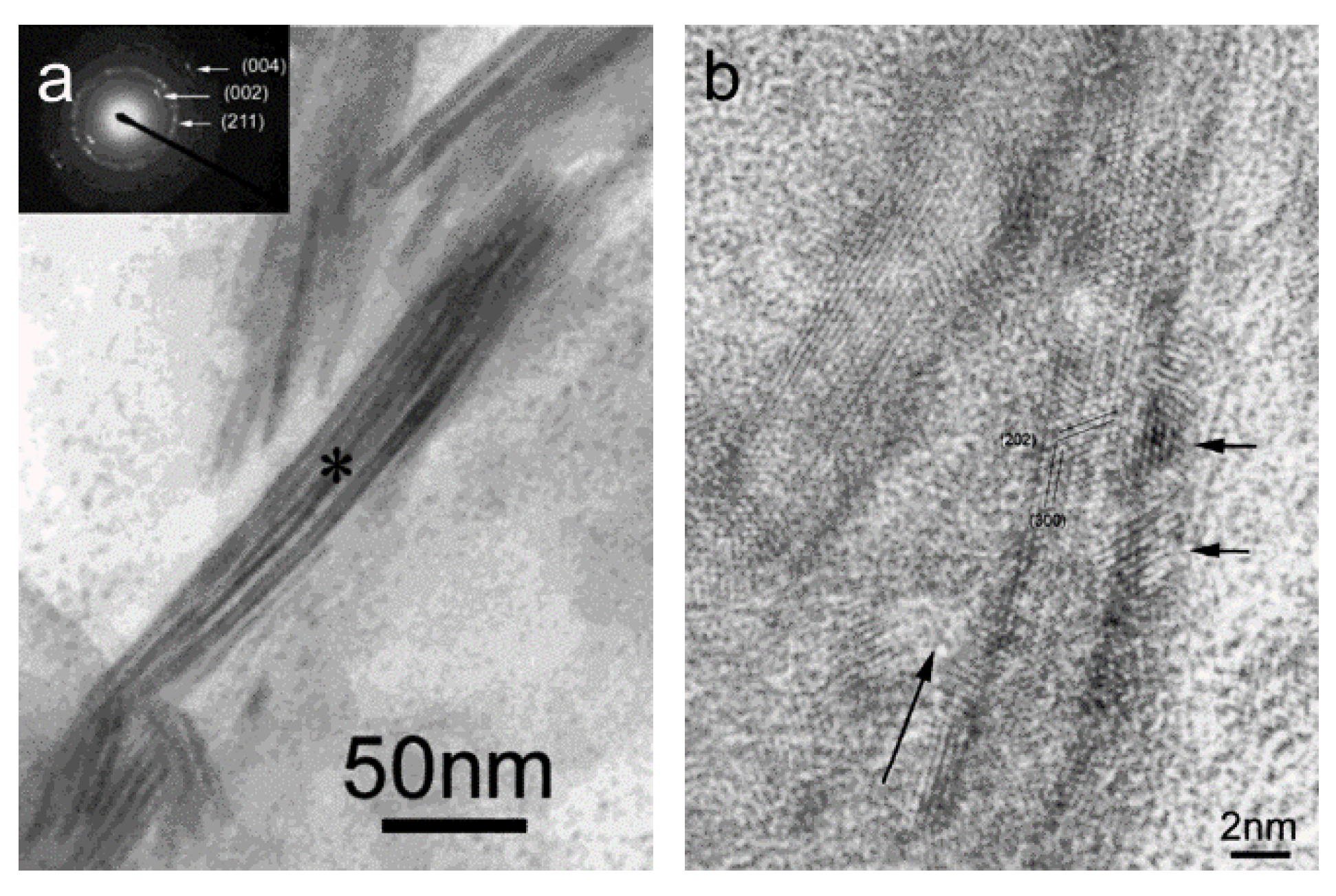
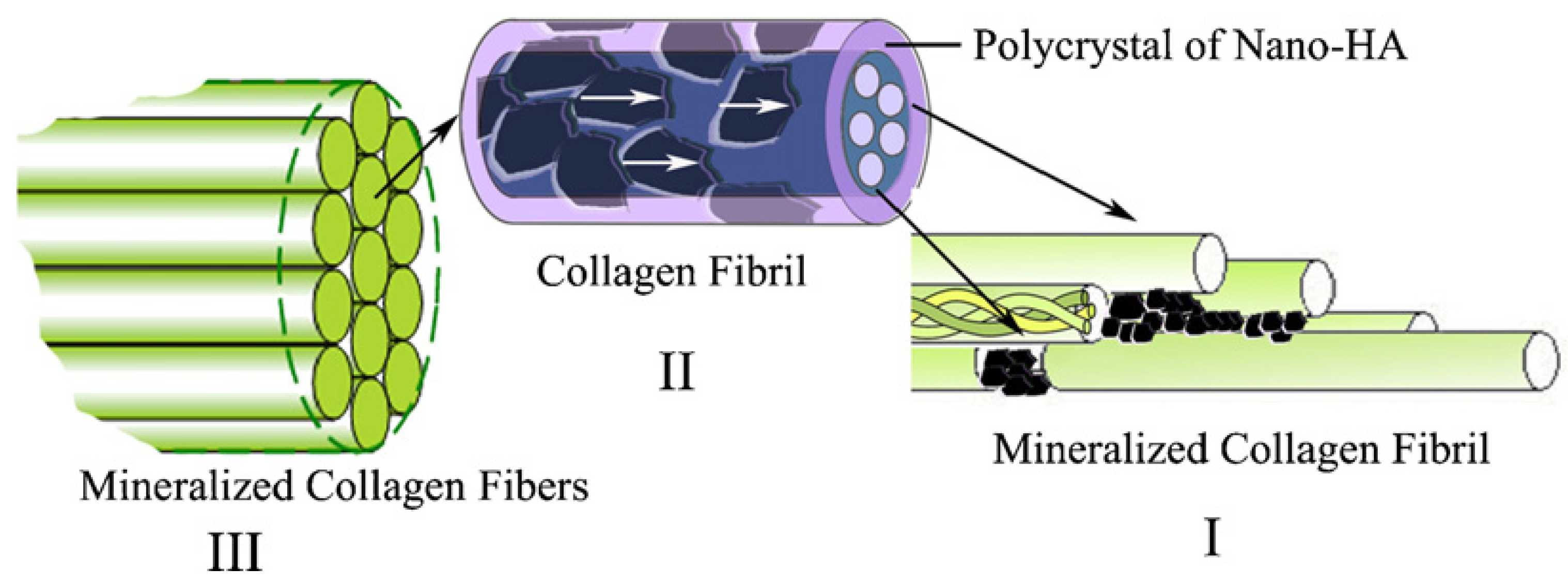
4. Commercially Available Mineralized Collagen Products
| Product | Company | Composition | Porosity | Pore Size | Form | Claimed mechanisms of action |
|---|---|---|---|---|---|---|
| Bio-Oss Collagen® | Geistlich, Switzerland | 10% type I collagen and 90% bovine mineral | 70%–75% | 300–1500 μm | Block |
|
| Bongold® | Allgens, China | Self-assembled type I bovine collagen and hydroxyapatite (HA) similar to the natural mineralized collagen | 70%–88% | 50–550 μm | Strip, granule, block, putty, sponge |
|
| CopiOs® | Zimmer, USA | Type I bovine collagen and 67% mineral | 93.39% | - | Sponge, paste |
|
| HEALOS® | Johnson & Johnson, USA | 70% type I bovine collagen and HA | > 95% | 4–200 μm | Strip |
|
| MOZAIK™ | Integra, USA | 20% type I collagen and 80% β-TCP | - | 12–350 μm | Strip, putty |
|
| MASTERGRAFT® Strip/Putty | Medtronic, USA | Bovine type I collagen and biphasic ceramics (15% HA and 85% β-TCP) | 89% | - | Strip |
|
| OSTEON™ | Dentium, Korea | 8% type I collagen and 92% mineral (30% HA and 70% β-TCP) | 70% | 500–1000 μm | Cylinder |
|
| OssiMend™ | Collagen Matrix, USA | 45% bovine type I collagen and 55% synthetic calcium phosphate | - | - | Strip, block, putty |
|
| Refit | HOYA, Japan | 20% type I bovine collagen and 80% HA | 95% | 100–500 μm | Block |
|
| SynOss™ Putty | Collagen Matrix, USA | Type I collagen and carbonate HA | - | - | Putty |
|
| Vitoss® FOAM | Stryker, USA | Type I bovine collagen and calcium phosphate | 90% | 1–1000 μm | Putty, strip, flow, morsels and shapes |
|
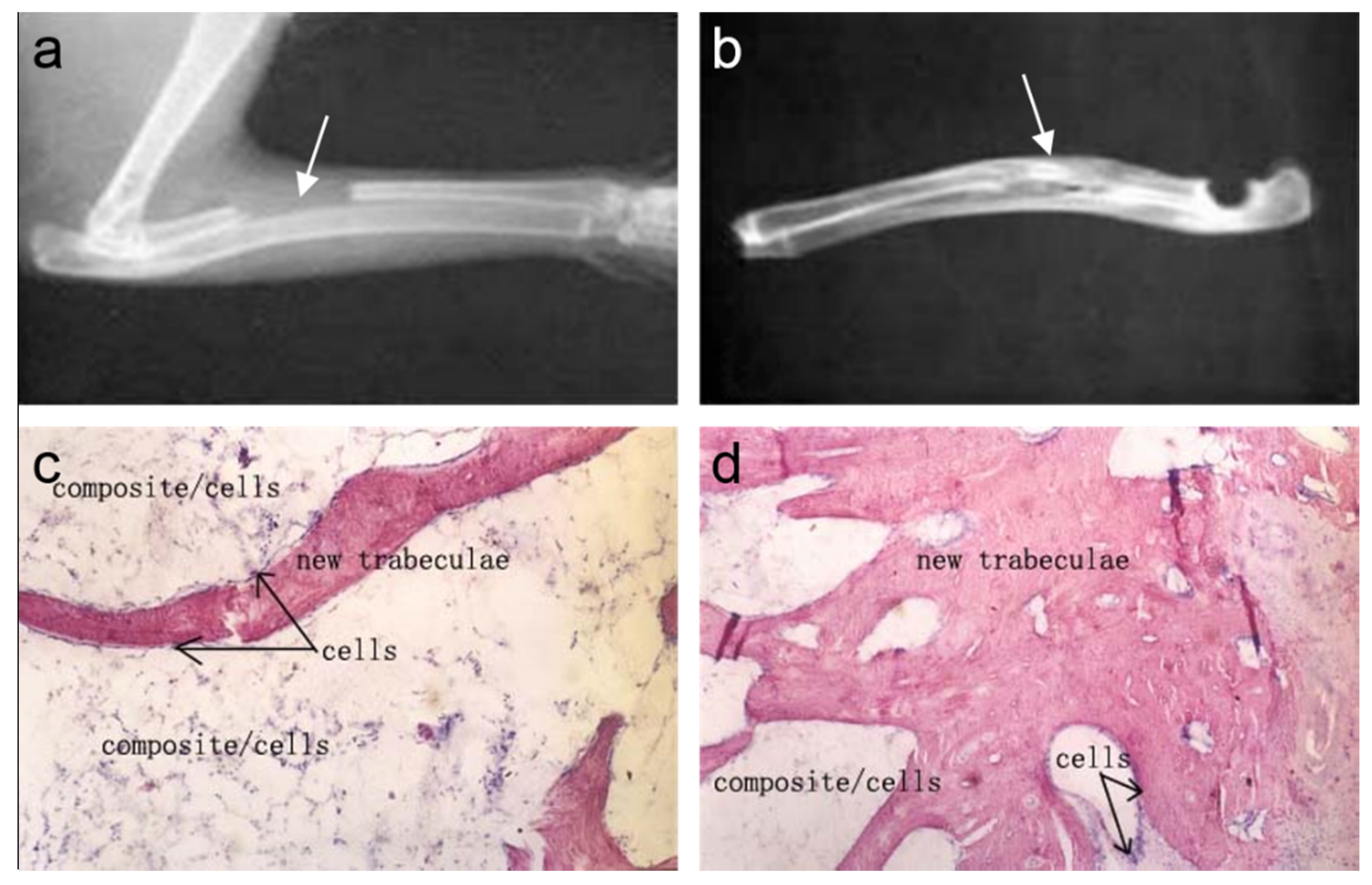
5. Clinical Applications of Mineralized Collagen Products
5.1. Restoration of Bone Defects in Orthopedic Applications
5.2. Bone Volume Augmentation in Dental Surgeries
5.3. Reconstruction of Skull Defects in Neurosurgical Applications
