Experimental and Numerical Study on Effect of Sample Orientation on Auto-Ignition and Piloted Ignition of Poly(methyl methacrylate)
Abstract
:1. Introduction
2. Experimental Details
2.1. Apparatus Methods
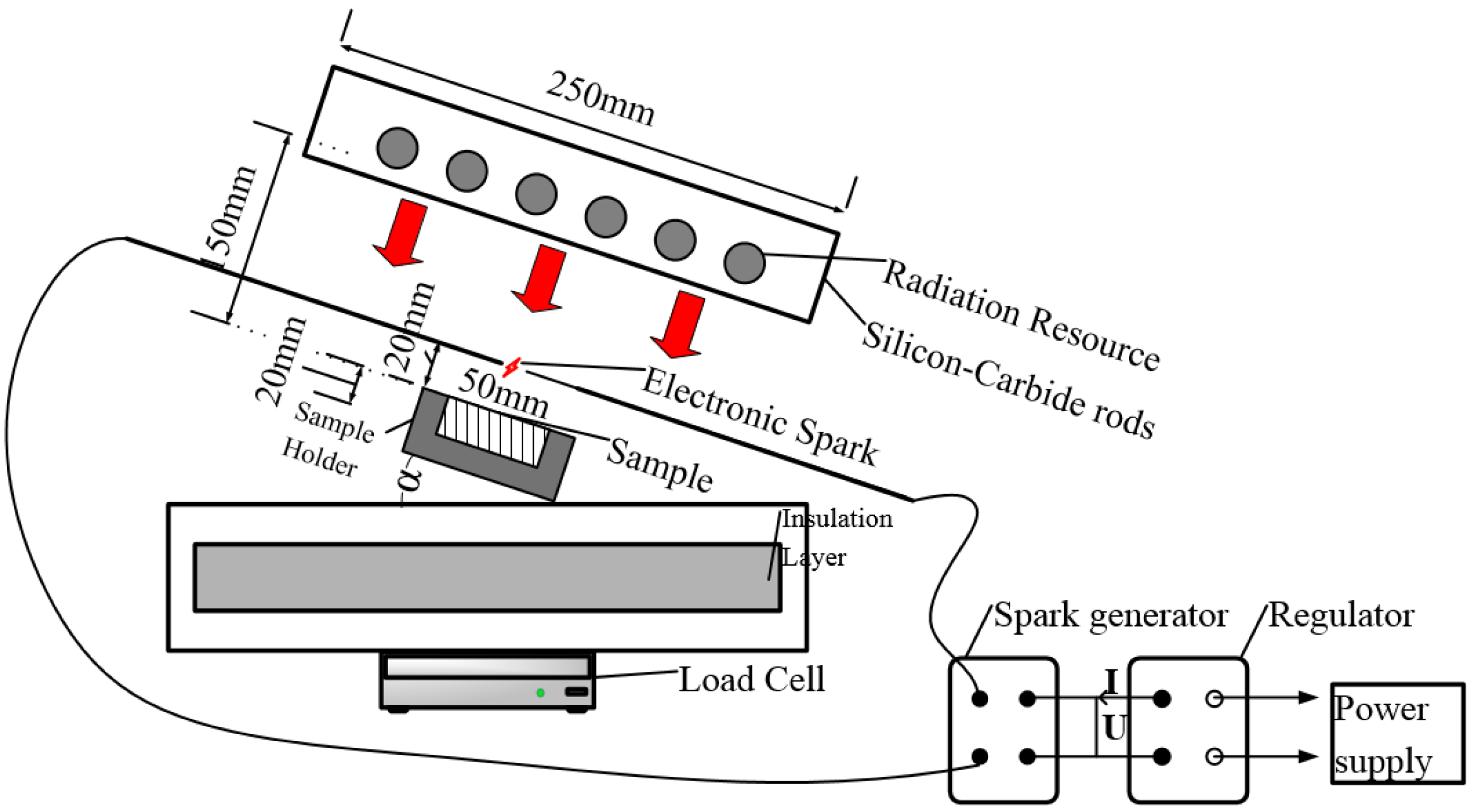

2.2. Procedure and Observed Fire Behavior

3. Numerical Modeling

| Orientation | 0° | 15° | 30° | 45° | 60° | 75° | 90° |
