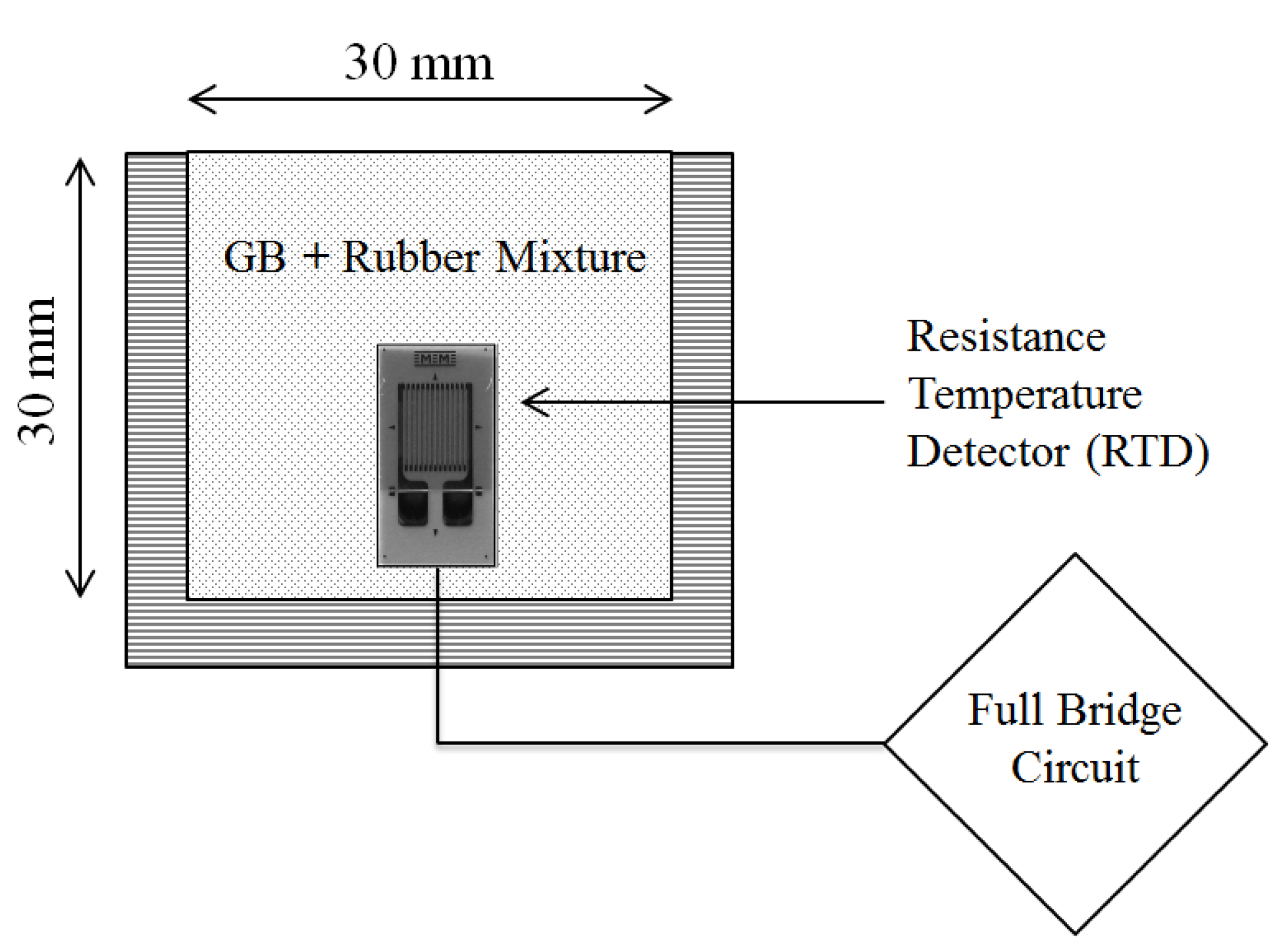
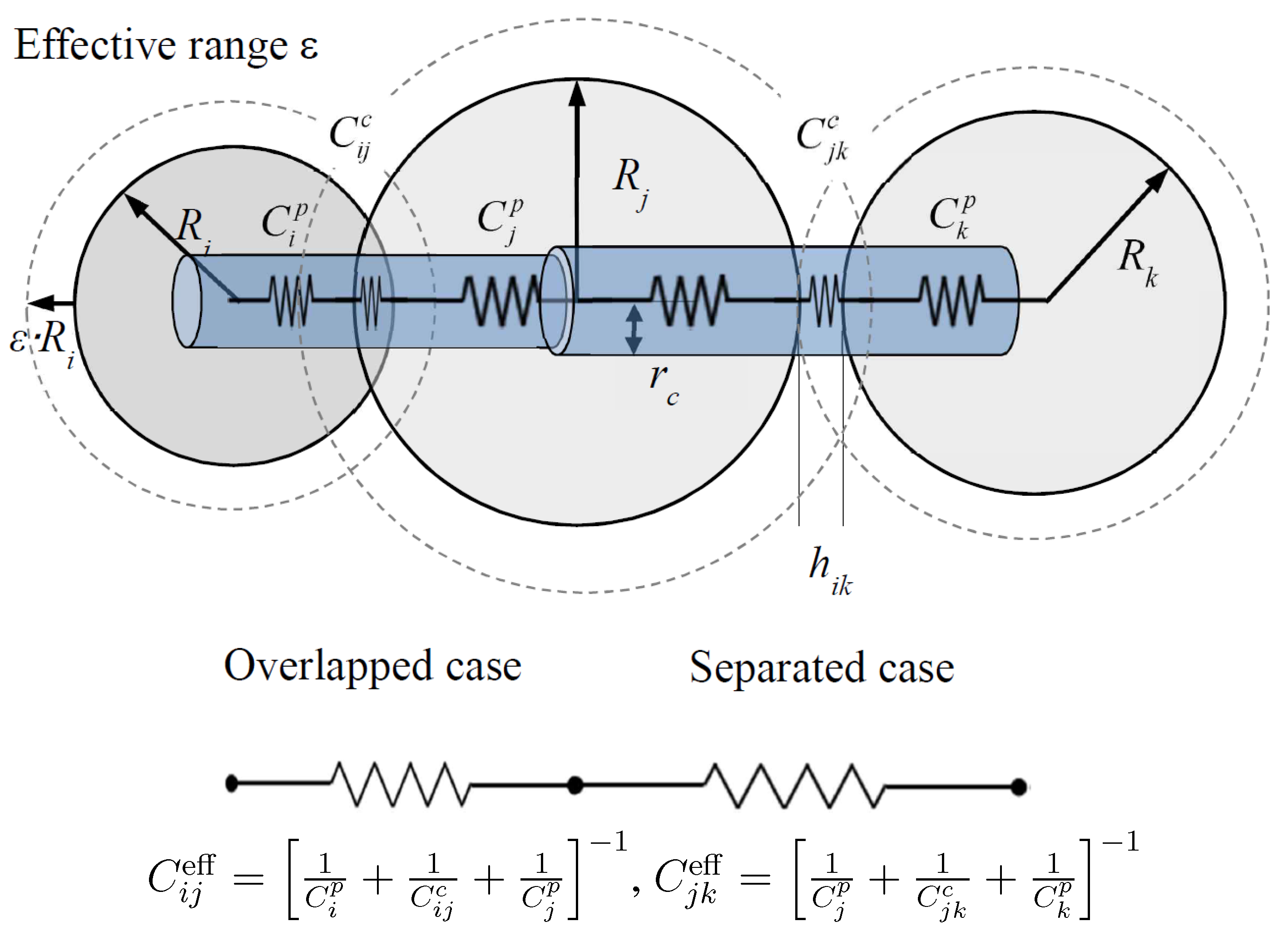
5.1. Sand-Rubber Mixture Test
Glass beads were used to investigate the influence of particle size ratio on thermal conductivity and, despite their spherical shapes, to simplify the comparative numerical simulation. Hence, a sand-rubber mixture, a widely-studied mixture with possible usefulness deriving from the manipulation of its properties [
4], was tested under similar experimental conditions (e.g., the same size ratio). Jumumjin sand (
= 0.5 mm,
= 1.16) was used to replace the glass beads, and the mixing conditions are summarized in
Table 4. The porosity values were similar to those of the glass bead-rubber mixture specimens.