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, F.Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Nalla, R.K.; Porter, A.E.; Daraio, C.; Minor, A.M.; Radmilovic, V.; Stach, E.A.; Tomsia, A.P.; Ritchie, R.O. Ultrastructural examination of dentin using focused ion-beam cross-sectioning and transmission electron microscopy. Micron 2005, 36, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G.; Pasteris, J.D.; Genin, G.M.; Daulton, T.L.; Thomopoulos, S. Mineral distributions at the developing tendon enthesis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci. Mater. Med. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.; McEwen, L.; McEwen, B.F. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Traub, W.; Wagner, H.D. Lamellar bone: Structure-function relations. J. Struct. Biol. 1999, 126, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Ai, X.; Zhang, S. Biomimetic self-assembly of apatite hybrid materials: From a single molecular template to bi-/multi-molecular templates. Biotechnol. Adv. 2014, 32, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking natural bio-mineralization processes: A new tool for osteochondral scaffold development. Trends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized scaffolds to enhance tissue regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; Miller, A.; Irving, T.C.; Fischetti, R.F.; Hammersley, A.P.; Wess, T.J. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069. [Google Scholar] [CrossRef]
- Stamov, D.R.; Stock, E.; Franz, C.M.; Jahnke, T.; Haschke, H. Imaging collagen type I fibrillogenesis with high spatiotemporal resolution. Ultramicroscopy 2015, 149, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ottani, V.; Martini, D.; Franchi, M.; Ruggeri, A.; Raspanti, M. Hierarchical structures in fibrillar collagens. Micron 2002, 33, 587–596. [Google Scholar] [CrossRef]