|---|---|---|---|---|---|---|---|
| g(x) | 0 | −2.539 | −4.905 | −6.937 | −8.496 | −9.476 | −9.81 |
| g(z) | −9.81 | −9.476 | −8.496 | −6.937 | −4.905 | −2.539 | 0 |
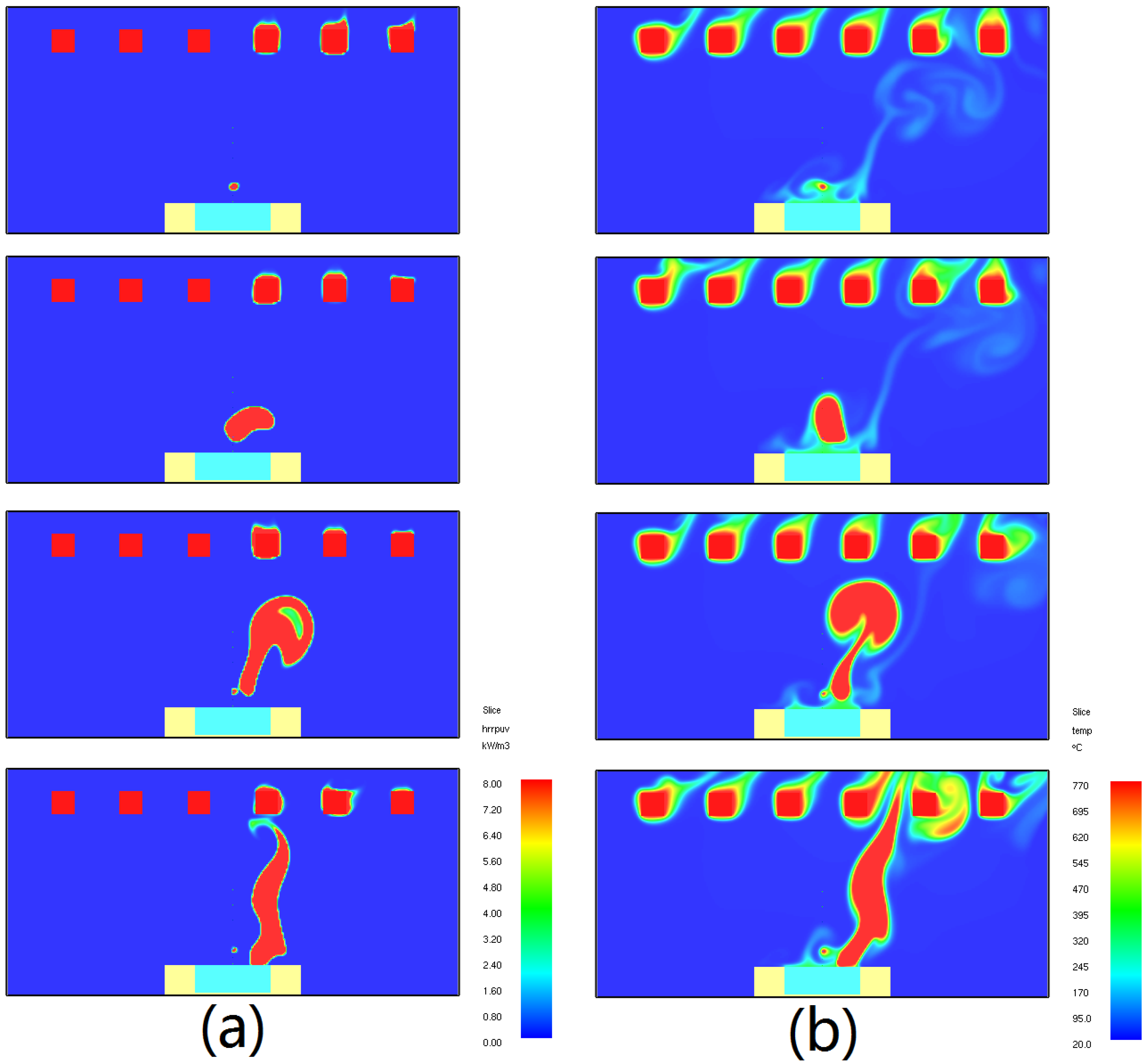
4. Results and Discussion
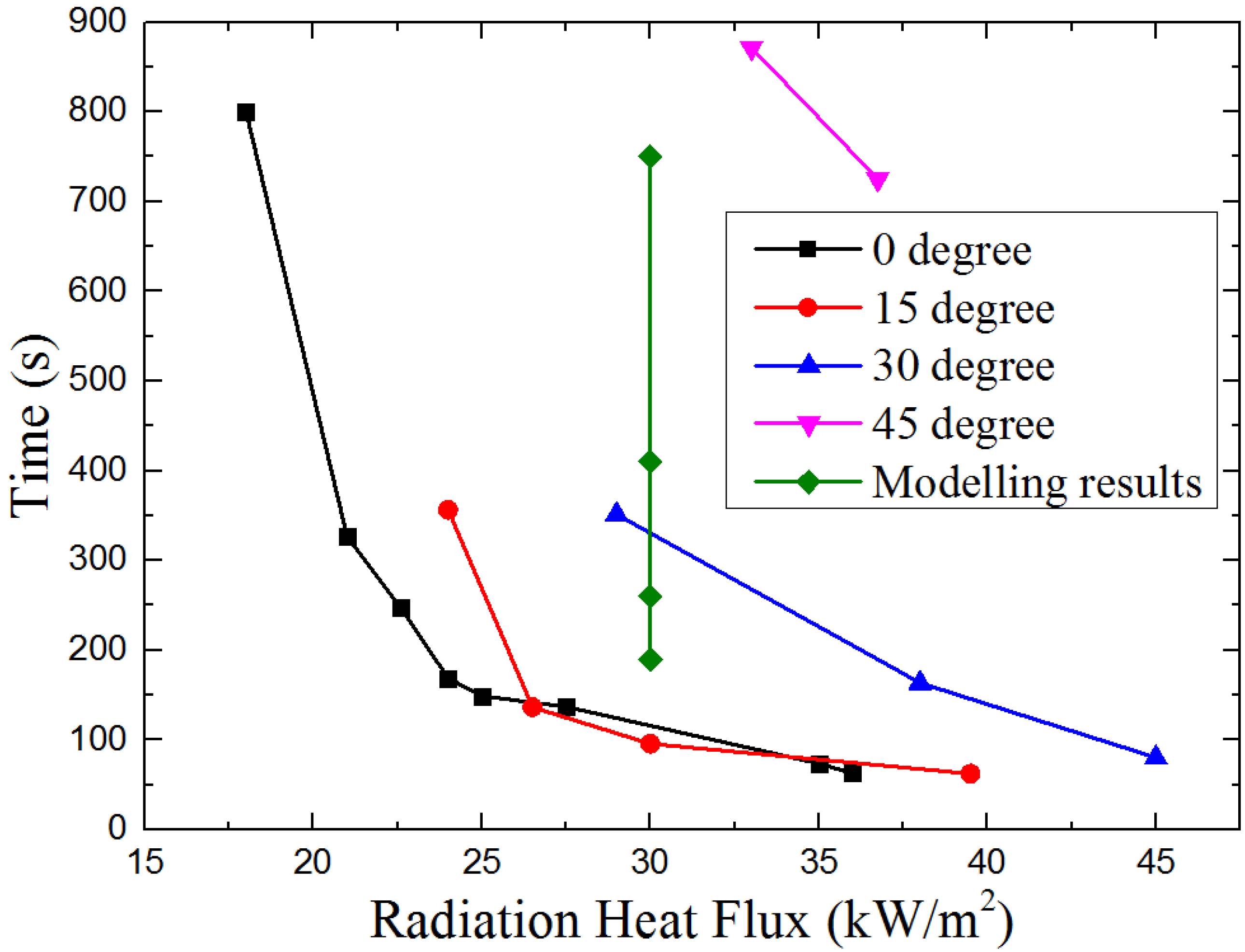
4.1. Ignition Time
4.1.1. Piloted Ignition


4.1.2. Auto-Ignition

