Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility
Abstract
:1. Introduction
2. Results and Discussion
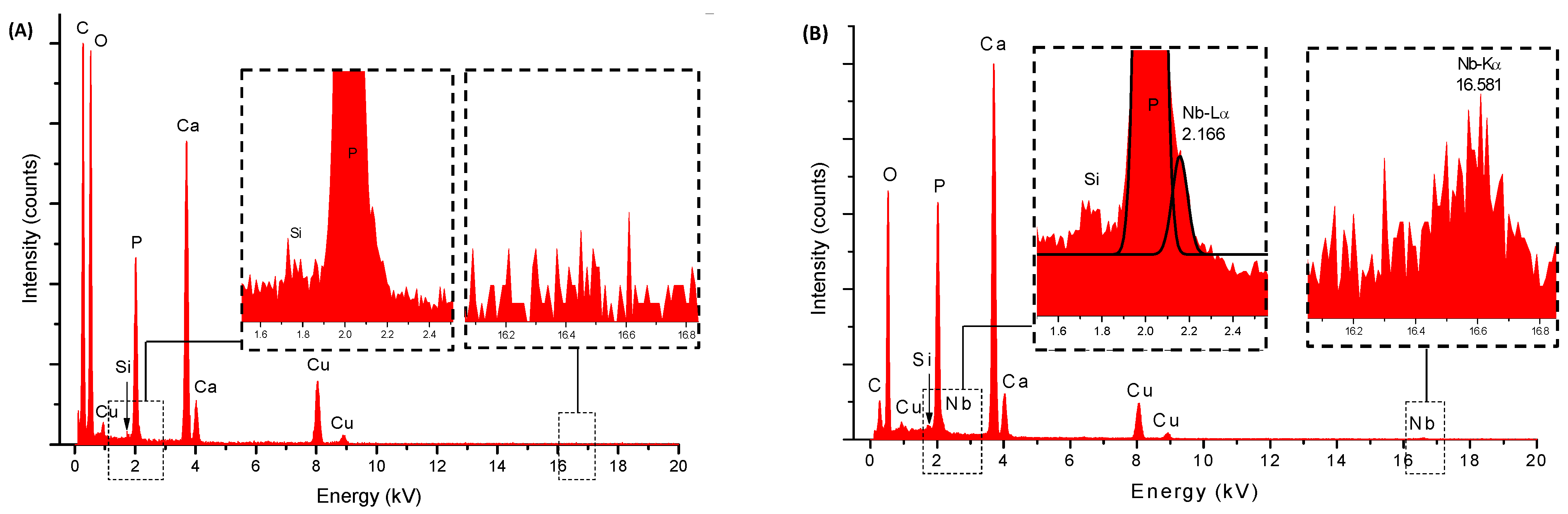
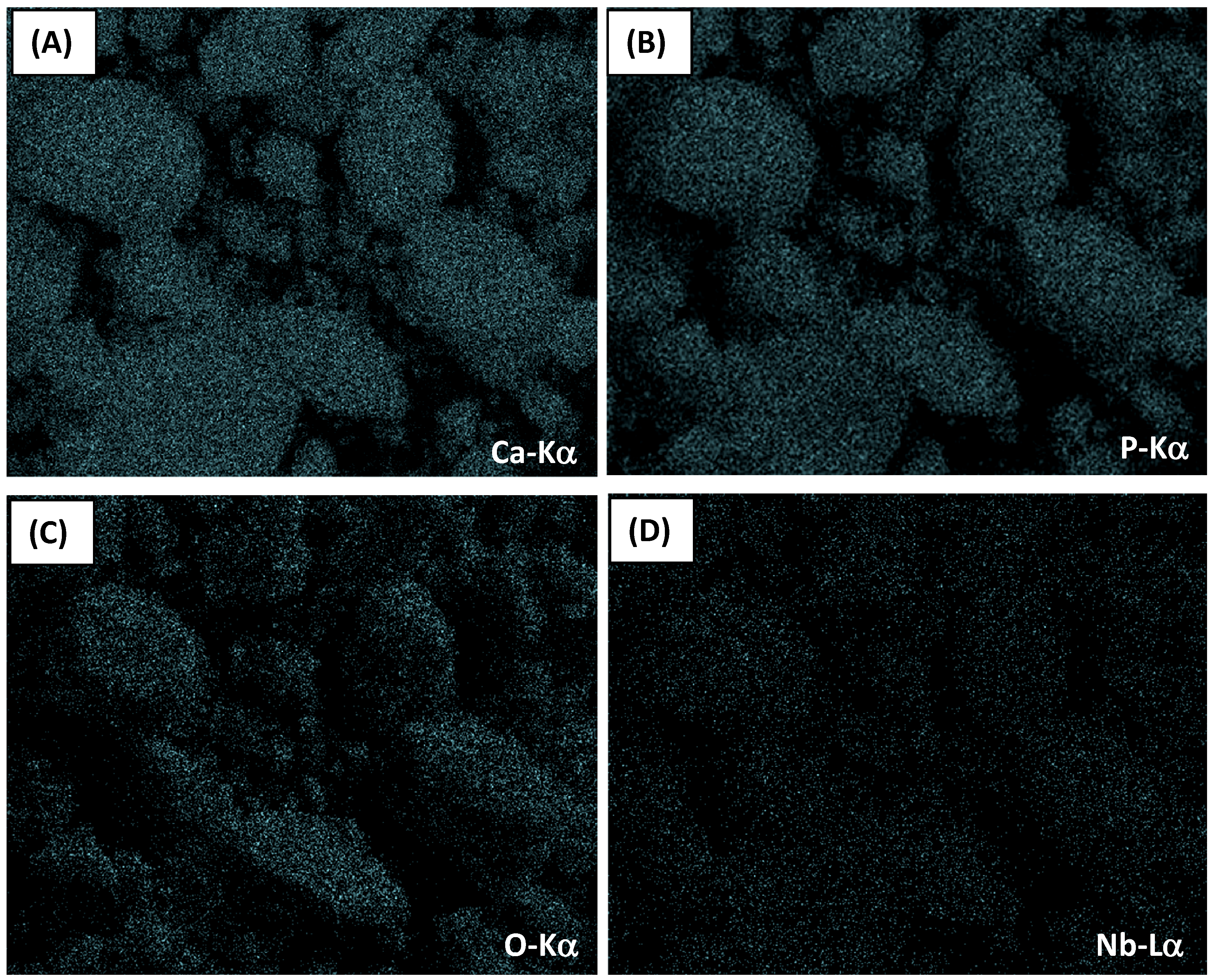
2.1. Energy Dispersive X-ray Spectroscopy Analysis (EDX) and Wavelength Dispersive X-ray Fluorescence Spectrometry (WD-XRF)

2.2. X-ray Diffraction (XRD) Analysis
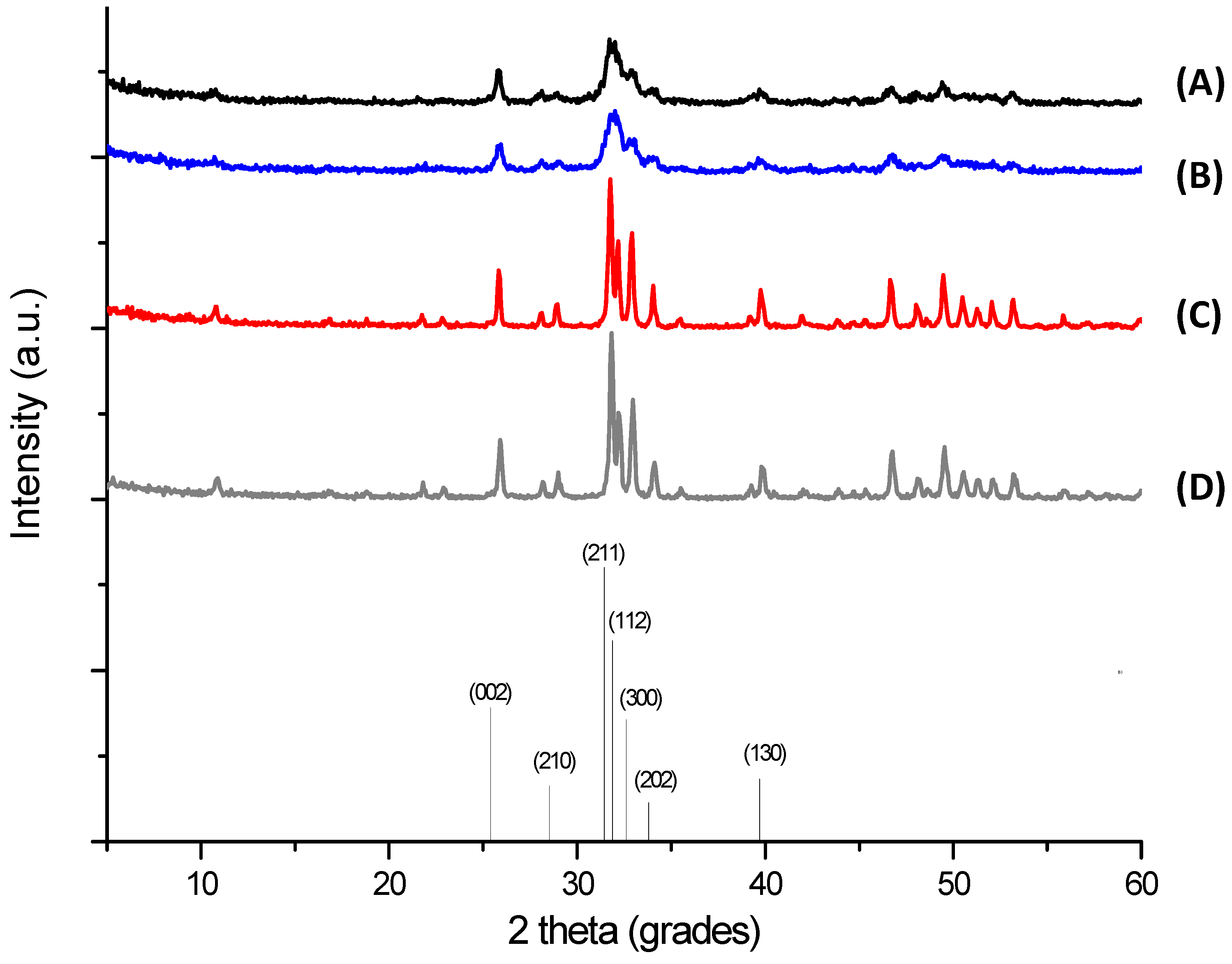
| Sample | Crystallite size [Å] * | Lattice parameters [Å] * | Unit cell volume [Å3] | |
|---|---|---|---|---|
| - | - | a = b | c | - |
| HA_110 | 94.7 ± 4.1 | - | - | - |
| Nb-HA_110 | 86.9 ± 3.4 | - | - | - |
| HA_900 | 365.3 ± 6.3 | 9.419 ± 0.004 | 6.874 ± 0.003 | 528.1 |
| Nb-HA_900 | 365.3 ± 6.5 | 9.432 ± 0.006 | 6.892 ± 0.000 | 531.0 |
2.3. Thermal Analysis


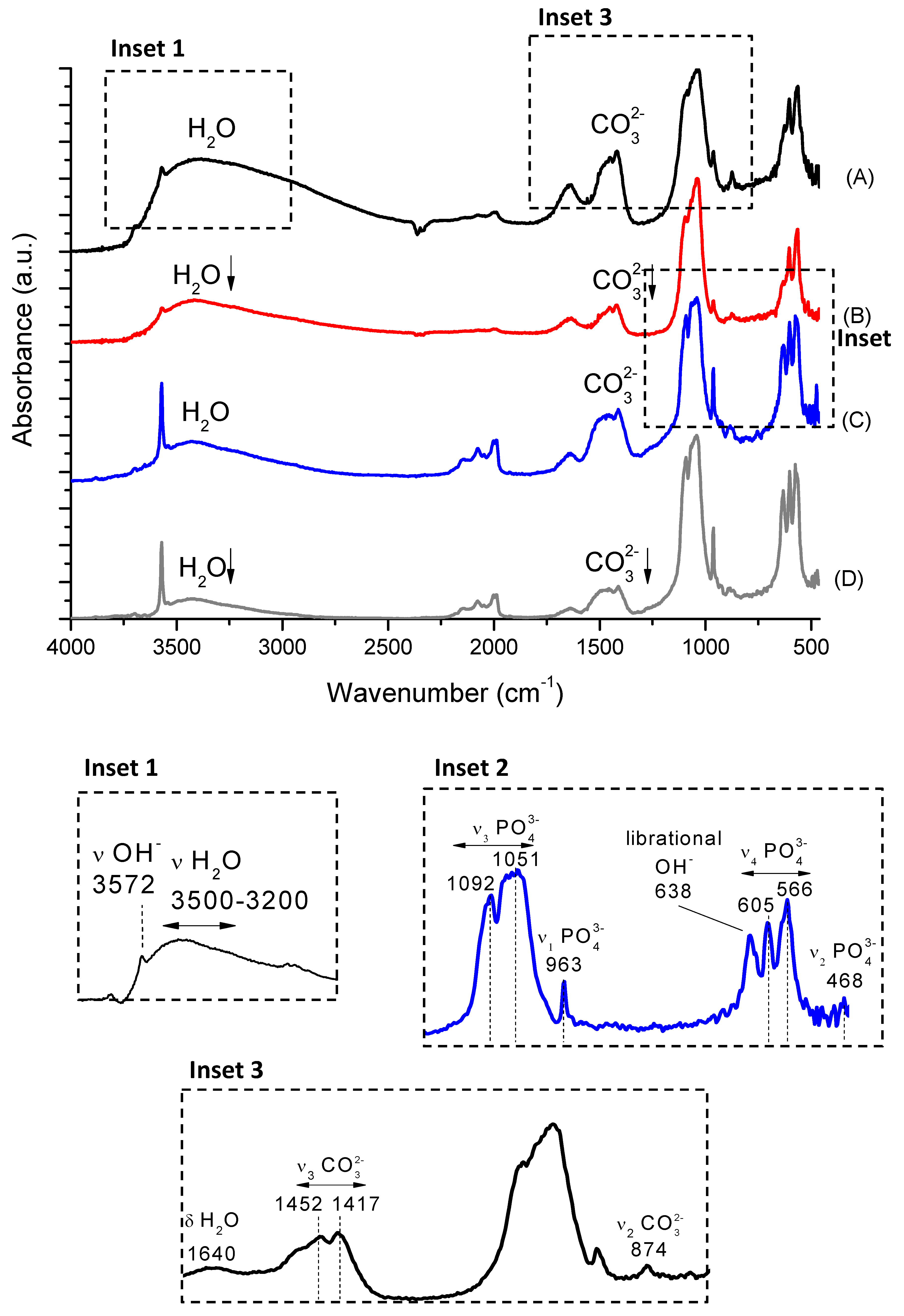
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
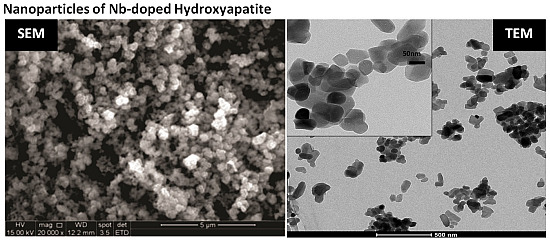
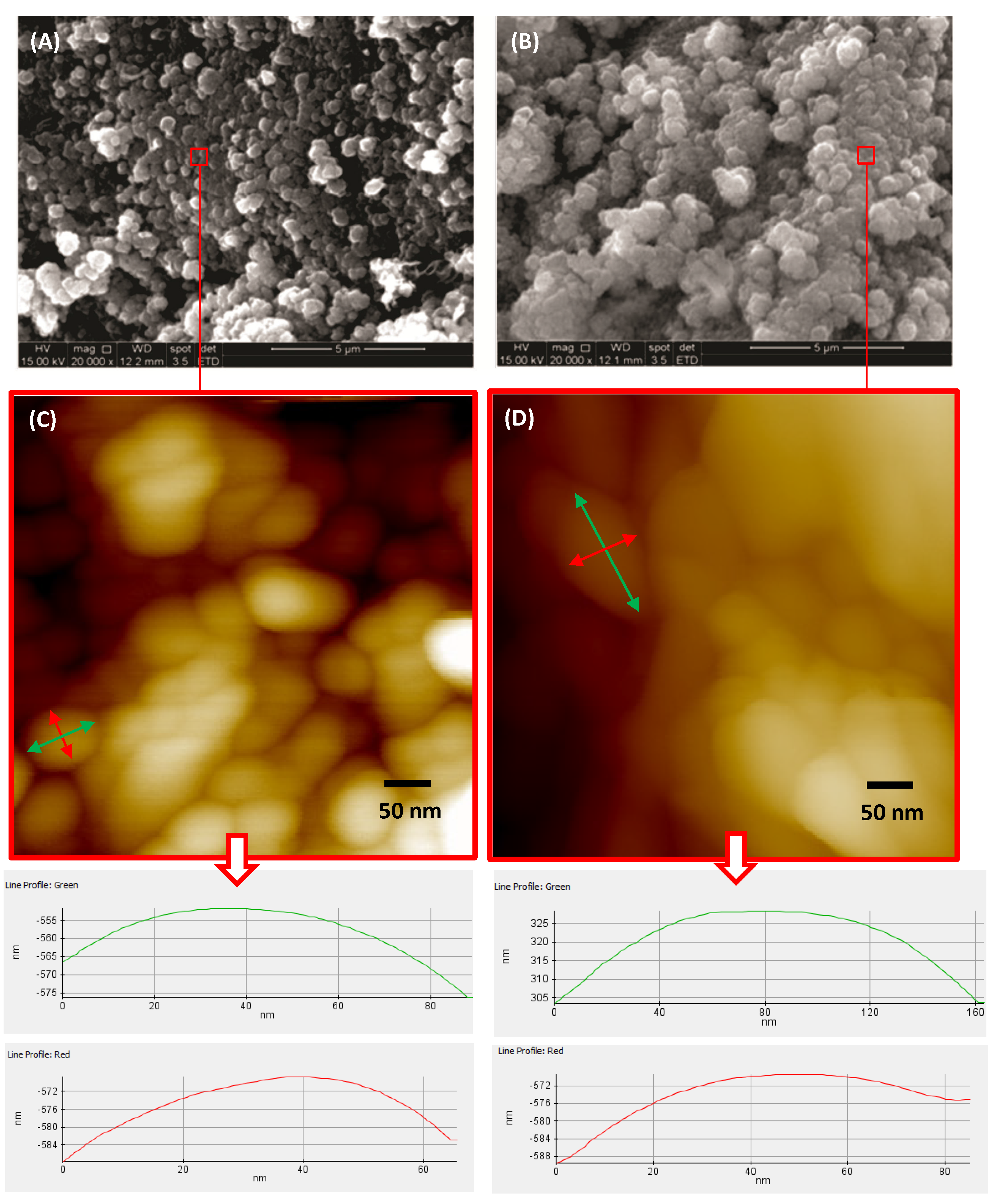
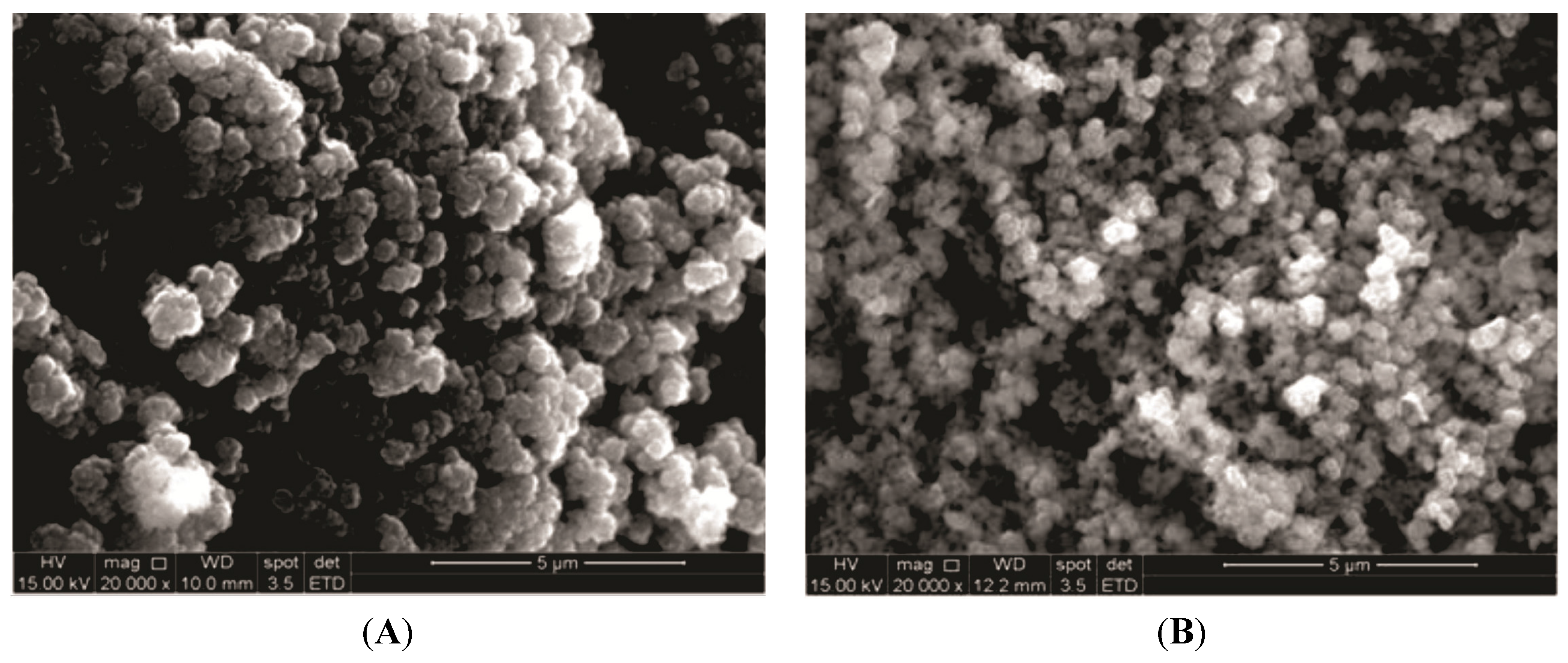
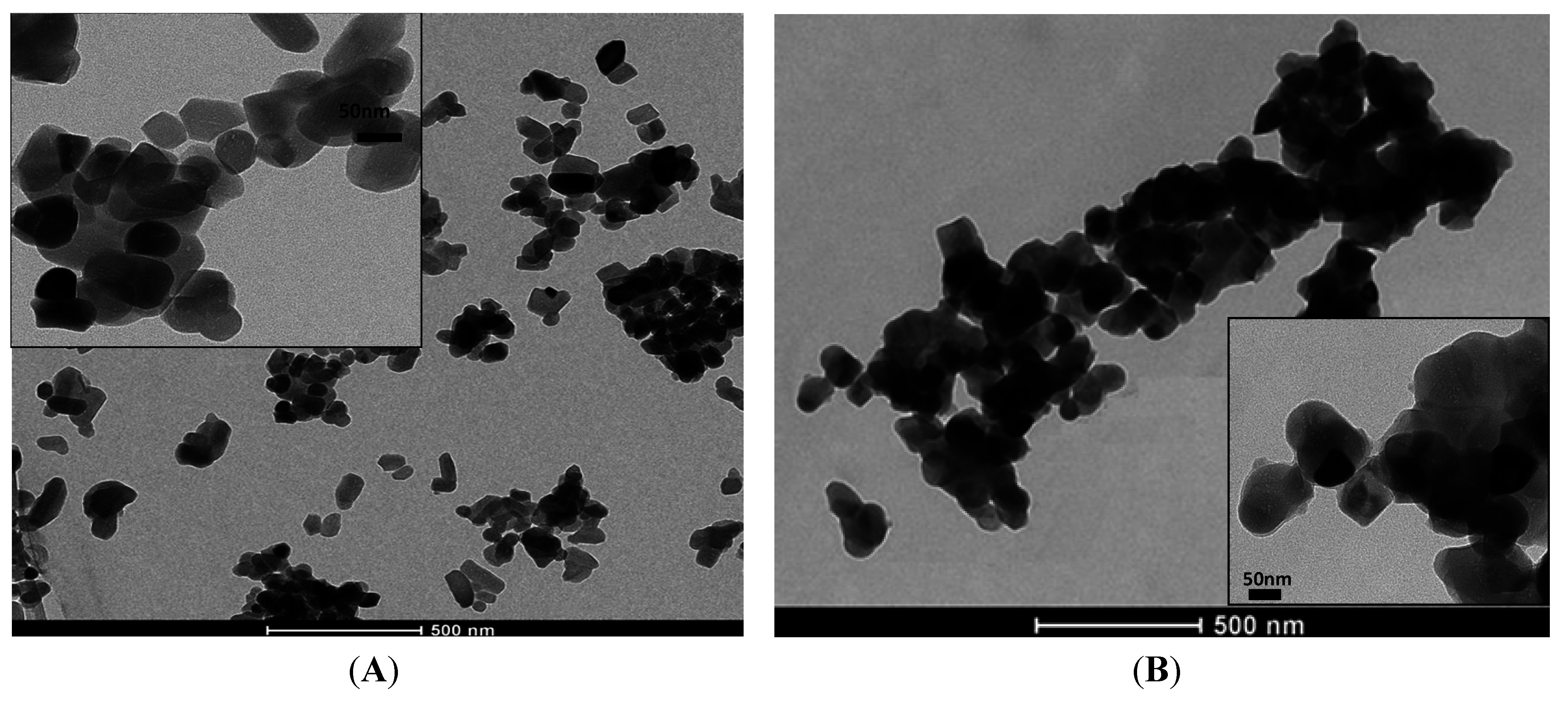
2.5. Morphological Analysis
2.6. Brunauer–Emmett–Teller (BET) Method
