The Modification of a Tetrafunctional Epoxy and Its Curing Reaction
Abstract
:1. Introduction
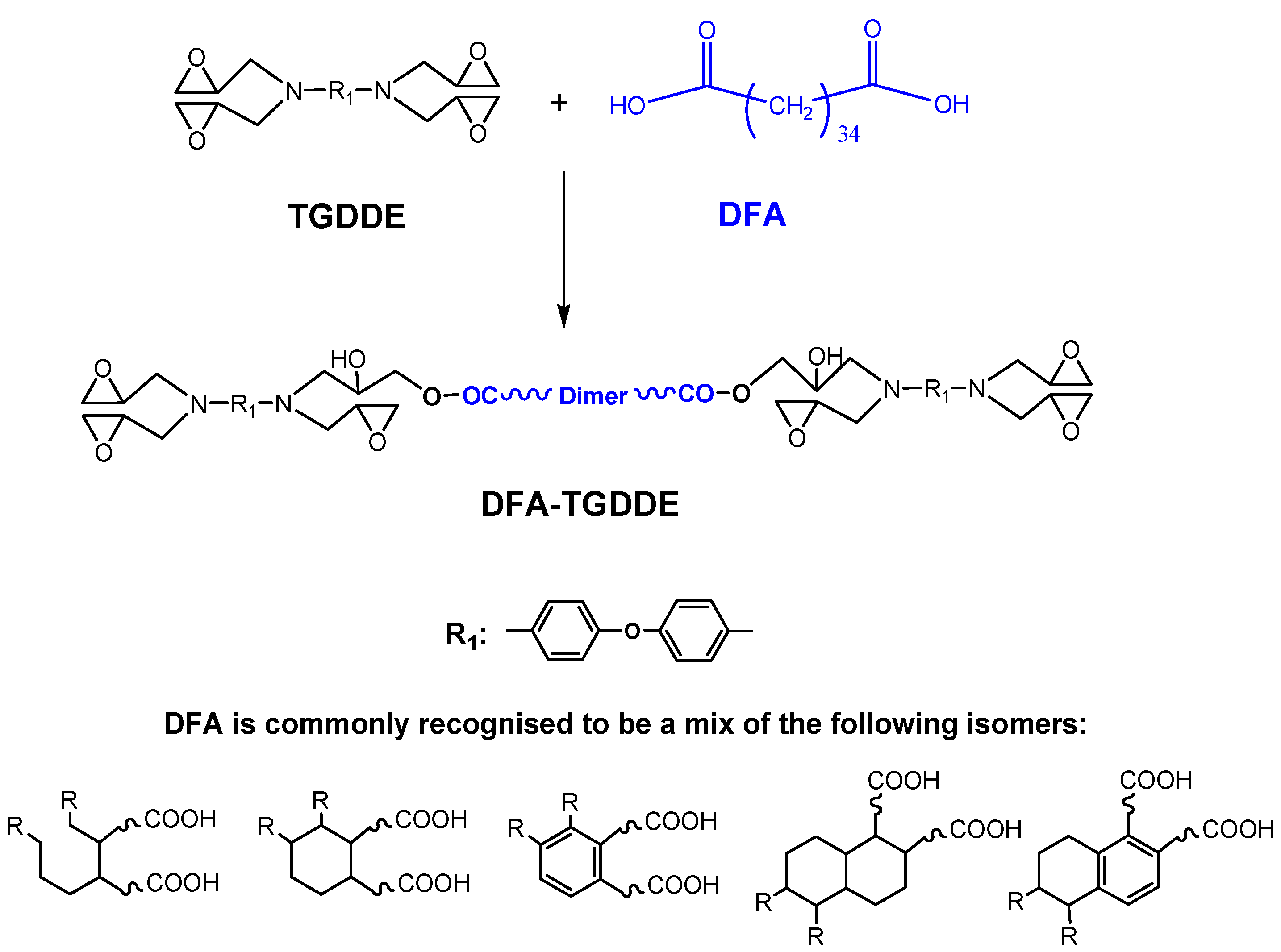
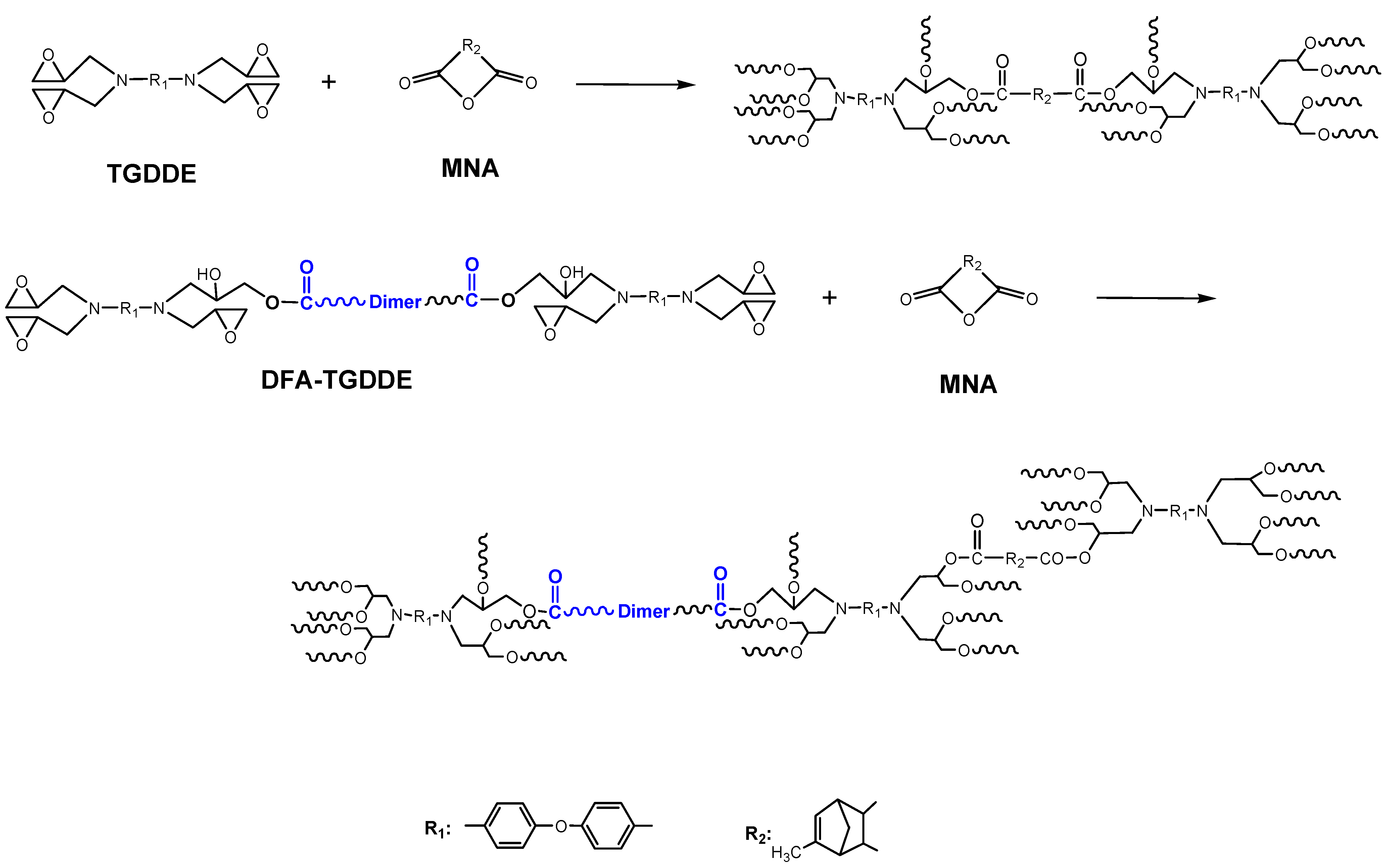

