A Novel Technique for the Connection of Ceramic and Titanium Implant Components Using Glass Solder Bonding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Specimens
2.2. Bonding Process

2.3. Storage Regimes and Mechanical Testing

2.4. Fractographic Analysis
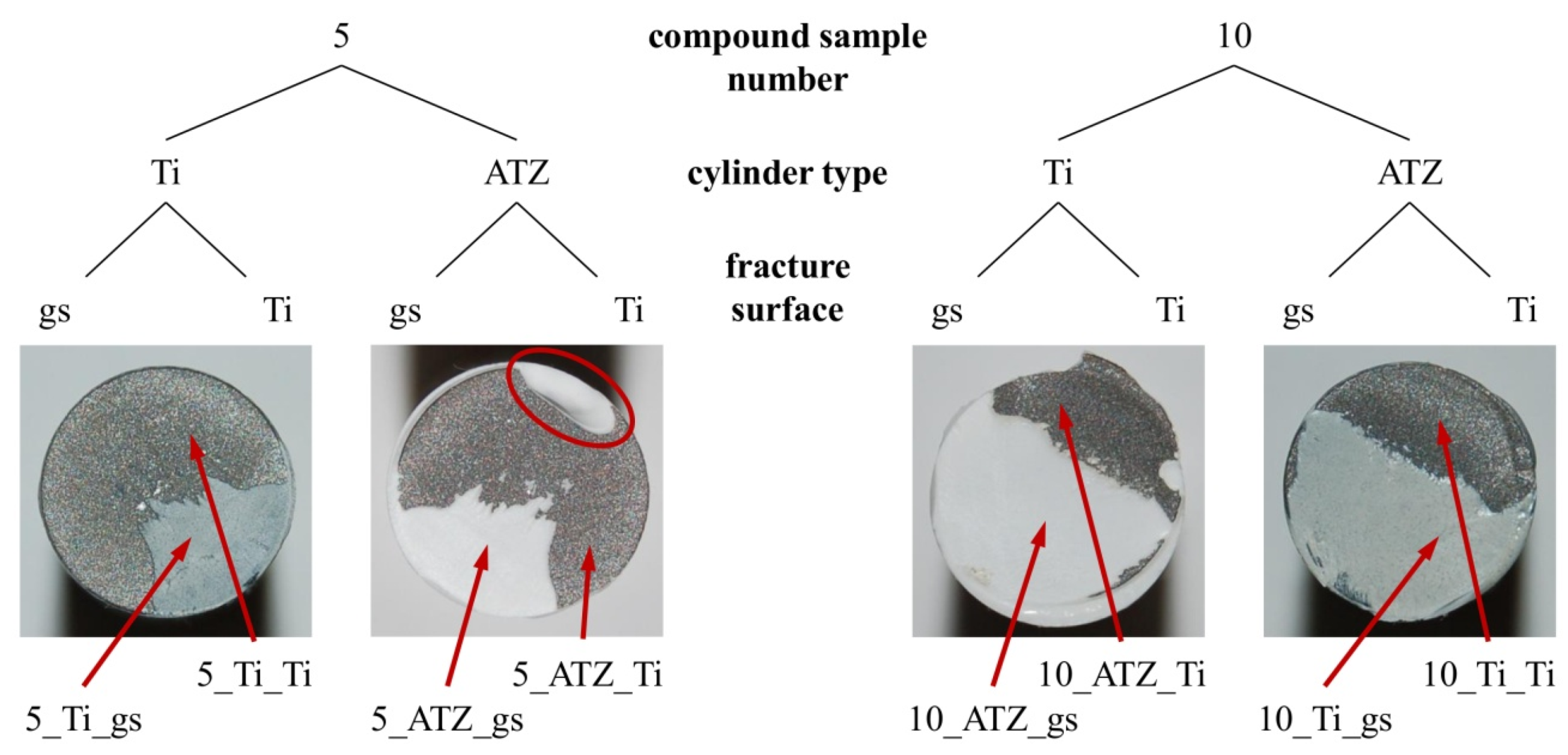
3. Results and Discussion
3.1. Mechanical Testing
| Storage regime | Glass solder M (SD) [MPa] | Adhesive glue M (SD) [MPa] | Mean differences (95% CI) [MPa] |
|---|---|---|---|
| (a) | 118 (33) | 30 (4) | 88 (67–110) |
| (b) | 104 (40) | 17 (7) | 87 (66–108) |
| (c) | 117 (36) | 13 (2) | 104 (82–125) |
| (d) | 119 (23) | 18 (1) | 101 (80–122) |

3.2. Fractographic Analysis
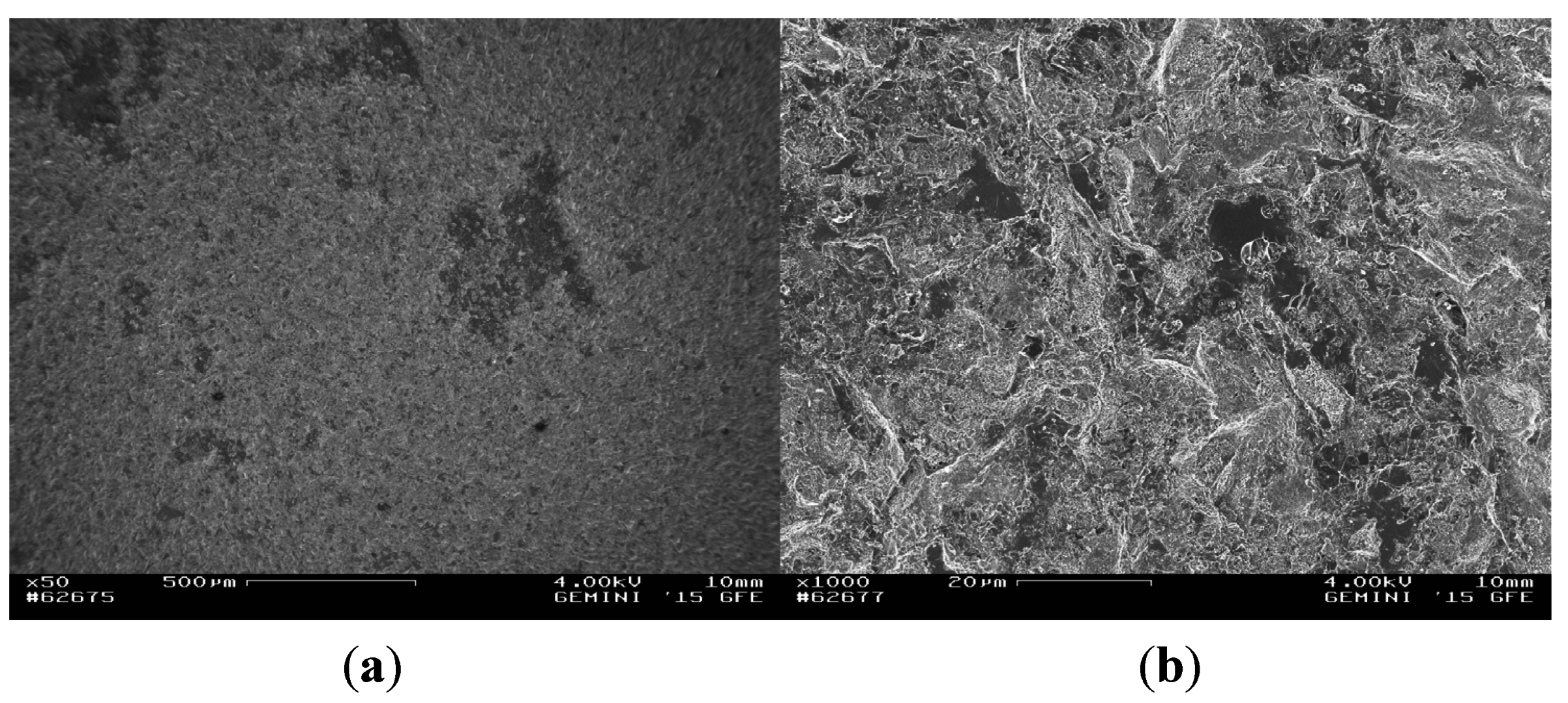
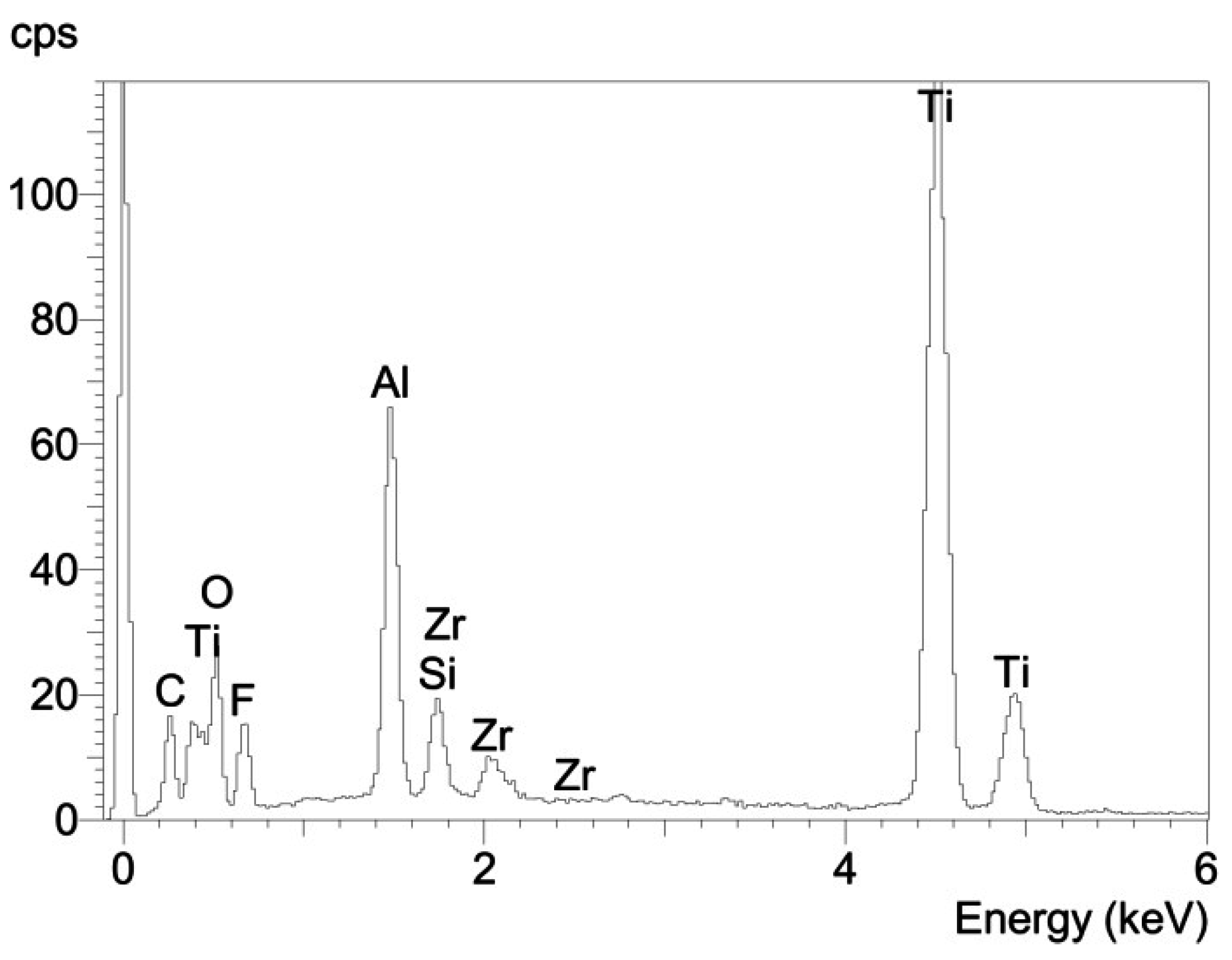

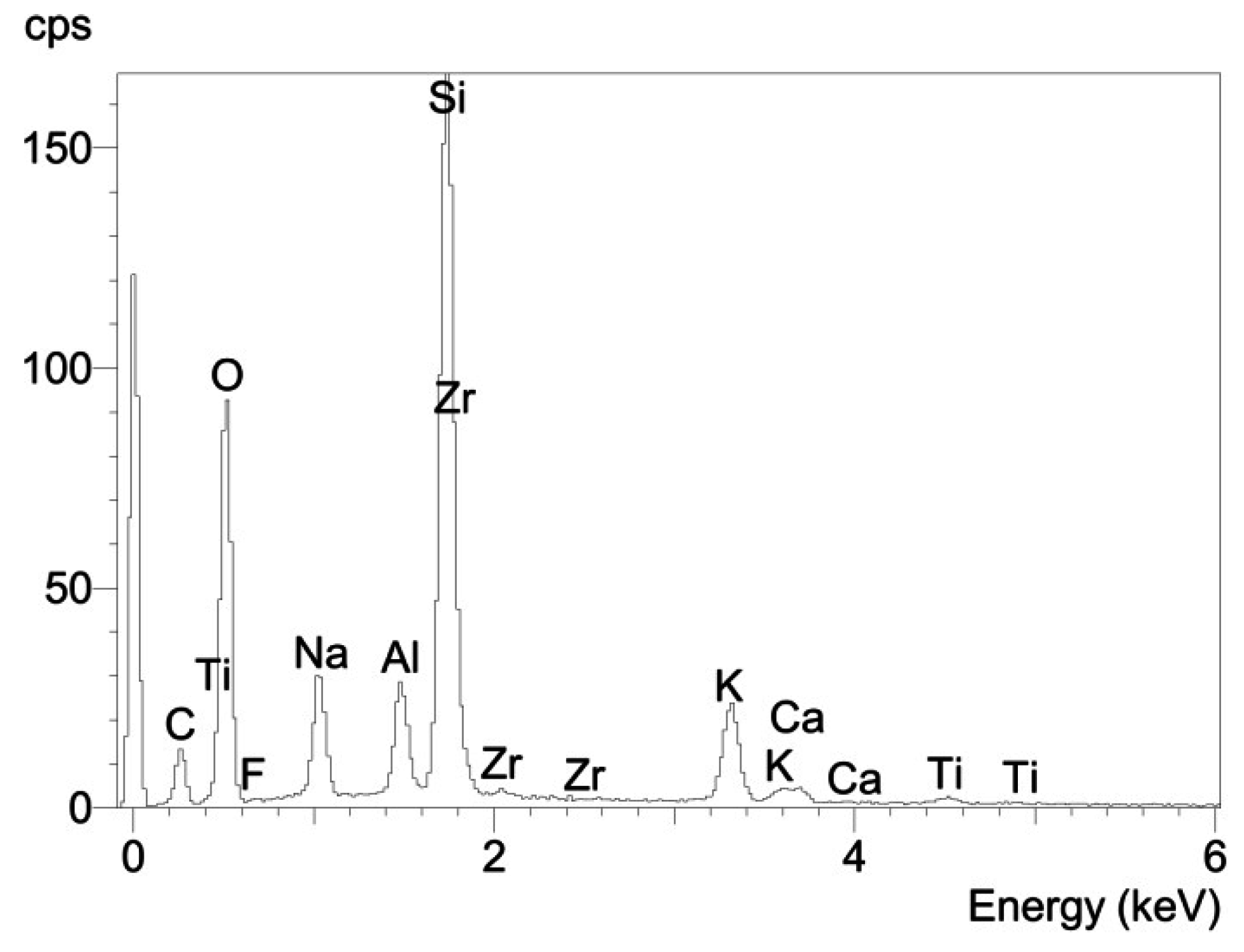