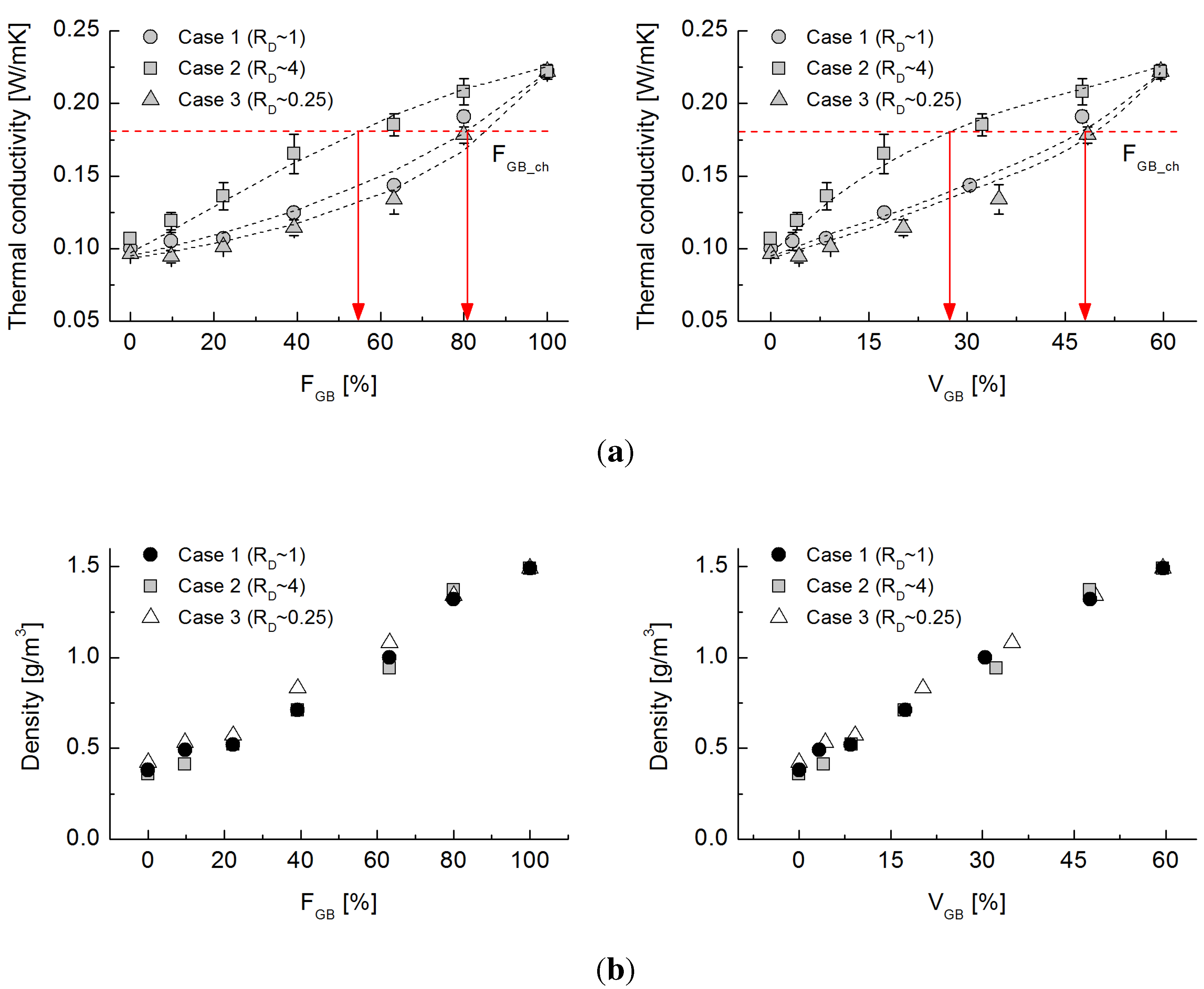
Figure 8 shows the evolution of thermal conductivity with the varying volumetric fraction of sand. Although the tested sand is mainly comprised of quartz, whose thermal conductivity ranges from 3 to 8 W/mK and is higher than that of the glass bead raw material, the maximum thermal conductivity under dry conditions is similar to the experimental results shown in
Figure 4. Case 2, which has the largest rubber particles, shows a quasi-linear trend with increasing sand fraction, whereas the behaviors of Cases 1 and 3 are similar to those in the glass bead-rubber mixture experiments. Despite the different particle shape, the particle size ratio still has the predominant effect on thermal conduction in natural sands.