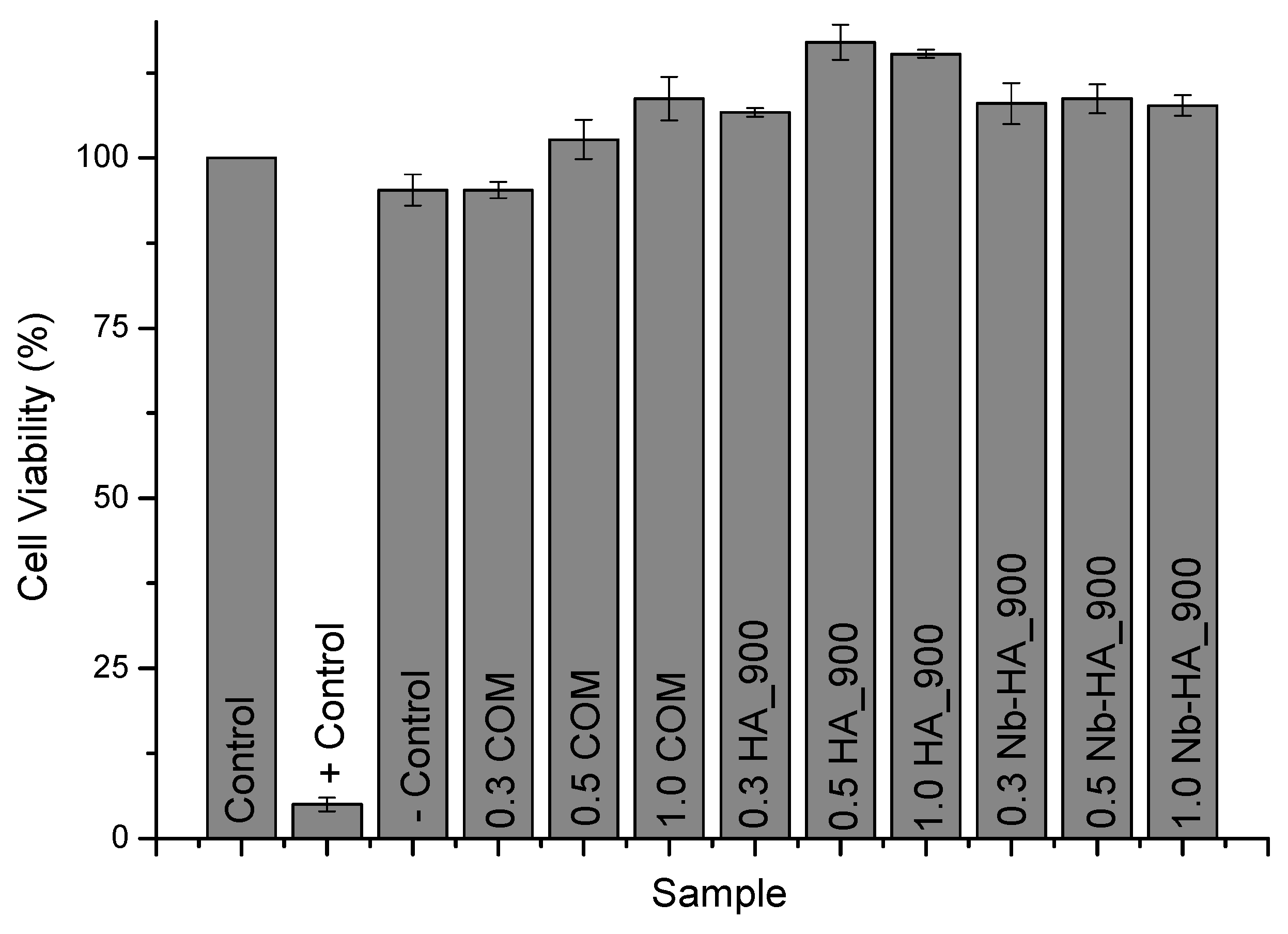
2.7. In Vitro Cytocompatibility Assays

3. Experimental Section
3.1. Materials
3.2. Synthesis of Calcium Phosphate
3.3. Characterization of Calcium Phosphates
3.4. In Vitro Characterization Assays by MTT and Resazurin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J. R. Soc. Interface 2009, 6, S349–S360. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Costa, H.S. Nanostructured poly(vinyl alcohol)/bioactive glass and poly (vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem. Eng. J. 2008, 137, 72–83. [Google Scholar] [CrossRef]
- Santos, M.H.; Heneine, L.G.D.; Mansur, H.S. Synthesis and characterization of calcium phosphate/collagen biocomposites doped with Zn2+. Mater. Sci. Eng. C 2008, 28, 563–571. [Google Scholar] [CrossRef]
- Mansur, H.S.; Costa, H.S.; Mansur, A.A.P.; Pereira, M. 3D-macroporous hybrid scaffolds for tissue engineering: Network design and mathematical modeling of the degradation kinetics. Mater. Sci. Eng. C 2012, 32, 404–415. [Google Scholar] [CrossRef]