- Ramshaw, J.A.; Shah, N.K.; Brodsky, B. Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Traub, W. Bone structure: From angstroms to microns. Fed. Am. Soc. Exp. Biol. J. 1992, 6, 879–885. [Google Scholar]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.J.; Silver, F.H. Mineral deposition in the extracellular matrices of vertebrate tissues: Identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs 2009, 189, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Lee, J.D.; Tanaka, J. Nucleation of hydroxyapatite crystal through chemical interaction with collagen. J. Am. Ceram. Soc. 2000, 83, 2890–2892. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.L.; Liao, S.S.; Cui, F.Z. Nucleation sites of calcium phosphate crystals during collagen mineralization. J. Am. Ceram. Soc. 2003, 86, 1052–1054. [Google Scholar] [CrossRef]
- Piez, K.A. Molecular and aggregate structures of the collagens. In Extracellular Matrix Biochemistry; Piez, K.A., Reddi, A.H., Eds.; Elsevier: Amsterdam, The Netherland, 1984; pp. 1–39. [Google Scholar]
- Kühn, K. The classical collagens: Types I, II, and III. In Structure and Function of Collagen Types; Mayne, R., Burgeson, R.E., Eds.; Academic Press, Inc.: Orlando, FL, USA, 1987; pp. 1–42. [Google Scholar]
- McEwen, B.F.; Song, M.J.; Landis, W.J. Quantitative determination of the mineral distribution in different collagen zones of calcifying tendon using high voltage electron microscopic tomography. J. Comput. Assist. Microsc. 1991, 3, 201–210. [Google Scholar] [PubMed]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.J.; Hodgens, K.J.; Song, M.J.; Arena, J.; Kiyonaga, S.; Marko, M.; Owen, C.; McEwen, B.F. Mineralization of collagen may occur on fibril surfaces: Evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J. Struct. Biol. 1996, 117, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cui, F.Z.; Wang, X.; Wang, Y. New evidence of surface mineralization of collagen fibrils in wild type zebrafish skeleton by AFM and TEM. Mater. Sci. Eng. C 2007, 27, 46–50. [Google Scholar] [CrossRef]