Table 4.
Volumetric fraction and porosity of sand-rubber mixture.
Table 4.
Volumetric fraction and porosity of sand-rubber mixture.
| Vol.Fraction,
(%) | Porosity |
|---|
| Case 1 | Case 2 | Case 3 |
|---|
| 0 | 0.62 | 0.61 | 0.62 |
| 20 | 0.59 | 0.55 | 0.58 |
| 40 | 0.55 | 0.55 | 0.55 |
| 60 | 0.50 | 0.51 | 0.50 |
| 80 | 0.48 | 0.46 | 0.49 |
| 100 | 0.41 | 0.41 | 0.41 |
Figure 8.
(a) Evolution of thermal conductivity values with varying volumetric fractions of sand for Cases 1 to 3; (b) density values with varying volumetric fractions of sand for Cases 1 to 3.
Figure 8.
(a) Evolution of thermal conductivity values with varying volumetric fractions of sand for Cases 1 to 3; (b) density values with varying volumetric fractions of sand for Cases 1 to 3.
5.2. Effect of Particle Contact
Heat transfer occurs mainly at direct contacts between conductive particles. To further investigate the effect of contact conduction, the electrical conduction of mixtures was tested. Electrical current flows only through particles that are in direct contact. The electrical current results therefore corroborate the significance of particle contact conditions determined by mixture fraction, with the effect of less conductive particles being eliminated. This corresponds to the case when the cutoff range parameter is not applicable (i.e., hard-core percolation).
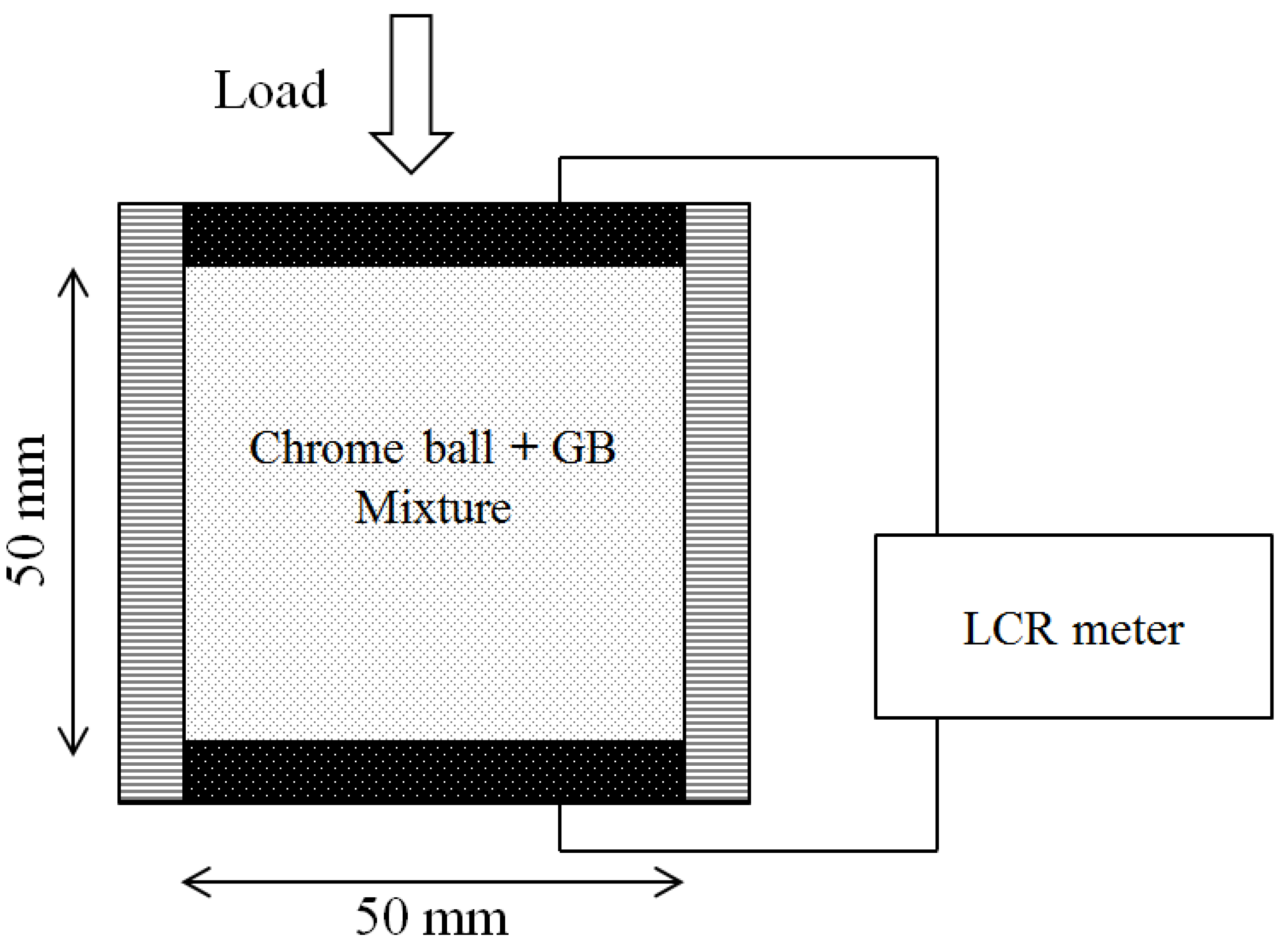
Chrome balls were selected as electrically-conductive particles, and glass beads were used as insulators in these additional tests, in contrast to their conductive role in the thermal conductivity tests [