- Reddi, A.H.; Wientroub, S.; Muthukumaran, N. Biologic principles of bone induction. Orthop. Clin. N. Am. 1987, 18, 207–212. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3–56. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.S.; Mansur, A.A.P.; Barbosa-Stancioli, E.F.; Pereira, M.M.; Mansur, H.S. Morphological, mechanical, and biocompatibility characterization of macroporous alumina scaffolds coated with calcium phosphate/PVA. J. Mater. Sci. 2008, 43, 510–524. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Rueger, J.M. Bone replacement materials-state of the art and the way ahead. Der Orthop. 1998, 27, 72–79. [Google Scholar] [CrossRef]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone graft substitutes: Facts, fictions and applications. J. Bone Joint Surg. Am. 2001, 83, 98–103. [Google Scholar] [PubMed]
- Finkemeier, C.G. Bone grafting and bone graft substitutes. J. Bone Joint Surg. Am. 2002, 84, 454–464. [Google Scholar] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Layrolle, P.; Daculsi, G. A review of bioceramics and fibrin sealant. Eur. Cells Mater. 2004, 8, 1–11. [Google Scholar]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Jarcho, M.; Kay, J.L.; Gumaer, R.H.; Drobeck, H.P. Tissue, cellular, and subcellular events at a bone-ceramic apatite interface. J. Bioeng. 1977, 1, 79–92. [Google Scholar] [PubMed]
- LeGeros, R.Z.; LeGeros, J.P. Dense hydroxyapatite. In An Introduction to Bioceramics; Hench, L.L., Wilson, J., Eds.; World Scientific: Singapore, 1993; pp. 139–180. [Google Scholar]
- Kokubo, T.; Shigematsu, M.; Nagashima, Y.; Tashiro, M.; Nakamura, T.; Yamamuro, T.; Higashi, S. Apatite and wollastonite-containing glass-ceramics for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982, 60, 260–268. [Google Scholar]
- Cox, S.C. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C 2014, 35, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tamai, M.; Isama, K.; Nakaoka, R.; Tsuchiya, T. Synthesis of a novel b-tricalcium phosphate/hydroxyapatite biphasic calcium phosphate containing niobium ions and evaluation of its osteogenic properties. J. Artif. Org. 2007, 10, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, D.N. Production and characterization of niobate apatite. J. Mater. Res. Technol. 2013, 2, 24–29. [Google Scholar] [CrossRef]