| Orientation | a | b | R-Square | |
|---|---|---|---|---|
| 0° | 0.00397 | −0.3728 | 0.97382 | 9.39 kW/m2 |
| 15° | 0.00367 | −0.04108 | 0.90971 | 11.19 kW/m2 |
| 30° | 0.00307 | −0.05143 | 0.94509 | 16.75 kW/m2 |
| 45° | 0.000684 | −0.0155 | --------- | 22.63 kW/m2 |
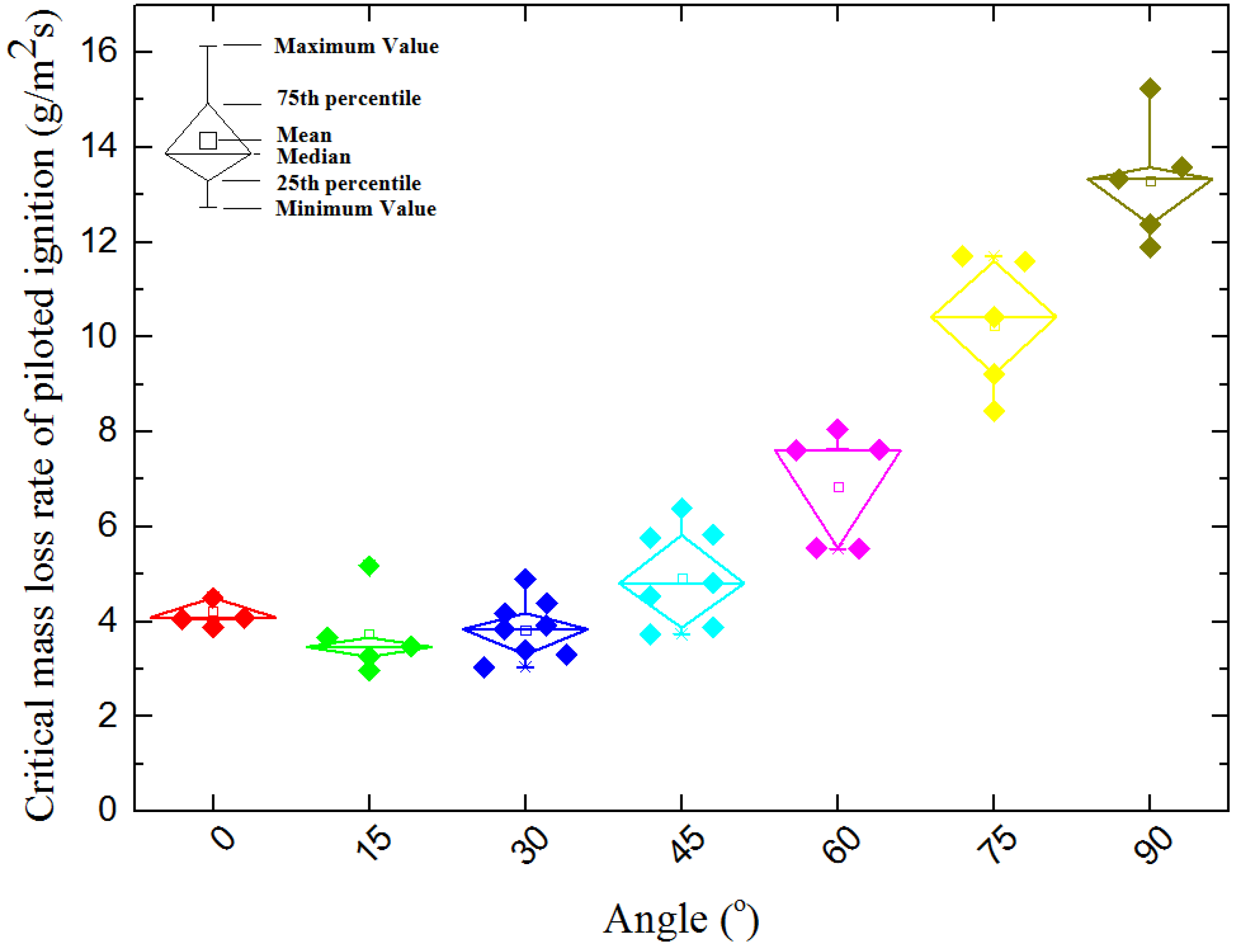
4.2. Mass Loss Rate
4.2.1. Piloted Ignition