- Su, X.; Sun, K.; Cui, F.Z.; Landis, W.J. Organization of apatite crystals in human woven bone. Bone 2003, 32, 150–162. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Wang, X.M.; Cui, F.Z.; Ge, J.; Wang, Y. Hierarchical structural comparisons of bones from wild-type and liliput dtc232 gene-mutated Zebrafish. J. Struct. Biol. 2004, 145, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Rubin, M.A.; Rubin, J.; Jasiuk, I. SEM and TEM study of the hierarchical structure of C57BL/6J and C3H/HeJ mice trabecular bone. Bone 2004, 35, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Nanostructured biomaterials for regeneration. Adv. Funct. Mater. 2008, 18, 3568–3582. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.; Sone, E.D. In vitro models of collagen biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M. Hydroxyapatite/collagen bone-like nanocomposite. Biol. Pharm. Bull. 2013, 36, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Constantz, B.R.; Gunasekaran, S. Mineralized Collagen. U.S. Patent 5,231,169,1993, 27 July 1993. [Google Scholar]
- Bradt, J.H.; Mertig, M.; Teresiak, A.; Pompe, W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 1999, 11, 2694–2701. [Google Scholar] [CrossRef]
- Pederson, A.W.; Ruberti, J.W.; Messersmith, P.B. Thermal assembly of a biomimetic mineral/collagen composite. Biomaterials 2003, 24, 4881–4890. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-assembled collagen/hydroxyapatite composite materials. Chem. Eng. J. 2010, 160, 794–800. [Google Scholar] [CrossRef]
- Maas, M.; Guo, P.; Keeney, M.; Yang, F.; Hsu, T.M.; Fuller, G.G.; Martin, C.R.; Zare, R.N. Preparation of mineralized nanofibers: Collagen fibrils containing calcium phosphate. Nano Lett. 2011, 11, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C. Biomimetic collagen/hydroxyapatite composite scaffolds: Fabrication and characterizations. J. Bionic Eng. 2014, 11, 600–609. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.S.; Cui, F.Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Wang, R.Z.; Cui, F.Z.; Lu, H.B.; Wen, H.B.; Ma, C.L.; Li, H.D. Synthesis of nanophase hydroxyapatite/collagen composite. J. Mater. Sci. Lett. 1995, 14, 490–492. [Google Scholar] [CrossRef]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Du, C.; Cui, F.Z.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Tissue response to nano-hydroxyapatite/collagen composite implants in marrow cavity. J. Biomed. Mater. Res. 1998, 42, 540–548. [Google Scholar] [CrossRef]
- Macmillan Publishers Limited. Polishing, sensing, switching and synthesizing. Nat. Mater. 2003, 2. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.S.; Beniash, E. Bioinspired synthesis of mineralized collagen fibrils. Cryst. Growth Des. 2008, 8, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.M.; Tian, L.L.; Cheng, Z.J.; Cui, F.Z. In situ remineralizaiton of partially demineralized human dentine mediated by a biomimetic non-collagen peptide. Soft Matter 2011, 7, 9673–9680. [Google Scholar] [CrossRef]
- Burwell, A.K.; Thula-Mata, T.; Gower, L.B.; Habelitz, S.; Kurylo, M.; Ho, S.P.; Chien, Y.C.; Cheng, J.; Cheng, N.F.; Gansky, S.A.; et al. Functional remineralization of dentin lesions using polymer-induced liquid-precursor process. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.D.; Ling, J. Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng. Part C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Y.; Tao, C.S.; Cui, H.; Wang, C.M.; Cui, F.Z. High-strength mineralized collagen artificial bone. Front. Mater. Sci. 2014, 8, 53–62. [Google Scholar] [CrossRef]
- Ling, L.E.; Feng, L.; Liu, H.C.; Wang, D.S.; Shi, Z.P.; Wang, J.C.; Luo, W.; Lv, Y. The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 1732–1745. [Google Scholar] [CrossRef] [PubMed]
- 510(k) summary of K012751. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf/k012751.pdf (accessed on 23 July 2015).