- Obata, A. Effects of niobium ions released from calcium phosphate invert glasses containing Nb2O5 on osteoblast-like cell functions. ACS Appl. Mater. Interfaces 2012, 4, 5684–5690. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.L.; Holloway, J.A.; Nakkula, R.J.; Walters, J.D. Effect of niobium content on the microstructure and thermal properties of fluorapatite glass-ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 18–24. [Google Scholar]
- Legeros, R.Z. Calcium Phosphates in Oral Biology and Medicine. In Monographs in Oral Science; Myers, H.M., Ed.; Karger AG: New York, NY, USA, 1991; Volume 15, p. 200. [Google Scholar]
- Ramesh, S.; Aw, K.L.; Tolouei, R.; Amiriyan, M.; Tan, C.Y.; Hamdi, M.; Purbolaksono, J.; Hassan, M.A.; Teng, W.D. Sintering properties of hydroxyapatite powders prepared using different methods. Ceram. Int. 2013, 39, 111–119. [Google Scholar]
- Gross, A.K.; Gross, V.; Berndt, C.C. Thermal analysis of amorphous phases in hydroxyapatite coatings. J. Am. Ceram. Soc. 1998, 81, 106–112. [Google Scholar]
- Liao, C.J.; Lin, F.H.; Chen, K.S.; Sun, J.S. Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials 1999, 20, 1807–1813. [Google Scholar] [CrossRef]
- Lafon, J.-P.; Champion, E.; Bernache-Assollant, D.; Gibert, R.; Danna, A.-M. Thermal decomposition of carbonated calcium phosphate apatites. J. Therm. Anal. Calorim. 2003, 72, 1127–1134. [Google Scholar] [CrossRef]
- Alqap, A.S.F.; Adzila, S.; Sopyan, I.; Hamdi, M.; Ramesh, S. Thermal analysis on hydroxyapatite synthesis through mechanochemical method. IFMBE Proc. 2011, 35, 108–111. [Google Scholar]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Evis, A.T.Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Kaynak, G.; Gümüşderelioğlu, M. Bone-like hydroxyapatite precipitated from 10×SBF-like solution by microwave irradiation. Mater. Sci. Eng. C 2015, 49, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Elliot, J.C. Structure and Chemistry of the Apatites and Other Calcium Phosphates; Elsevier: Amsterdam, The Netherlands, 1994; p. 389. [Google