2. Results and Discussion
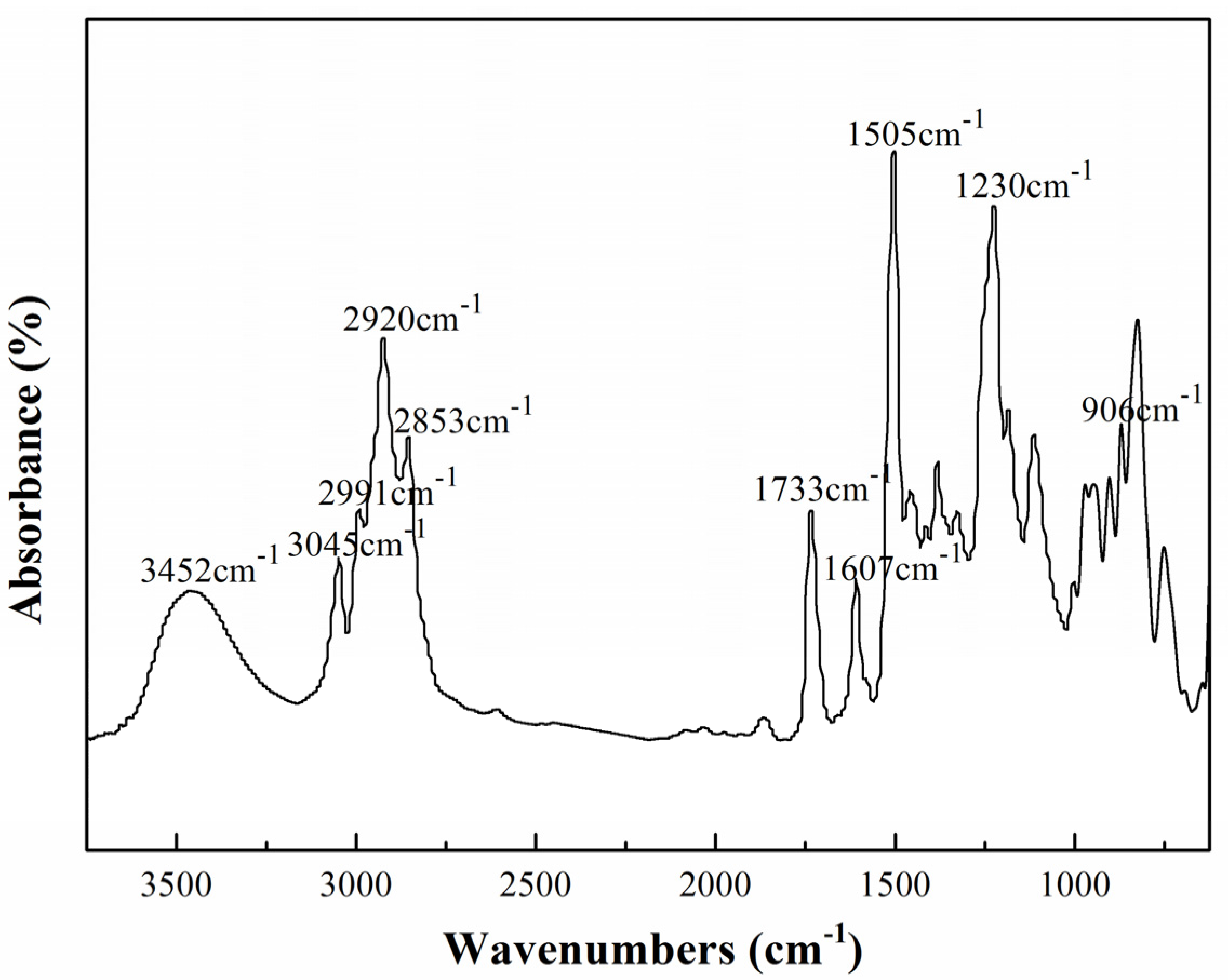
2.1. Modification of TGDDE with DFA