- 510(k) summary of K032288. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf3/K032288.pdf (accessed on 23 July 2015).
- 510(k) summary of K033679. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf3/k033679.pdf (accessed on 23 July 2015).
- Smucker, J.D.; Petersen, E.B.; Nepola, J.V.; Fredericks, D.C. Assessment of MASTERGRAFT® STRIP with bone marrow aspirate as a graft extender in a rabbit posterolateral fusion model. Iowa Orthop. J. 2012, 32, 61–68. [Google Scholar] [PubMed]
- 510(k) summary of K082166. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf8/k082166.pdf (accessed on 23 July 2015).
- 510(k) summary of K081784. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf8/k081784.pdf (accessed on 23 July 2015).
- Kim, D.M.; Nevins, M.L.; Lin, Z.; Fateh, A.; Kim, S.W.; Schupbach, P.; Nevins, M. The clinical and histologic outcome of dental implant in large ridge defect regenerated with alloplast: a randomized controlled preclinical trial. J. Oral Implantol. 2013, 39, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.M.; Fu, T.Y.; Jia, X.J.; Hou, J.W.; Gao, C.; Ma, Y.Z.; Qiu, Z.Y.; Cui, F.Z. Clinical observations on repair of non-infected bone nonunion by using mineralized collagen graft. J. Biomater. Tissue Eng. 2014, 4, 1107–1112. [Google Scholar] [CrossRef]
- Lian, K.; Lu, H.; Guo, X.; Cui, F.; Qiu, Z.; Xu, S. The mineralized collagen for the reconstruction of intra-articular calcaneal fractures with trabecular defects. Biomatter 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, L.; Cui, F.Z.; Qu, Y.; Lian, X.J.; Wang, X.M.; Wang, Y. Clinical evaluation of mineralized collagen as a bone graft substitute for anterior cervical intersomatic fusion. J. Biomater. Tissue Eng. 2012, 2, 170–176. [Google Scholar] [CrossRef]
- Hvistendahl, M. China’s push in tissue engineering. Science 2012, 338, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Karp, J.M. Controlling cell fate in vivo. ChemBioChem 2009, 10, 2308–2310. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Teng, Y.; Zhang, J.; Guo, H.; Antezana, J.P.E.; Cui, F.; He, X. Clinical and radiographic analysis of mineralized collagen in posterior lumbar interbody fusion. J. Biomater. Tissue Eng. 2014, 4, 288–294. [Google Scholar] [CrossRef]
- Carter, J.D.; Swearingen, A.B.; Chaput, C.D.; Rahm, M.D. Clinical and radiographic assessment of transforaminal lumbar interbody fusion using HEALOS collagen-hydroxyapatite sponge with autologous bone marrow aspirate. Spine J. 2009, 9, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.B.; Huang, K.; Teng, Y.; Qu, Y.Z.; Cui, W.; Huang, Z.F.; Sun, T.F.; Guo, X.D. Use of mineralized collagen bone graft substitutes and dorsal locking plate in treatment of elder metaphyseal comminuted distal radius fracture. Front. Mater. Sci. 2014, 8, 87–94. [Google Scholar] [CrossRef]
- Neen, D.; Noyes, D.; Shaw, M.; Gwilym, S.; Fairlie, N.; Birch, N. Healos and bone marrow aspirate used for lumbar spine fusion: A case controlled study comparing healos with autograft. Spine 2006, 31, E636–E640. [Google Scholar] [CrossRef] [PubMed]
- Ploumis, A.; Albert, T.J.; Brown, Z.; Mehbod, A.A.; Transfeldt, E.E. Healos graft carrier with bone marrow aspirate instead of allograft as adjunct to local autograft for posterolateral fusion in degenerative lumbar scoliosis: a minimum 2-year follow-up study: Clinical article. J. Neurosurg. Spine 2010, 13, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction alveolar ridge preservation: Biological basis and treatments. Int. J. Dent. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Lang, N.P. Ridge preservation after tooth extraction. Clin. Oral Implant. Res. 2012, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fickl, S.; Zuhr, O.; Wachtel, H.; Stappert, C.F.; Stein, J.M.; Hürzeler, M.B. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J. Clin. Periodontol. 2008, 35, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Ding, Y.; Zhou, H.; Qin, R.; Hou, R.; Zhang, G.; Hu, K. Alveolar ridge preservation with deproteinized bovine bone graft and collagen membrane and delayed implants. J. Craniofac. Surg. 2014, 25, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft—A 5-year retrospective study. Clin. Oral Implant. Res. 2015, 26, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Nevins, M.L.; Schupbach, P.; Kim, S.W.; Lin, Z.; Kim, D.M. A prospective, randomized controlled preclinical trial to evaluate different formulations of biphasic calcium phosphate in combination with a hydroxyapatite collagen membrane to reconstruct deficient alveolar ridges. J. Oral Implantol. 2013, 39, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.L.; Camelo, M.; Schupbach, P.; Kim, D.M.; Camelo, J.M.; Nevins, M. Human histologic evaluation of mineralized collagen bone substitute and recombinant platelet-derived growth factor-BB to create bone for implant placement in extraction socket defects at 4 and 6 months: A case series. Int. J. Periodontics Restor. Dent. 2009, 29, 129–139. [Google Scholar]
- Sam, G.; Pillai, B.R.M. Evolution of barrier membranes in periodontal regeneration—“Are the third generation membranes really here?”. J. Clin. Diagn. Res. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; Maté-Sánchez, J.E.; Bruno, N.; Velasquez, P.; de Aza, P.N. Enhanced bone regeneration with a novel synthetic bone substitute in combination with a new natural cross-linked collagen membrane: radiographic and histomorphometric study. Clin. Oral Implant. Res. 2015, 26, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Stricker, A.; Fleiner, J.; Stübinger, S.; Fleiner, H.; Buser, D.; Bosshardt, D.D. Ridge preservation after ridge expansion with simultaneous guided bone regeneration: A preclinical study. Clin. Oral Implant. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dujovny, M.; Aviles, A.; Cuevas, P. Bone-like polyethelyne burr-hole cover. Neurol. Res. 2005, 27, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Easwer, H.V.; Rajeev, A.; Varma, H.K.; Vijayan, S.; Bhattacharya, R.N. Cosmetic and radiological outcome following the use of synthetic hydroxyapatite porous-dense bilayer burr-hole buttons. Acta Neurochir. 2007, 149, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Cokluk, C.; Senel, A.; Iyigün, O.; Aydin, K.; Rakunt, C.; Celik, F. Reconstruction of burr hole by using autologous button-shaped graft harvested from inner table of craniotomy flap: Technique and clinical result. Minim. Invasive Neurosurg. 2003, 46, 372–373. [Google Scholar] [PubMed]
- Wang, K.W.; Zhang, X.Q.; Liu, X.H. The clinical study on the repair bone defects with nano-hap/collagen composites. Chin. J. Pediatr. Surg. 2005, 26, 476–478. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.-Y.; Cui, Y.; Tao, C.-S.; Zhang, Z.-Q.; Tang, P.-F.; Mao, K.-Y.; Wang, X.-M.; Cui, F.-Z. Mineralized Collagen: Rationale, Current Status, and Clinical Applications. Materials 2015, 8, 4733-4750. https://doi.org/10.3390/ma8084733
Qiu Z-Y, Cui Y, Tao C-S, Zhang Z-Q, Tang P-F, Mao K-Y, Wang X-M, Cui F-Z. Mineralized Collagen: Rationale, Current Status, and Clinical Applications. Materials. 2015; 8(8):4733-4750. https://doi.org/10.3390/ma8084733
Chicago/Turabian StyleQiu, Zhi-Ye, Yun Cui, Chun-Sheng Tao, Zi-Qiang Zhang, Pei-Fu Tang, Ke-Ya Mao, Xiu-Mei Wang, and Fu-Zhai Cui. 2015. "Mineralized Collagen: Rationale, Current Status, and Clinical Applications" Materials 8, no. 8: 4733-4750. https://doi.org/10.3390/ma8084733






