Fine Structure in Multi-Phase Zr8Ni21-Zr7Ni10-Zr2Ni7 Alloy Revealed by Transmission Electron Microscope
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
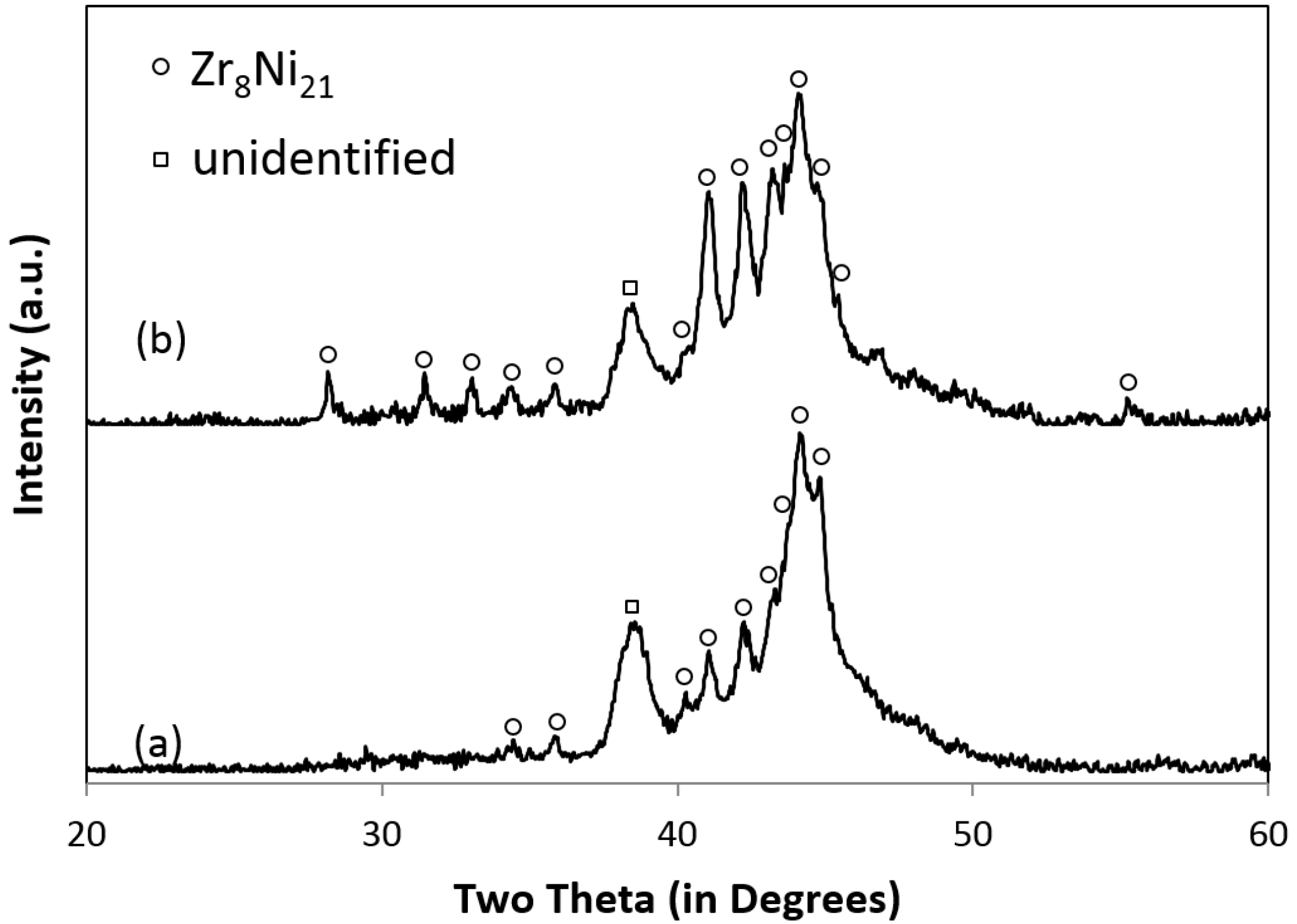
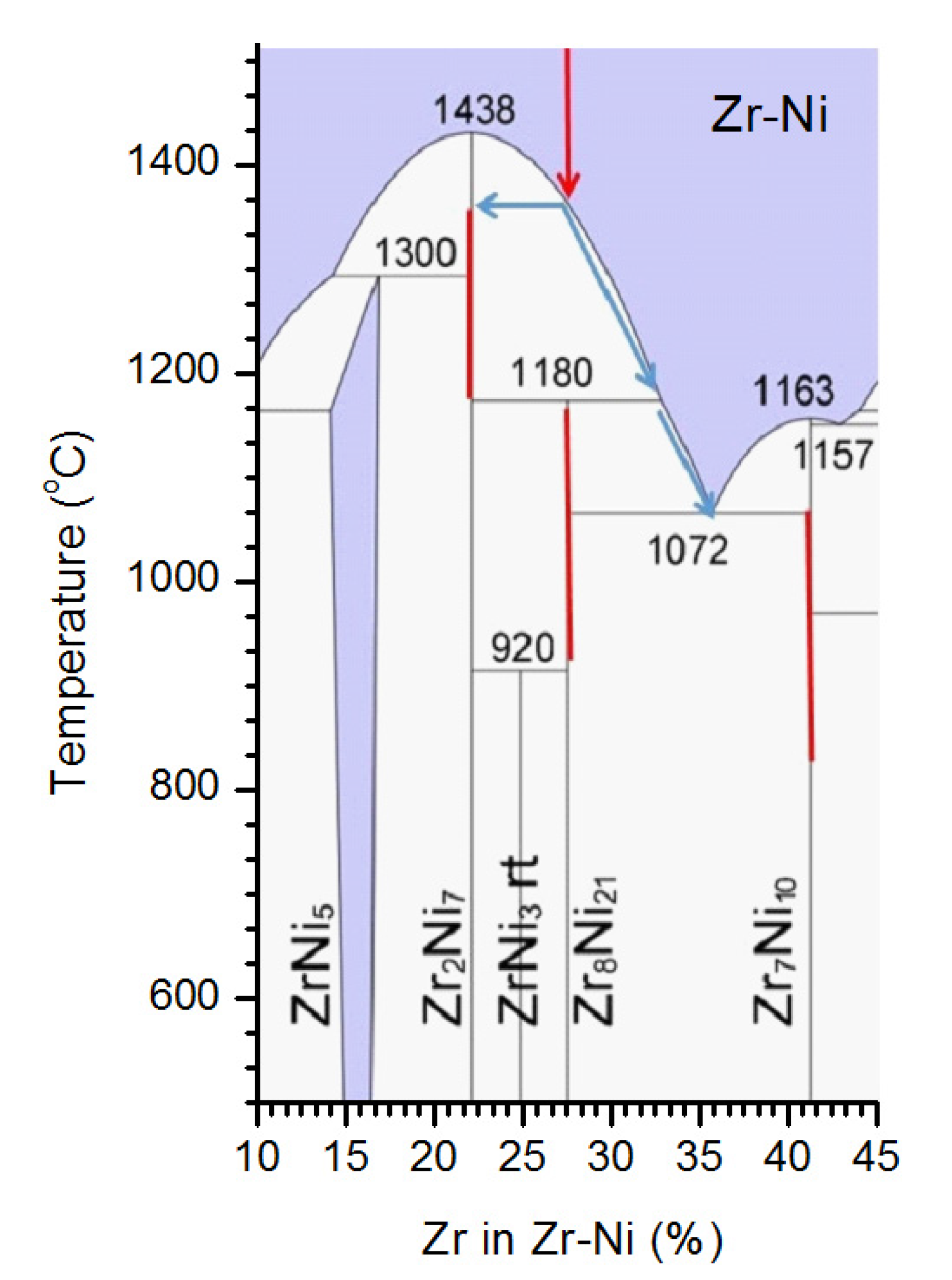
3.1. Phases, Microstructure, and Formation Path

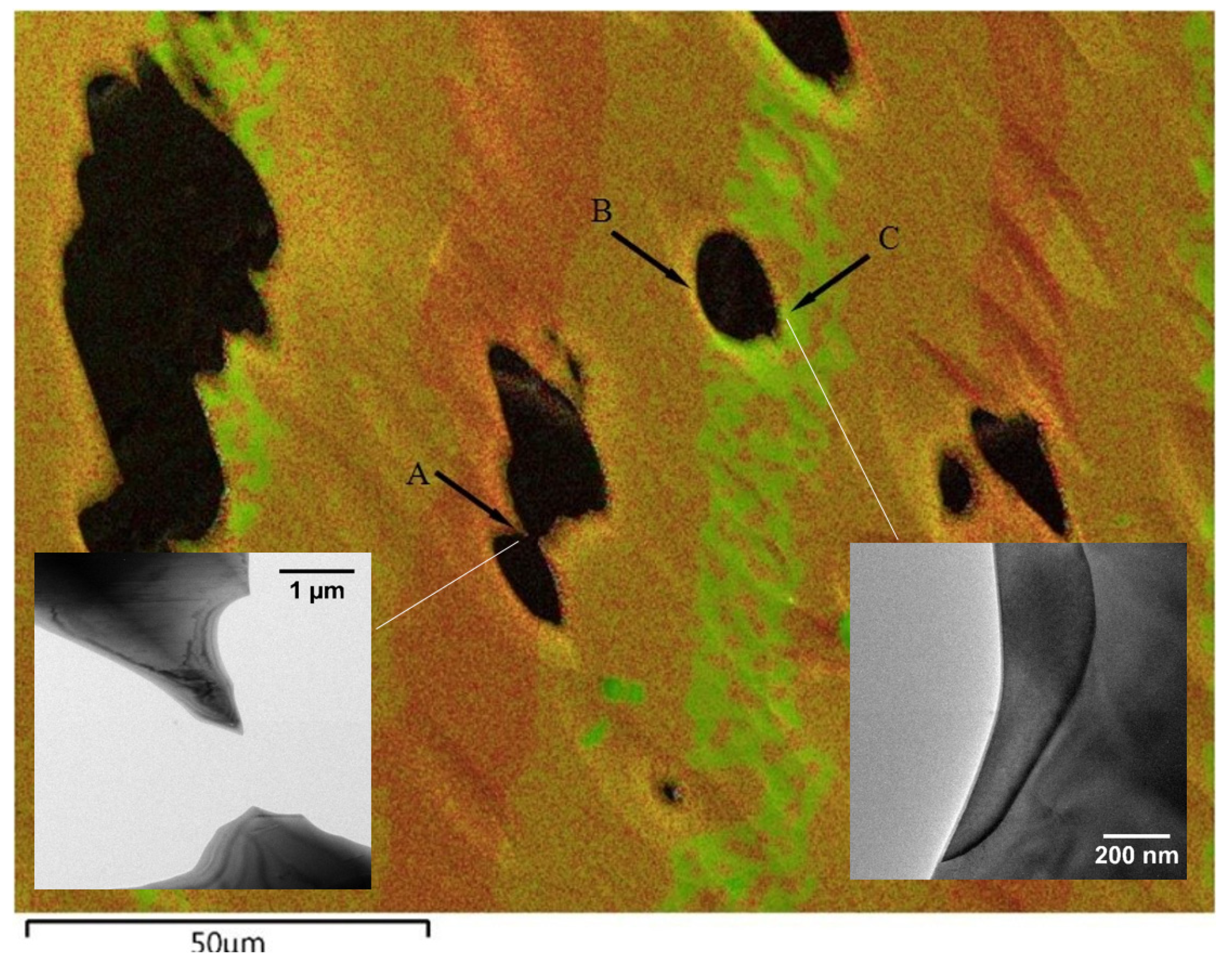
| Phases from Figure 2 | EDS Zr at% | EDS Ni at% | Corresponding Ni-Zr phases | Stoichiometric Ni at% |
|---|---|---|---|---|
| Spot A (Region 1) | 22 (±2) | 78 (±2) | Zr2Ni7 | 77.8 |
| Spot B (Region 2) | 27 (±2) | 73 (±2) | Zr8Ni21 | 72.7 |
| Spot C (Region 3) | 40 (±2) | 60 (±2) | Zr7Ni10 | 58.8 |
| Spot D | 93 (±2) | 7 (±2) | – | – |
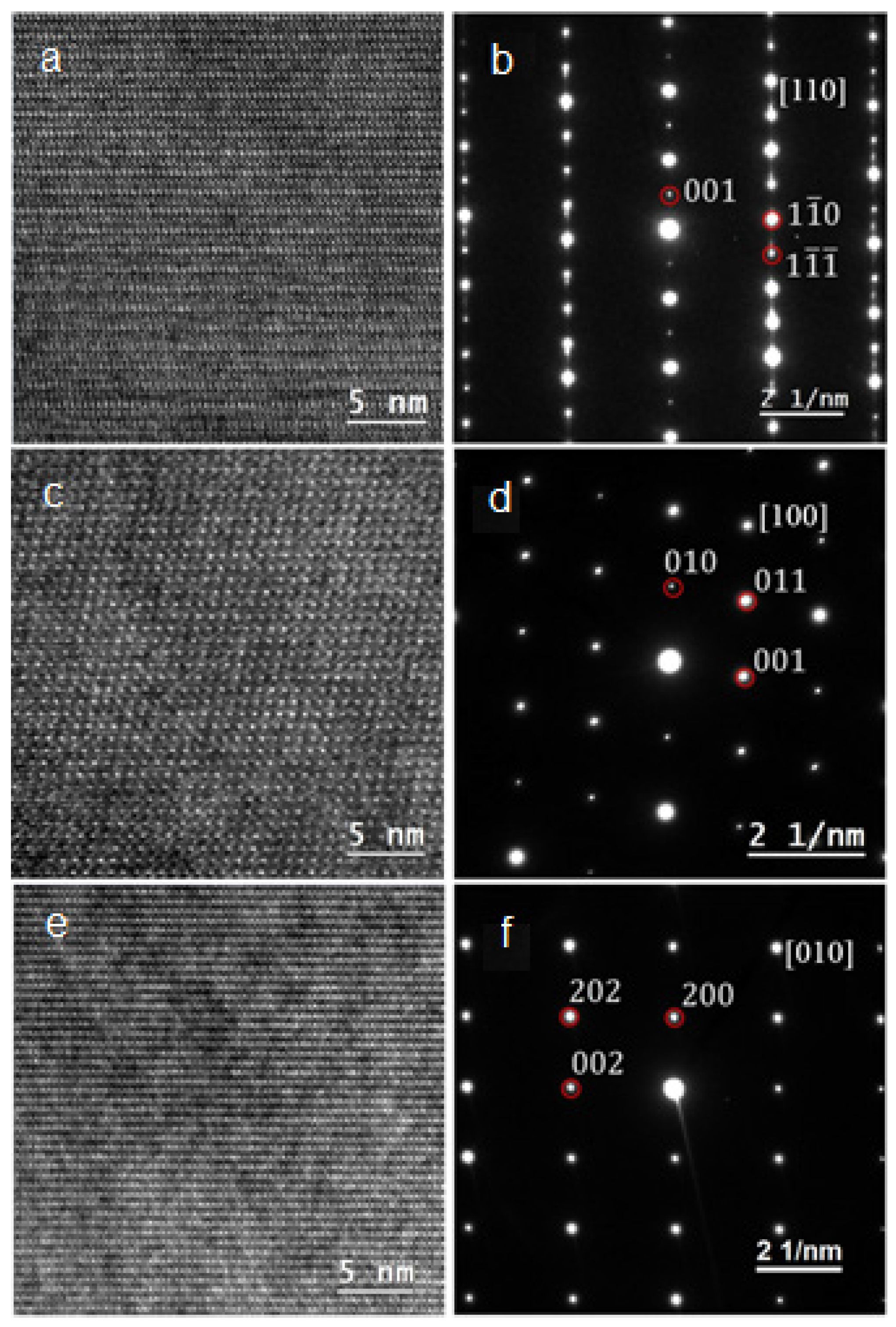
3.2. Defects and Crystallography of the Zr2Ni7 Phase