17]. The balls and beads were mixed at 20% volumetric fraction increments. A vertical pressure of 112 kPa was applied to each mixture to ensure that the particles were in contact. The top and bottom stainless steel plates served as two-point electrodes. Subsequent measurement of the potential decrease between the two electrodes using an LCRmeter allowed the electrical resistance to be derived (
Figure 9). The particle size ratios and the volumetric fractions of the tested mixtures are summarized in
Table 5 and
Table 6.
Figure 9.
Experimental configuration for measuring the electrical conductivity of granular mixtures. The electrical conductance is obtained with two-point electrodes at the top and bottom plates.
Figure 9.
Experimental configuration for measuring the electrical conductivity of granular mixtures. The electrical conductance is obtained with two-point electrodes at the top and bottom plates.
Table 5.
Cases in chrome ball-glass bead mixture experimentation.
Table 5.
Cases in chrome ball-glass bead mixture experimentation.
| Case | Particle Diameter | |
|---|
| Chrome Ball | Glass Bead |
|---|
| Case 1 | 1∼0.84 | 1.19∼0.3 | ∼1 |
| Case 2 | 1∼1.68 | 2.38∼0.3 | ∼2 |
| Case 3 | 1∼0.4 | 0.6∼0.3 | ∼0.5 |
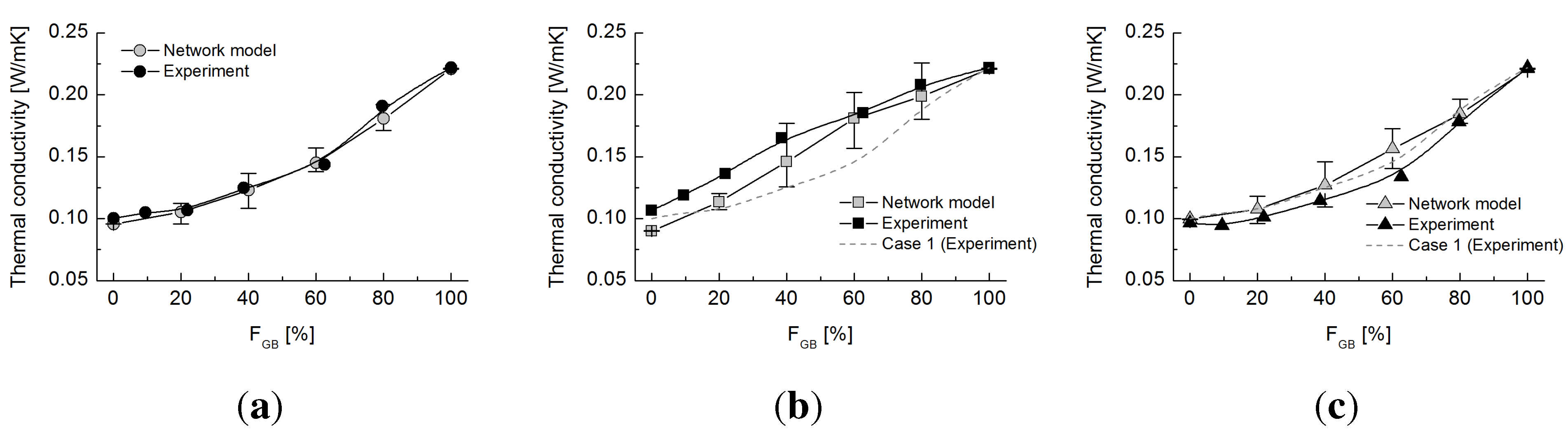
Figure 10 shows the experimentally-measured electrical conductivity values, the values estimated using the network model and the coordination number for each of the three tested cases. Similar to the thermal studies, electrical conductivity values at
= 0 (0 W/mK) and 100% (32 W/mK) were first matched, and then, a curvature parameter