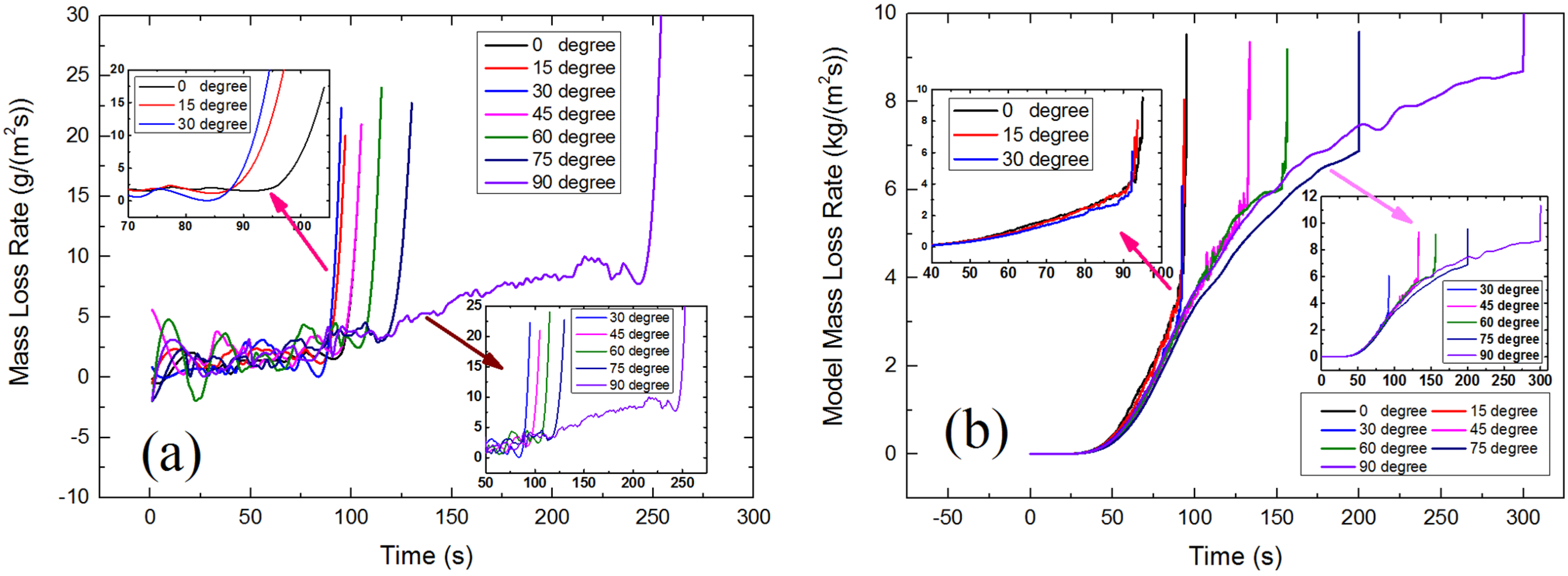
4.2.2. Auto-Ignition
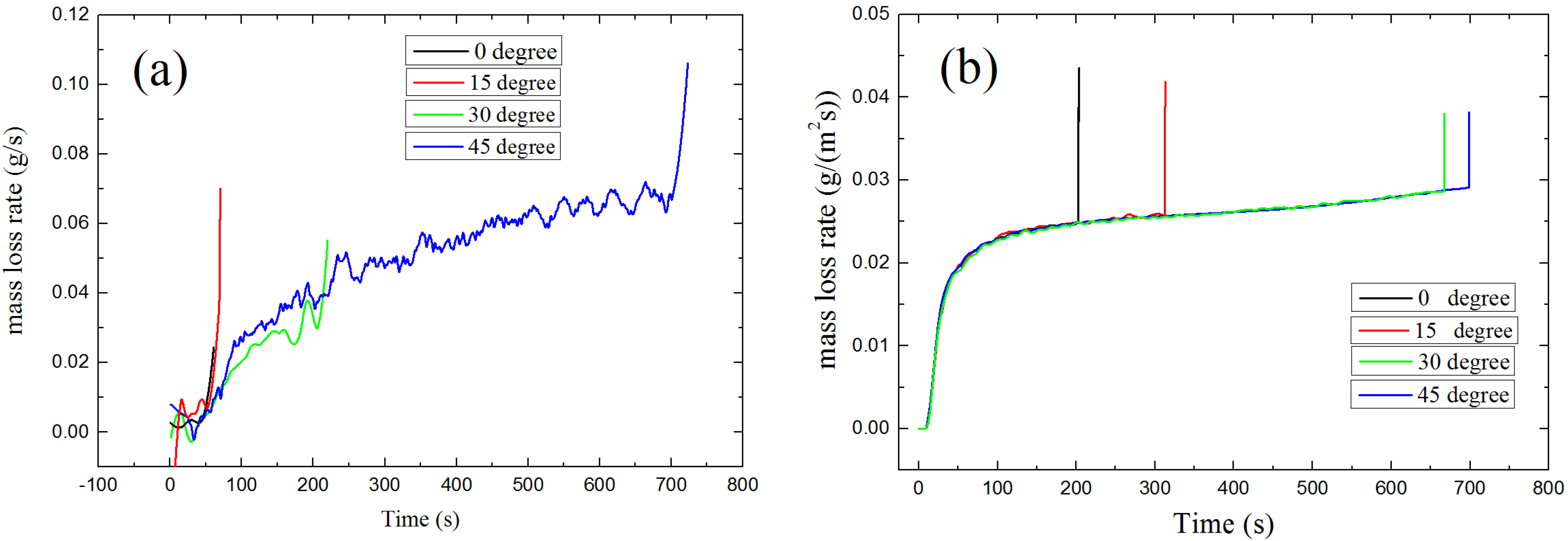
4.3. Heat Flux
4.3.1. Radiant Heat Flux

4.3.2. Convective Heat Flux
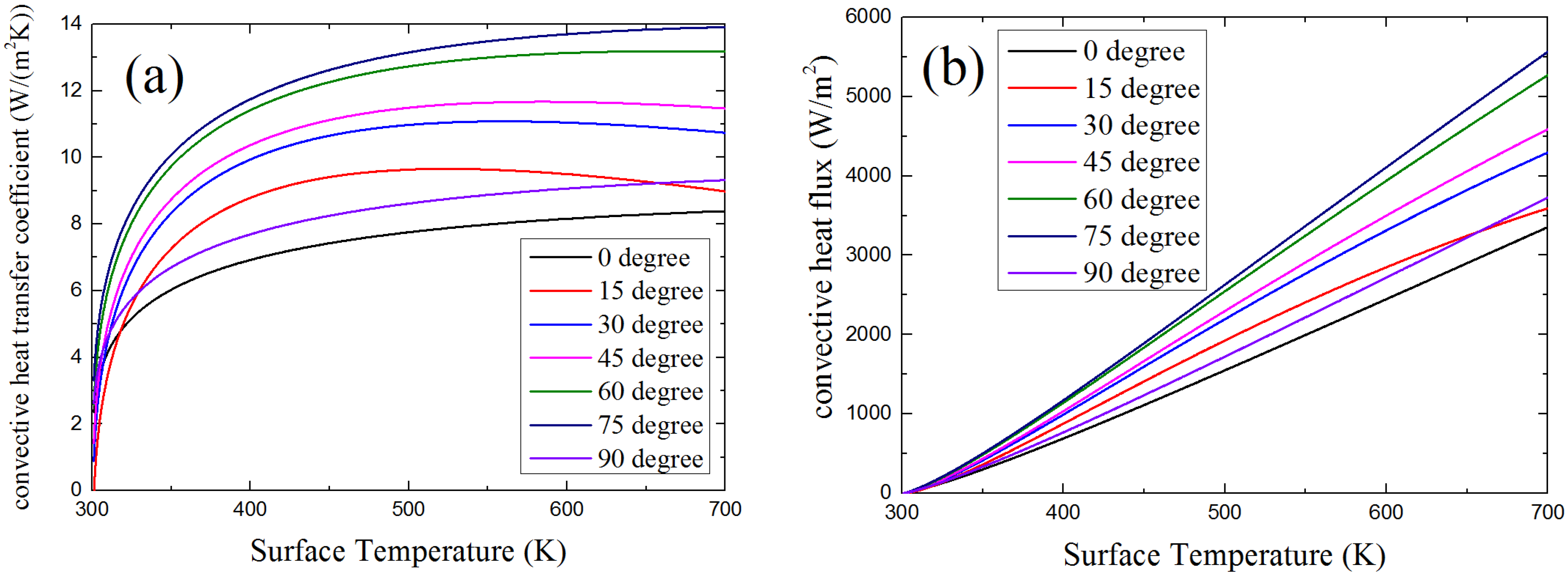
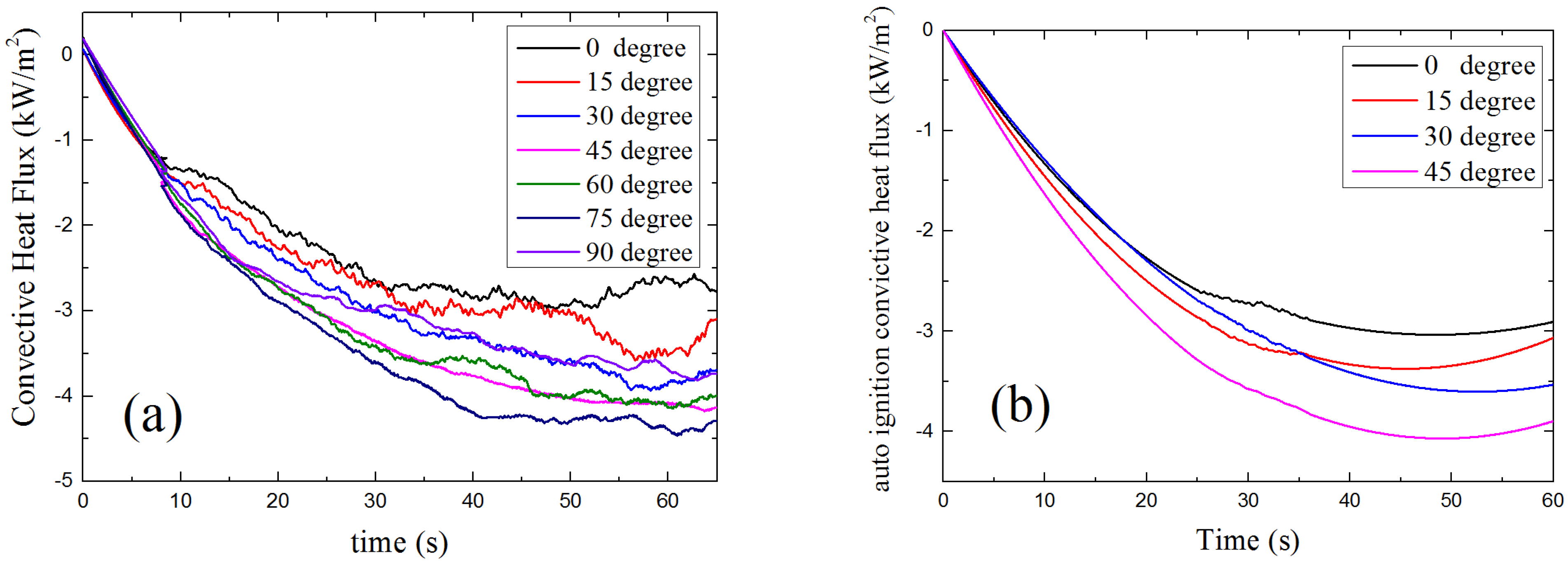
4.3.3. Net Heat Flux

4.4. Surface Temperature
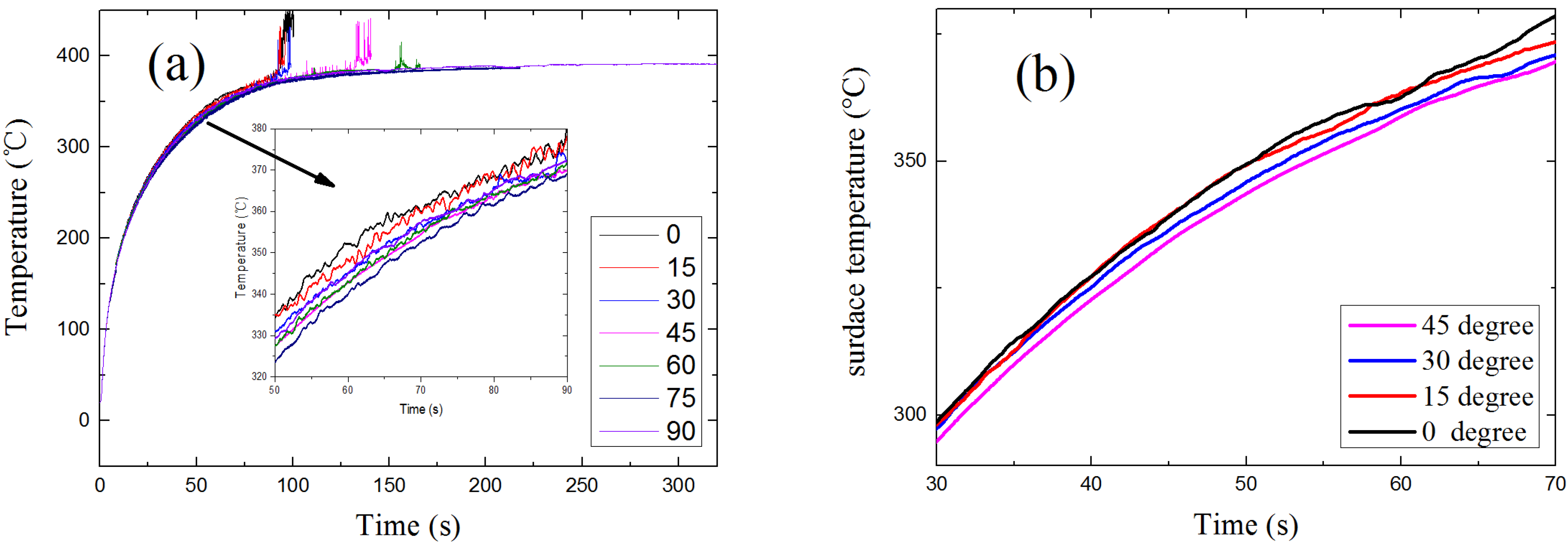
4.5. Fuel Concentration

4.6. Discussion
5. Conclusions
- (1)
- In piloted ignition conditions, both the ignition time and critical mass loss rate at ignition have a U-shape relationship with the sample orientation angle α. The U-shape relationships indicate there is a specific α which makes the ignition time and critical mass loss rate is at a minimum. In auto-ignition conditions, both the ignition time and critical mass loss rate at ignition decrease with sample orientation angle α, but the critical heat flux increases from 9.39 to 22.63 kW/m2 as α increases from 0° to 45°.
- (2)
- Both the convictive heat flux loss and the inflow of radiant heat flux grows with sample orientation angle as α increases from 0° to 75°. As a result of the combination of the convective and radiant heat flux, the total incident heat flux at the solid surface decreases with α which makes the surface temperature of solid decrease. This makes the auto-ignition harder to occur with the increasing of the orientation angle. Because of the laminar air flow of vertical placed samples, the angle of α = 90° does not obey the same rule.