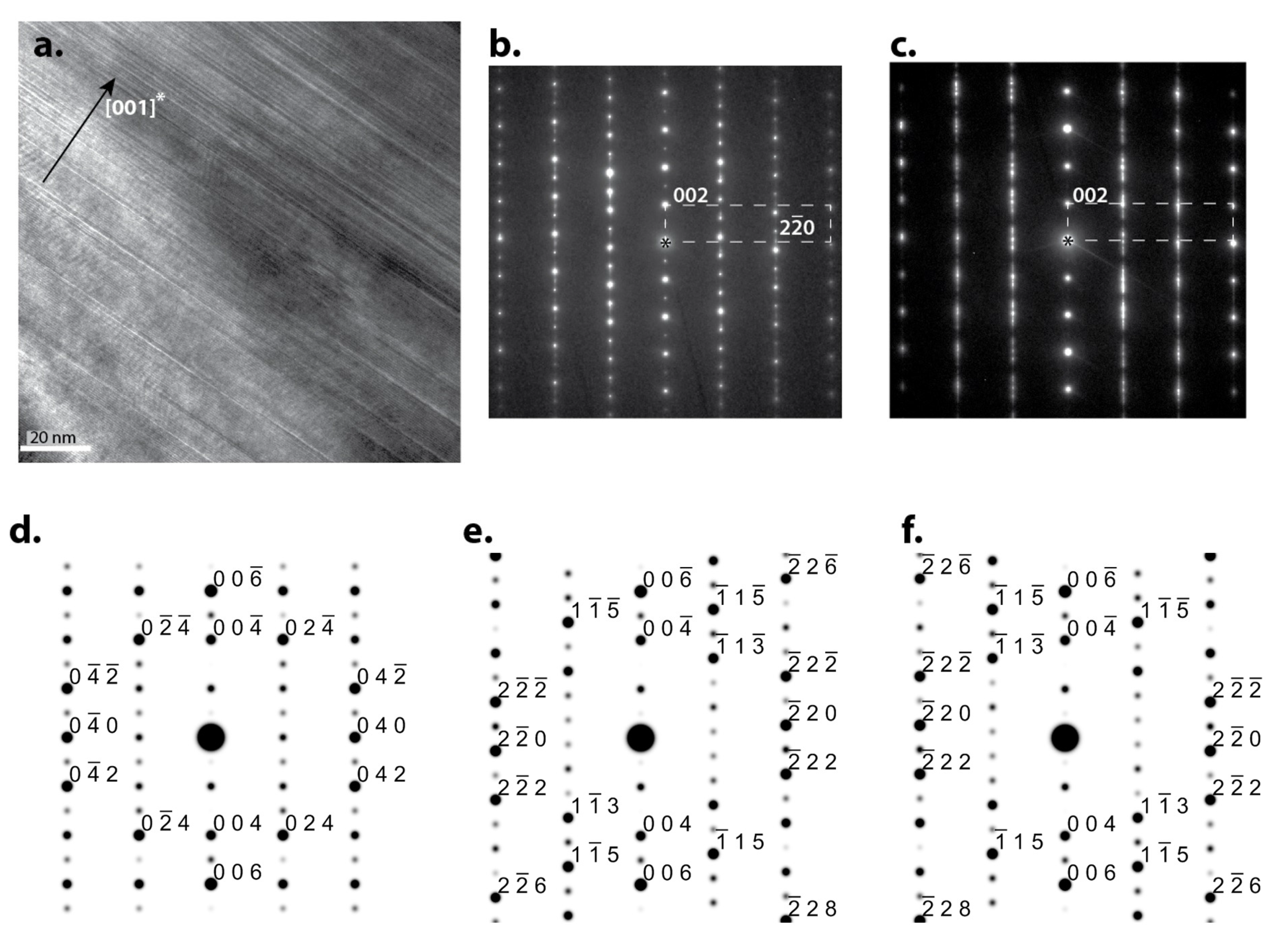
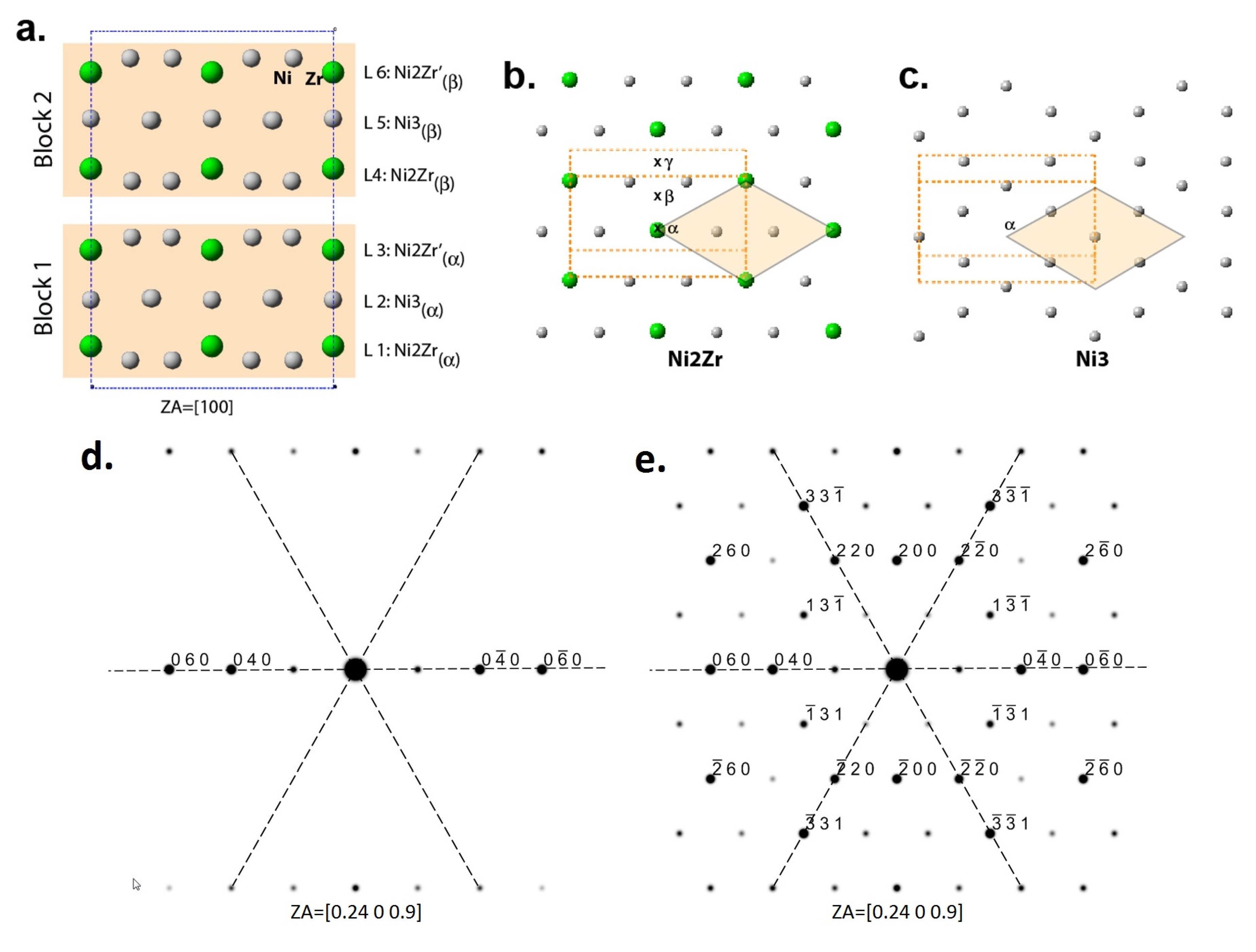
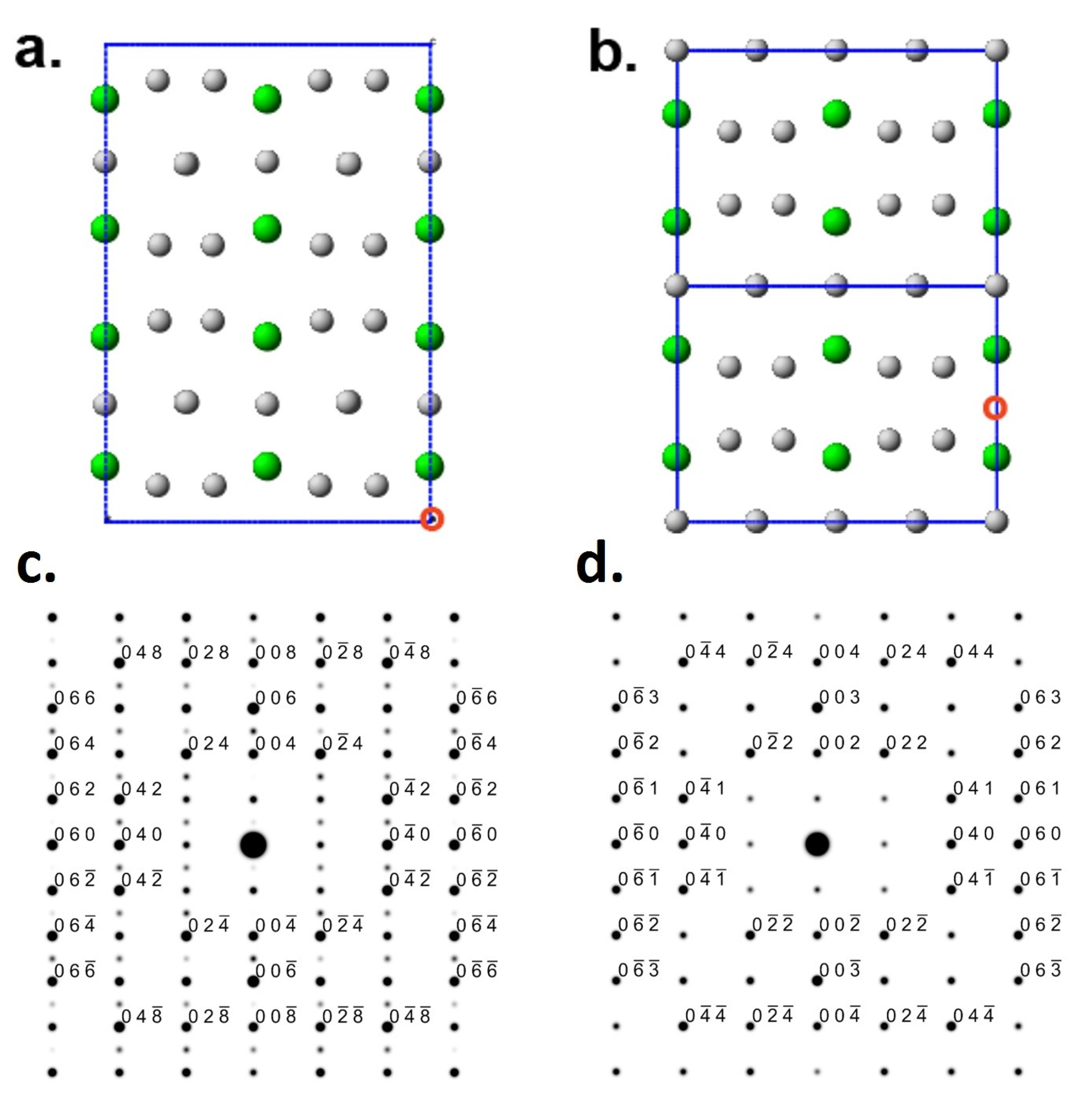
3.3. Software Simulation of Zr2Ni7 Fine Crystallites

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fetcenko, M.A.; Ovshinsky, S.R.; Young, K.; Reichman, B.; Fierro, C.; Koch, J.; Martin, F.; Mays, W.; Ouchi, T.; Sommers, B.; Zallen, A. High catalytic activity disordered VTiZrNiCrCoMnAlSn hydrogen storage alloys for nickel-metal hydride batteries. J. Alloys Compd. 2002, 330–332, 752–759. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. The role of Mn in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2009, 477, 749–758. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Yasuoka, S.; Ishida, J.; Nei, J.; Koch, J. Different failure modes for V-containing and V-free AB2 metal hydride alloys. J. Power Sources 2014, 251, 170–177. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Fetcenko, M.A. A nickel metal hdride battery for electric vehicles. J. Ross. Science 1993, 260, 176–181. [Google Scholar]
- Young, K.; Fetcenko, M.A.; Koch, J.; Morii, K.; Shimizu, T. Studies of Sn, Co, Al, and Fe additives in C14/C15 Laves alloys for NiMH battery application by orthogonal arrays. J. Alloys Compd. 2009, 486, 559–569. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Yang, J.; Fetcenko, M.A. Studies of off-stoichiometric AB2 metal hydride alloy: Part 1. Structural characteristics. Int. J. Hydrogen Energy 2011, 36, 11137–11145. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Young, K.; Young, M.; Chang, S.; Huang, B. Synergetic effects in electrochemical properties of ZrVxNi4.5−x (x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5) metal hydride alloys. J. Alloys Compd. 2013, 560, 33–41. [Google Scholar] [CrossRef]
- Young, M.; Chang, S.; Young, K.; Nei, J. Hydrogen storage properties of ZrVxNi3.5−x (x = 0.0–0.9) metal hydride alloys. J. Alloys Compd. 2013, 580, S171–S174. [Google Scholar] [CrossRef]
- Wright, R.B.; Jolley, J.G.; Owens, M.; Cocke, D.L. Catalytic hydrogenation activity of a ZrNi3 intermetallic alloy. J. Vac. Sci. Technol. 1987, A5, 586–589. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La and Hf): Structural characteristics. J. Alloys Compd. 2012, 516, 144–152. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La, and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrog. Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Yu, J.Y.; Lei, Y.Q.; Chen, C.P.; Wu, J.; Wang, Q.D. The electrochemical properties of hydrogen storage Zr-based Laves phase alloys. J. Alloys Compd. 1995, 231, 578–581. [Google Scholar] [CrossRef]
- Züttel, A.; Chartouni, D.; Nützenadel, C.; Gross, K.; Schlapbach, L. Bulk and surface properties of crystalline and amorphous Zr36(V0.33Ni0.66)64 alloy as active electrode material. J. Alloys Compd. 1998, 266, 321–326. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Liu, B. Strucural and electrochemical properties of TixZr7-xNi10. J. Alloys Compd. 2009, 480, 521–528. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Nei, J.; Fetcenko, M.A. Studies of Ti1.5Zr5.5V0.5(MxNi1−x)9.5 (M = Cr, Mn, Fe, Co, Cu, Al): Part 2. Hydrogen storage and electrochemical properties. J. Alloys Compd. 2010, 501, 245–254. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Nei, J.; Fetcenko, M.A. Studies of Ti1.5Zr5.5V0.5(MxNi1−x)9.5 (M = Cr, Mn, Fe, Co, Cu, Al): Part 1. Structural characteristics. J. Alloys Compd. 2010, 501, 236–244. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Castro, E.B.; Real, S.G.; Peretti, H.A.; Visintin, A.; Triaca, W.E. Electrochemical characterization of AB2 alloys used for negative electrodes in Ni/MH batteries. Int. J. Hydrogen Energy 2008, 33, 3576–3580. [Google Scholar] [CrossRef]
- Prina, M.; Bowman, R.C., Jr.; Kulleck, J.G. Degradation study of ZrNiH1.5 for use as actuators in gas gap heat switches. J. Alloys Compd. 2004, 373, 104–114. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Regmi, R.; Lawes, G.; Salley, S.O.; Ng, K.Y.S. Gaseous phase hydrogen storage and electrochemical properties of Zr8Ni21, Zr7Ni10, Zr9Ni11 and ZrNi metal hydride alloys. Int. J. Hydrogen Energy 2012, 37, 16042–16055. [Google Scholar] [CrossRef]
- Escobar, D.; Srinivasan, S.; Goswami, Y.; Stefanakos, E. Hydrogen storage behavior of ZrNi 70/30 and ZrNi 30/70 composites. J. Alloys Compd. 2008, 458, 223–230. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Castro, E.B.; Peretti, H.A.; Visintin, A. Study of the different ZrxNiy phases of Zr-based AB2 materials. Int. J. Hydrogen Energy 2010, 35, 9879–9887. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF) database; MSDS No. 04-002-9932; International Centre for Diffraction Data: Newtown Square, PA, USA, 2011.