χ = 0.016 was determined to capture the experimental trend. The electrical conduction occurs only via the chrome balls. As observed in the thermal conduction study, Case 2 (with glass beads larger than chrome balls) has a higher electrical conductivity than Cases 1 and 3. However, the difference is smaller than that in the thermal tests. This seems to be due to the size ratio in the electrical experiment being smaller than that in the thermal test. Furthermore, the insulating materials (
i.e., the glass beads) do not participate in conduction. Hence, the results are highly analogous to the thermal estimation, assuming that the rubber particles are completely adiabatic, and are similar to the results displayed in
Figure 7a.
Table 6.
Volumetric fraction and porosity of chrome ball-glass bead mixture.
Table 6.
Volumetric fraction and porosity of chrome ball-glass bead mixture.
| Vol. Fraction,
(%) | Porosity |
|---|
| Case 1 | Case 2 | Case 3 |
|---|
| 0 | 0.36 | 0.36 | 0.36 |
| 20 | 0.36 | 0.36 | 0.36 |
| 40 | 0.38 | 0.36 | 0.37 |
| 60 | 0.37 | 0.39 | 0.38 |
| 80 | 0.39 | 0.38 | 0.38 |
| 100 | 0.39 | 0.39 | 0.39 |
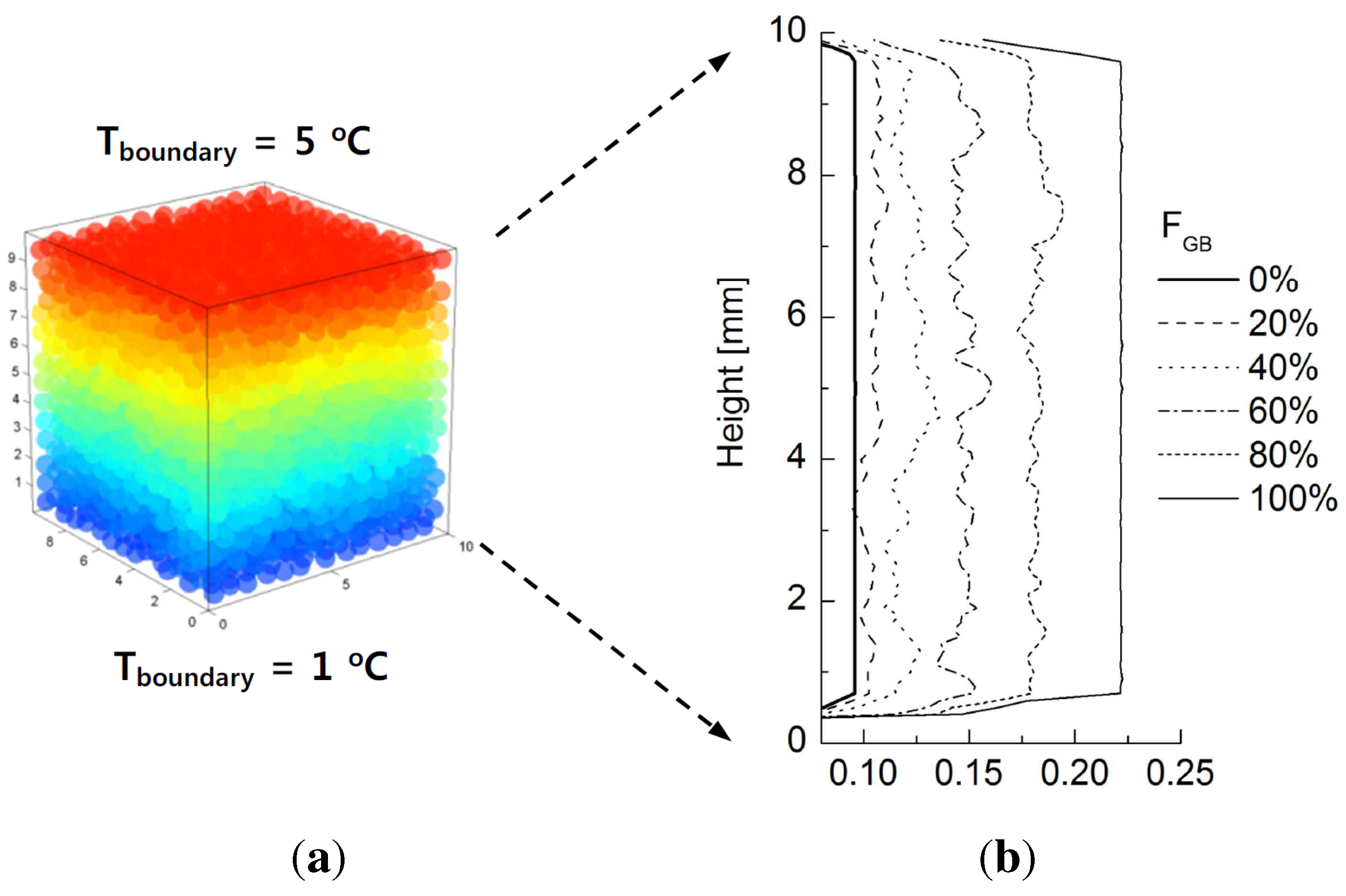
Figure 11a shows the potential distribution of chrome ball particles for Case 1 at
= 60% at steady state. The electrical conductivity computed across the given cross-sectional areas with respect to height for different values of
are plotted in
Figure 11b. Unlike thermal conduction, electrical conduction occurs only when the chrome balls are connected from top to bottom. Therefore, little variation in the electrical conductivity profiles with height is observed, unlike in