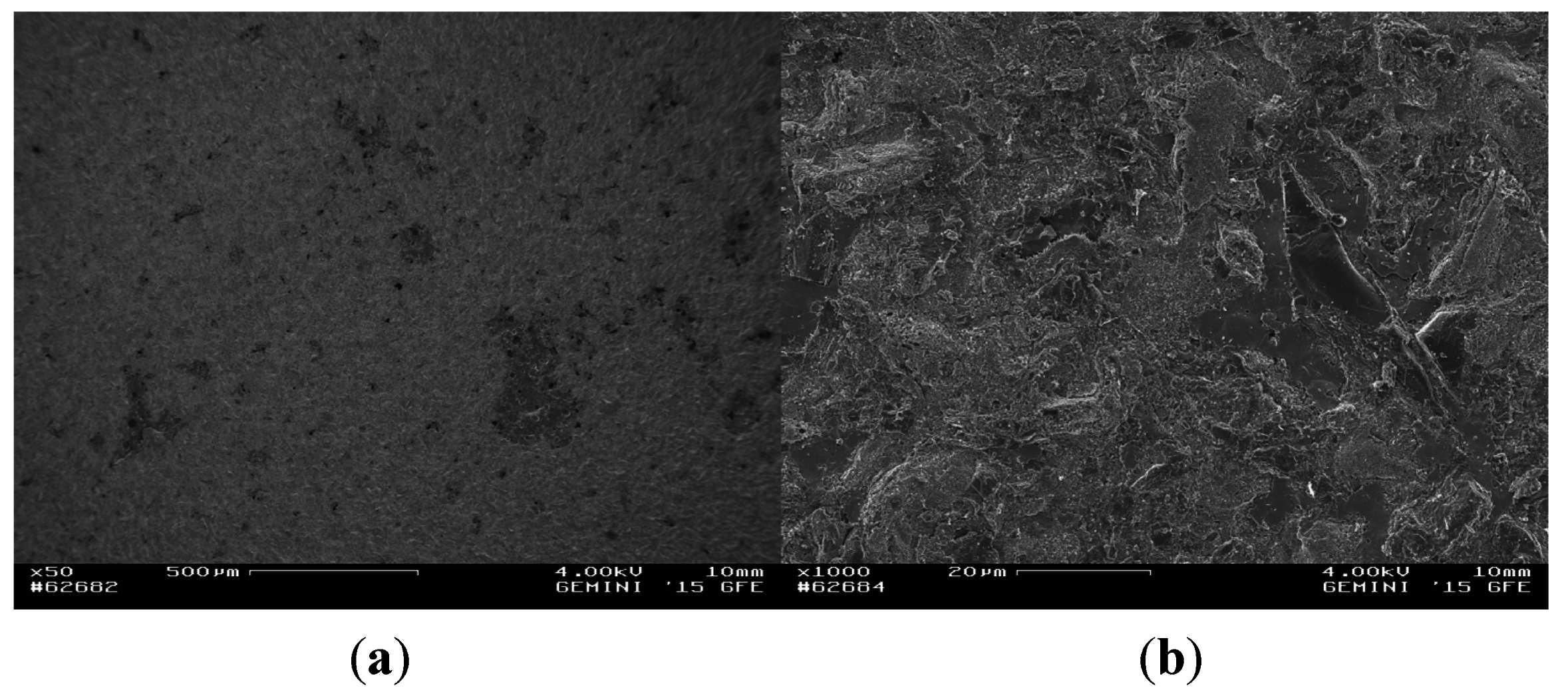
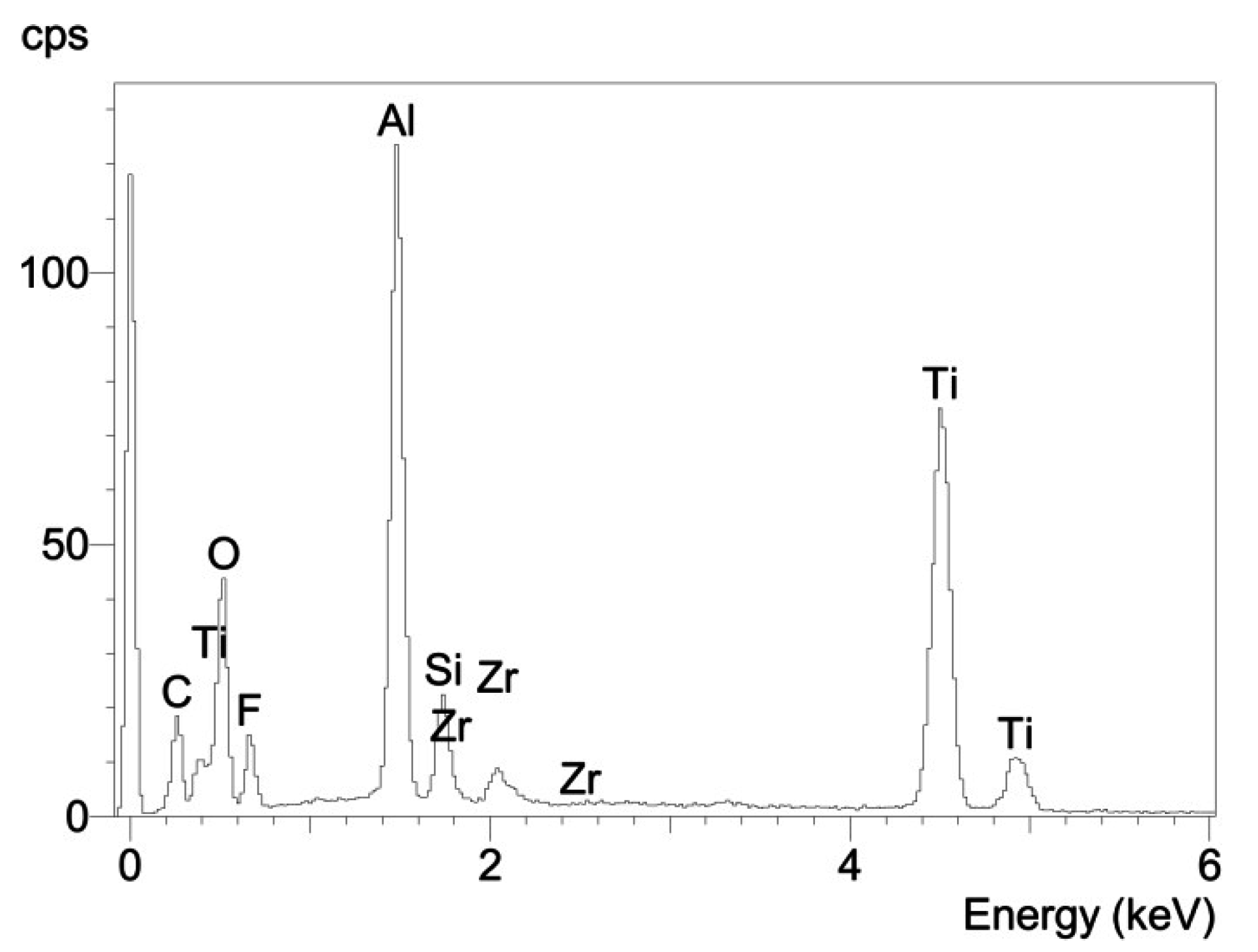


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, P.; Laino, L.; La Noce, M.; Piattelli, A.; De Rosa, A.; Iezzi, G.; Laino, G.; Paino, F.; Papaccio, G.; Tirino, V. Surface biocompatibility of differently textured titanium implants with mesenchymal stem cells. Dent. Mater. 2015, 31, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Kuebler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Le Guehennec, L.; Lopez-Heredia, M.A.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Baechle, M.; Han, J.S.; Hueren, D.; Huebner, U.; Butz, F. In vitro reaction of human osteoblasts on alumina-toughened zirconia. Clin. Oral Implant. Res. 2009, 20, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. Bristol. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.; Bergschmidt, P.; Fritsche, A.; Ansorge, S.; Thomas, P.; Mittelmeier, W. Alternative materials and solutions in total knee arthroplasty for patients with metal allergy. Orthopade 2008, 37, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bankoglu Güngör, M.; Aydin, C.; Yilmaz, H.; Gül, E.B. An Overview of zirconia dental implants: Basic properties and clinical application of three cases. J. Oral Implantol. 2014, 40, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Burtscher, D.; Grunert, I.; Kniha, H.; Steinhauser, E. Failure analysis of fractured dental zirconia implants. Clin. Oral Implant. Res. 2012, 23, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.F.A.; Sailer, I.; Zhang, Y.; Coelho, P.G.; Guess, P.C.; Zembic, A.; Kohal, R.J. Performance of zirconia for dental healthcare. Materials 2010, 3, 863–896. [Google Scholar] [CrossRef]
- Atabaki, M. Recent progress in joining of ceramic powder metallurgy products to metals. Metall. Mater. Eng. 2010, 16, 255–268. [Google Scholar]
- Takahashi, M.; Okabe, N.; Zhu, X.; Kagawa, K. Strength estimation of ceramic-metal joints with various interlayer thickness. Fatigue Fract. Eng. Mater. Struct. 2003, 26, 391–398. [Google Scholar] [CrossRef]
- Lemus-Ruiz, J.; Guevara-Laureano, A.O.; Zarate-Medina, J.; Arellano-Lara, A.; Ceja-Cárdenas, L. Interface behavior of Al2O3/Ti joints produced by liquid state bonding. Appl. Radiat. Isotop. 