- Jungbauer, M.; Hühn, S.; Egoavil, R.; Tan, H.; Verbeeck, J.; Van Tendeloo, G.; Moshnyaga, V. Atomic layer epitaxy of ruddlesden-popper SrO(SrTiO3)n films by means of metalorganic aerosol deposition. Appl. Phys. Lett. 2014, 105, 251603. [Google Scholar] [CrossRef]
- Burriel, M.; Casas-Cabanas, M.; Zapata, J.; Tan, H.; Verbeeck, J.; Solis, C.; Roqueta, J.; Skinner, S.J.; Kilner, J.A.; Van Tendeloo, G.; Santiso, J. Influence of the microstructure on the high-temperature transport properties of GdBaCo2O5.5+δ epitaxial films. Chem. Mater. 2010, 22, 5512–5520. [Google Scholar] [CrossRef]
- Tan, H.; Turner, S.; Yücelen, E.; Verbeeck, J.; Van Tendeloo, G. 2D atomic mapping of oxidation states in transition metal oxides by scanning transmission electron microscopy and electron energy-loss spectroscopy. Phys. Rev. Lett. 2011, 107, 107602. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, H.; Hadermann, J.; Abakumov, A.M.; Kalyuzhnaya, A.S.; Rozova, M.G.; Tsirlin, A.A.; Nath, R.; Tan, H.; Verbeeck, J.; Antipov, E.V.; et al. Synthesis, crystal structure and magnetic properties of the Sr2Al0.78Mn1.22O5.2 anion-deficient layered perovskite. J. Solid State Chem. 2009, 182, 356–363. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Tian, G.; Li, Y.; Tan, H.; Van Tendeloo, G.; Zhu, G.; Qiu, S.; Yang, X.; Su, B. Multimodal zeolite-beta-based catalysts with a hierarchical, three-level pore structure. ChemSusChem 2011, 4, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Egoavil, R.; Béché, A.; Martinez, G.T.; Van Aert, S.; Verbeeck, J.; Van Tendeloo, G.; Rotella, H.; Boullay, P.; Pautrat, A.; et al. Mapping electronic reconstruction at the metal-insulator interface in LaVO3/SrVO3 heterostructures. Phys. Rev. B 2013, 88, 155123. [Google Scholar] [CrossRef]
- Eshelman, F.R.; Smith, J.F. The structure of Zr2Ni7. Acta Cryst. 1972, B28, 1594–1600. [Google Scholar] [CrossRef]
- Bsenko, L. The HfNi and ZrNi systems in the region 65–80 at.% Ni. L. J. Less Comm. Met. 1979, 63, 171–179. [Google Scholar] [CrossRef]
- Joubert, J.M.; Cerný, R.; Yvon, K.; Latroche, M.; Percheron-Guégan, A. Zirconium-Nickel, Zr7Ni10: Space group revision for the stoichiometric phase. Acta Cryst. 1997, C53, 1536–1538. [Google Scholar] [CrossRef]
- Parthé, E.; Lemaire, R. Structure block stacking in intermetallic compounds. I. The rhombohedral-hexagonal Mn+1X5n-1 and the monoclinic-hexagonal-trigonal-orthorhombic Mn+1X5n+2 structure series. Acta Cryst. 1975, B31, 1879–1889. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Bendersky, L.A.; Young, K.; Nei, J. Fine Structure in Multi-Phase Zr8Ni21-Zr7Ni10-Zr2Ni7 Alloy Revealed by Transmission Electron Microscope. Materials 2015, 8, 4618-4630. https://doi.org/10.3390/ma8074618
Shen H, Bendersky LA, Young K, Nei J. Fine Structure in Multi-Phase Zr8Ni21-Zr7Ni10-Zr2Ni7 Alloy Revealed by Transmission Electron Microscope. Materials. 2015; 8(7):4618-4630. https://doi.org/10.3390/ma8074618
Chicago/Turabian StyleShen, Haoting, Leonid A. Bendersky, Kwo Young, and Jean Nei. 2015. "Fine Structure in Multi-Phase Zr8Ni21-Zr7Ni10-Zr2Ni7 Alloy Revealed by Transmission Electron Microscope" Materials 8, no. 7: 4618-4630. https://doi.org/10.3390/ma8074618