Figure 6b, because chrome ball-chrome ball contacts are the only contributors to conduction. Furthermore, electrical conductivity is not measured below
, because there is not a continuously connected network of chrome balls connecting top to bottom. Combining the observations in
Figure 10 and
Figure 11 suggests that a fully developed contact networks of chrome balls emerges between
values of 20% to 40%.
Figure 10.
(a) Electrical conductivity values measured by two-point electrodes; (b) electrical conductivity values estimated by the network model; (c) coordination number estimated for virtual specimens with varying volumetric fractions of chrome balls for Cases 1 to 3.
Figure 10.
(a) Electrical conductivity values measured by two-point electrodes; (b) electrical conductivity values estimated by the network model; (c) coordination number estimated for virtual specimens with varying volumetric fractions of chrome balls for Cases 1 to 3.
Figure 11.
(a) Potential distribution ofthe chrome balls with respect to the height for Case 1:
= 60% at the steady-state condition. (b) Distribution of estimated electrical conductivity with respect to the height for different fractions of chrome balls.
Figure 11.
(a) Potential distribution ofthe chrome balls with respect to the height for Case 1:
= 60% at the steady-state condition. (b) Distribution of estimated electrical conductivity with respect to the height for different fractions of chrome balls.
To discover the volume fraction that permits complete contact between the two electrodes, similar electrical conductivity tests were conducted at each one-percentage-point interval between
= 20% and 40% by using DEM and the network model.