- (3)
- Influenced by the increasing incident heat flux at sample surface and different gas flow of volatiles, the fuel concentration at the pilot place has a U-shape and results to the U-shape profile of ignition time and critical mass loss rate of piloted ignition.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gong, J.H.; Yang, L.Z. Effects of low atmospheric pressure on combustion characteristics of polyethylene and polymethyl methacrylate. J. Fire Sci. 2012, 30, 224–239. [Google Scholar] [CrossRef]
- Blasi, C.D.; Branca, C. Pyrolytic behavior and products of some wood varieties. Combust. Flame 2001, 124, 165–177. [Google Scholar] [CrossRef]
- Spearpoint, M.J.; Quintiere, J.G. Predicting the piloted ignition of wood in the cone calorimeter using an integral model-effect of species, grain orientation and heat flux. Fire Saf. J. 2001, 36, 391–415. [Google Scholar] [CrossRef]
- Shields, T.J.; Silcock, G.W. The effects of geometry and ignition mode on ignition times obtained using a cone calorimeter and ISO ignitability apparatus. Fire Mater. 1993, 17, 25–32. [Google Scholar] [CrossRef]
- Beaulieu, P.A.; Dembsey, N.A. Effect of oxygen on flame heat flux in horizontal and vertical orientations. Fire Saf. J. 2008, 43, 410–428. [Google Scholar] [CrossRef]
- Fereres, S.; Lautenberger, C. Understanding ambient pressure effects on piloted ignition through numerical modeling. Combust. Flame 2012, 159, 3544–3553. [Google Scholar] [CrossRef]
- Rich, D.; Lautenberger, C. Mass flux of combustible solids at piloted ignition. Proc. Combust. Inst. 2007, 31, 2653–2660. [Google Scholar] [CrossRef]
- Atreya, A. Ignition of fires. Philos. Trans. Royal Soc. A 1998, 356, 2787–2813. [Google Scholar]
- Bilbao, R.; Mastral, J.F. A model for the prediction of the thermal degradation and ignition of wood under constant and variable heat flux. J. Anal. Appl. Pyrolysis 2002, 62, 63–82. [Google Scholar] [CrossRef]
- Wu, W.; Yang, L.Z. Experimental study of the effect of spark power on piloted ignition of wood at different altitudes. J. Fire Sci. 2011, 29, 465–475. [Google Scholar] [CrossRef]
- Babrauskas, V. Ignition of Wood: A Review of the State of the Art. J. Fire Prot. Eng. 2002, 12, 163–189. [Google Scholar] [CrossRef]
- Tsai, K.C. Orientation effect on cone calorimeter test results to assess fire hazard of materials. J. Hazard. Mater. 2009, 172, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Z.; Wang, Y.H. Experimental and numerical study of the effect of sample orientation on the pyrolysis and ignition of wood slabs exposed to radiation. J. Fire Sci. 2012, 30, 211–223. [Google Scholar] [CrossRef]
- Gotoda, H.; Manzello, S.L. Effects of sample orientation on nonpiloted ignition of thin poly(methyl methacrylate) sheets by a laser: 2. Experimental results. Combust. Flame 2006, 145, 820–835. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kashiwagi, T. Effects of sample orientation on nonpiloted ignition of thin poly(methyl methacrylate) sheet by a laser 1. Theoretical prediction. Combust. Flame 2005, 141, 149–169. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.H. Effects of sample orientation on pyrolysis and piloted ignition of wood. J. Fire Sci. 2014, 32, 483–497. [Google Scholar] [CrossRef]
- Delichatsios, M.A. Ignition Times for Thermally Thick and Intermediate Conditions in Flat and Cylindrical Geometries. In Proceedings of the Sixth International Symposium, Poitiers, France, 5–9 July 1999; pp. 233–244.
- Zhou, Y.P.; Yang, L.Z. Radiation attenuation characteristics of pyrolysis volatiles of solid fuels and their effect for radiant ignition model. Combust. Flame 2010, 157, 167–175. [Google Scholar] [CrossRef]
- Atreya, A. Convection Heat Transfer. In SFPE Handbook of Fire Protection Engineering, 3rd ed.; DiNenno, P.J., Drysdale, D., Eds.; The Society of Fire Protection Engineers: Bethesda, MD, USA, 2002; pp. 65–68. [Google Scholar]
- Gong, J. Experimental and Theoretical Study on Pyrolysis and Opposed-flow Flame Spread of Typical Non-charring Polymers. Doctoral thesis, University of Science and Technology of China, Hefei, China, 2014. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Zhou, X.-D.; Zhao, K.; Wu, Z.-B.; Yang, L.-Z. Experimental and Numerical Study on Effect of Sample Orientation on Auto-Ignition and Piloted Ignition of Poly(methyl methacrylate). Materials 2015, 8, 4004-4021. https://doi.org/10.3390/ma8074004
Peng F, Zhou X-D, Zhao K, Wu Z-B, Yang L-Z. Experimental and Numerical Study on Effect of Sample Orientation on Auto-Ignition and Piloted Ignition of Poly(methyl methacrylate). Materials. 2015; 8(7):4004-4021. https://doi.org/10.3390/ma8074004
Chicago/Turabian StylePeng, Fei, Xiao-Dong Zhou, Kun Zhao, Zhi-Bo Wu, and Li-Zhong Yang. 2015. "Experimental and Numerical Study on Effect of Sample Orientation on Auto-Ignition and Piloted Ignition of Poly(methyl methacrylate)" Materials 8, no. 7: 4004-4021. https://doi.org/10.3390/ma8074004