2015, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Dinu, M.; Braic, M.; Vitelaru, C.; Balaceanu, M.; Tarcolea, M.; Braica, V.; Baciub, F.; Cotrutb, C.M. The effect of TiSiN interlayers on the bond strength of ceramic to NiCr and CoCr alloys. Ceram Int. 2015, in press. [Google Scholar] [CrossRef]
- Wang, W.; Fan, D.; Huang, J.; Cui, B.; Chen, S.; Zhao, X. A new partial transient liquid-phase bonding process with powder-mixture interlayer for bonding Cf/SiC composite and Ti-6Al-4V alloy. Mater. Lett. 2015, 143, 237–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, D.; He, Z.Y.; Chen, X.C. Progress in joining ceramics to metals. J. Iron Steel Res. Int. 2006, 13, 1–5. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E. The effect of oral-like environment on dental implants’ fatigue performance. Clin. Oral Implant. Res. 2014, 25, e166–e170. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Zothner, A. Dentalimplantat. Patent DE102011015299A1, 27 September 2012. [Google Scholar]
- Mick, E.; Markhoff, J.; Mitrovic, A.; Jonitz, A.; Bader, R. New coating technique of ceramic implants with different glass solder matrices for improved osseointegration-mechanical investigations. Materials 2013, 6, 4001–4010. [Google Scholar] [CrossRef]
- Markhoff, J.; Mick, E.; Mitrovic, A.; Pasold, J.; Wegner, K.; Bader, R. Surface modifications of dental ceramic implants with different glass solder matrices: In vitro analyses with human primary osteoblasts and epithelial cells. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Weisse, B.; Affolter, C.; Terrasi, G.P.; Piskoty, G.; Koebel, S. Failure analysis of in vivo fractured ceramic femoral heads. Eng. Fail. Anal. 2009, 16, 1188–1194. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mick, E.; Tinschert, J.; Mitrovic, A.; Bader, R. A Novel Technique for the Connection of Ceramic and Titanium Implant Components Using Glass Solder Bonding. Materials 2015, 8, 4287-4298. https://doi.org/10.3390/ma8074287
Mick E, Tinschert J, Mitrovic A, Bader R. A Novel Technique for the Connection of Ceramic and Titanium Implant Components Using Glass Solder Bonding. Materials. 2015; 8(7):4287-4298. https://doi.org/10.3390/ma8074287
Chicago/Turabian StyleMick, Enrico, Joachim Tinschert, Aurica Mitrovic, and Rainer Bader. 2015. "A Novel Technique for the Connection of Ceramic and Titanium Implant Components Using Glass Solder Bonding" Materials 8, no. 7: 4287-4298. https://doi.org/10.3390/ma8074287