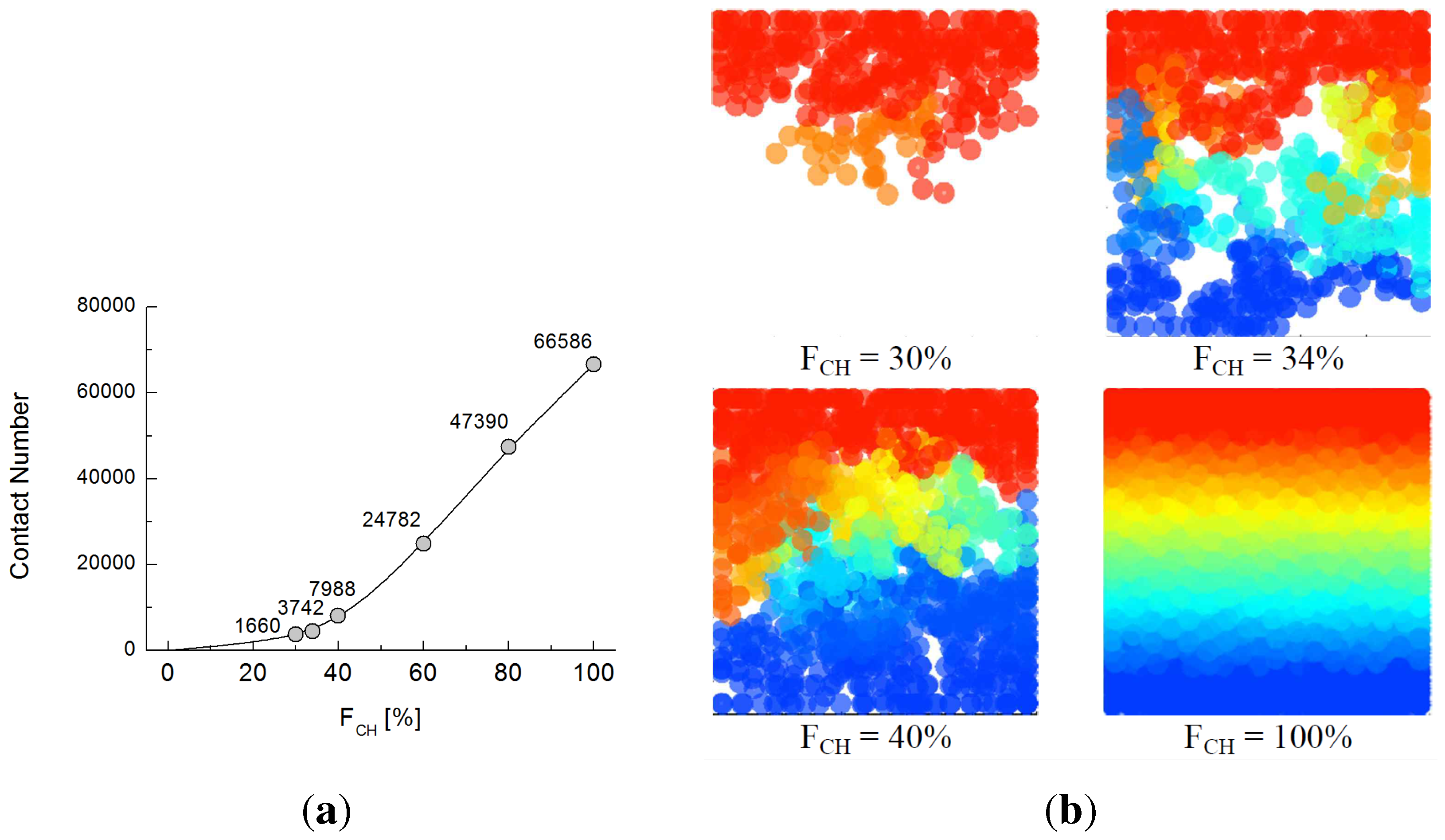
Figure 12a,b shows the increasing connected contact number of chrome balls from top to bottom and the 3D configuration of potentials of connected chrome particles at different volume fractions. A fully-developed connection is achieved when
exceeds 31%. The contact number of particles sharply increases with the increasing volumetric fraction of chrome balls, and the trend in the contact number of particles is similar to the trend in electrical conductivity. This shows that the contact number of conductive particles is the major factor determining conductivity.
Figure 12.
(a) Contact number along the volumetric fraction at Case 1; (b) connected chrome particles from the top to bottom boundary at different volume fractions for Case 1.
Figure 12.
(a) Contact number along the volumetric fraction at Case 1; (b) connected chrome particles from the top to bottom boundary at different volume fractions for Case 1.