Scholar]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.A.; Oliveira, H.S.; Mayrink, G.; Mansur, H.S.; Mansur, A.A.P.; Moreira, R.L. One-pot synthesis of CdS@Nb2O5 core-shell nanostructures with enhanced photocatalytic activity. Appl. Catal. B 2014, 152–153, 403–412. [Google Scholar] [CrossRef]
- Compton, O.C.; Osterloh, F.E. Niobate nanosheets as catalysts for photochemical water splitting into hydrogen and hydrogen peroxide. J. Phys. Chem. C 2009, 113, 479–485. [Google Scholar] [CrossRef]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef]
- Santos, M.H.; Valerio, P.; Goes, A.M.; Leite, M.F.; Heneine, L.G.D.; Mansur, H.S. Biocompatibility evaluation of hydroxyapatite/collagen nanocomposites doped with Zn2+. Biomed. Mater. 2007, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.H.; Oliveira, M.; Souza, L.P.F.; Mansur, H.S.; Vasconcelos, W.L. Synthesis control and characterization of HA prepared by wet process. Mater. Res. 2004, 7, 625–630. [Google Scholar] [CrossRef]
- Ruys, A.J.; Brandwood, A.; Milthorpe, B.K.; Dickson, M.R.; Zeigler, K.A.; Sorrell, C.C. The effects of sintering atmosphere on the chemical compatibility of hydroxyapatite and particulate additives at 1200 °C. Mater. Sci. Mater. Med. 1995, 6, 297–301. [Google Scholar] [CrossRef]
- Etoubleau, J.; Cambon, P.; Bougault, H.; Joron, J.-L. Precise quantitative determination of niobium at low level concentrations in geological samples by WD-XRF. Geostand. Newsl. 1999, 23, 187–195. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Silva, A.R.P.; Ciminelli, V.S.; Mansur, H.S. Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility. Materials 2015, 8, 4191-4209. https://doi.org/10.3390/ma8074191
Capanema NSV, Mansur AAP, Carvalho SM, Silva ARP, Ciminelli VS, Mansur HS. Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility. Materials. 2015; 8(7):4191-4209. https://doi.org/10.3390/ma8074191
Chicago/Turabian StyleCapanema, Nádia S. V., Alexandra A. P. Mansur, Sandhra M. Carvalho, Alexandra R. P. Silva, Virginia S. Ciminelli, and Herman S. Mansur. 2015. "Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility" Materials 8, no. 7: 4191-4209. https://doi.org/10.3390/ma8074191