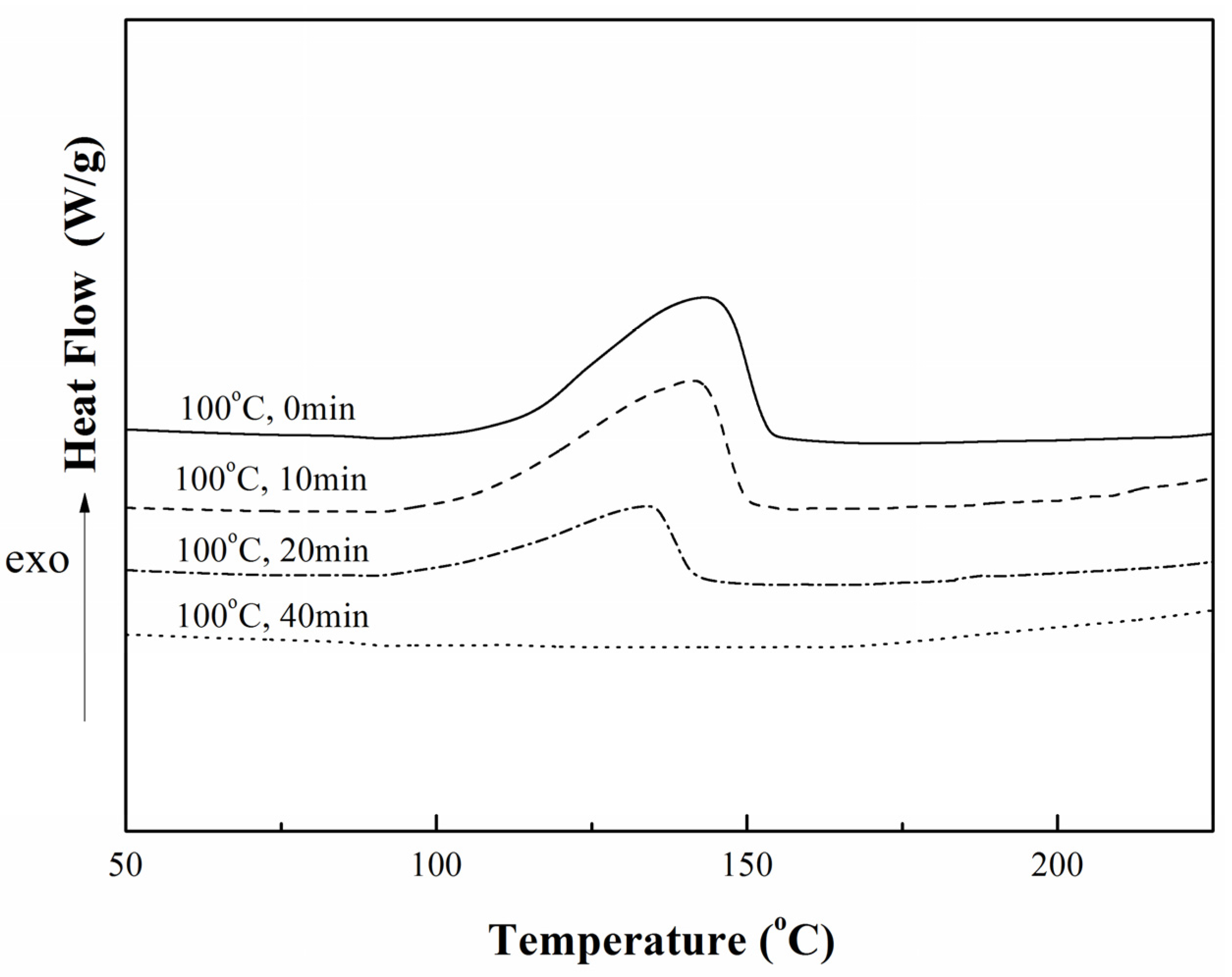
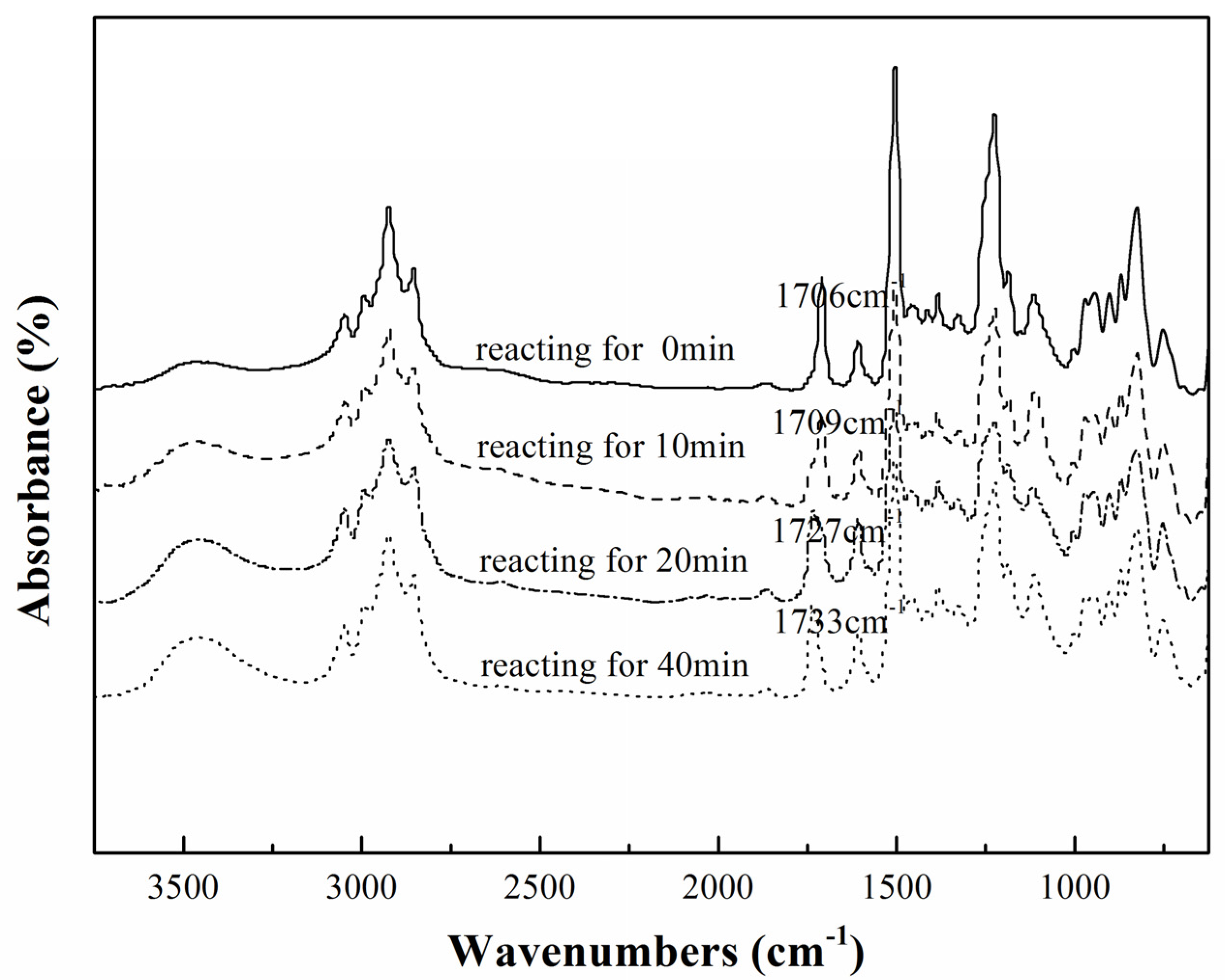
 ).
). 
2.2. Curing Reactions of TGDDE/MNA and DFA-TGDDE/MNA Systems
2.2.1. Curing Behaviors
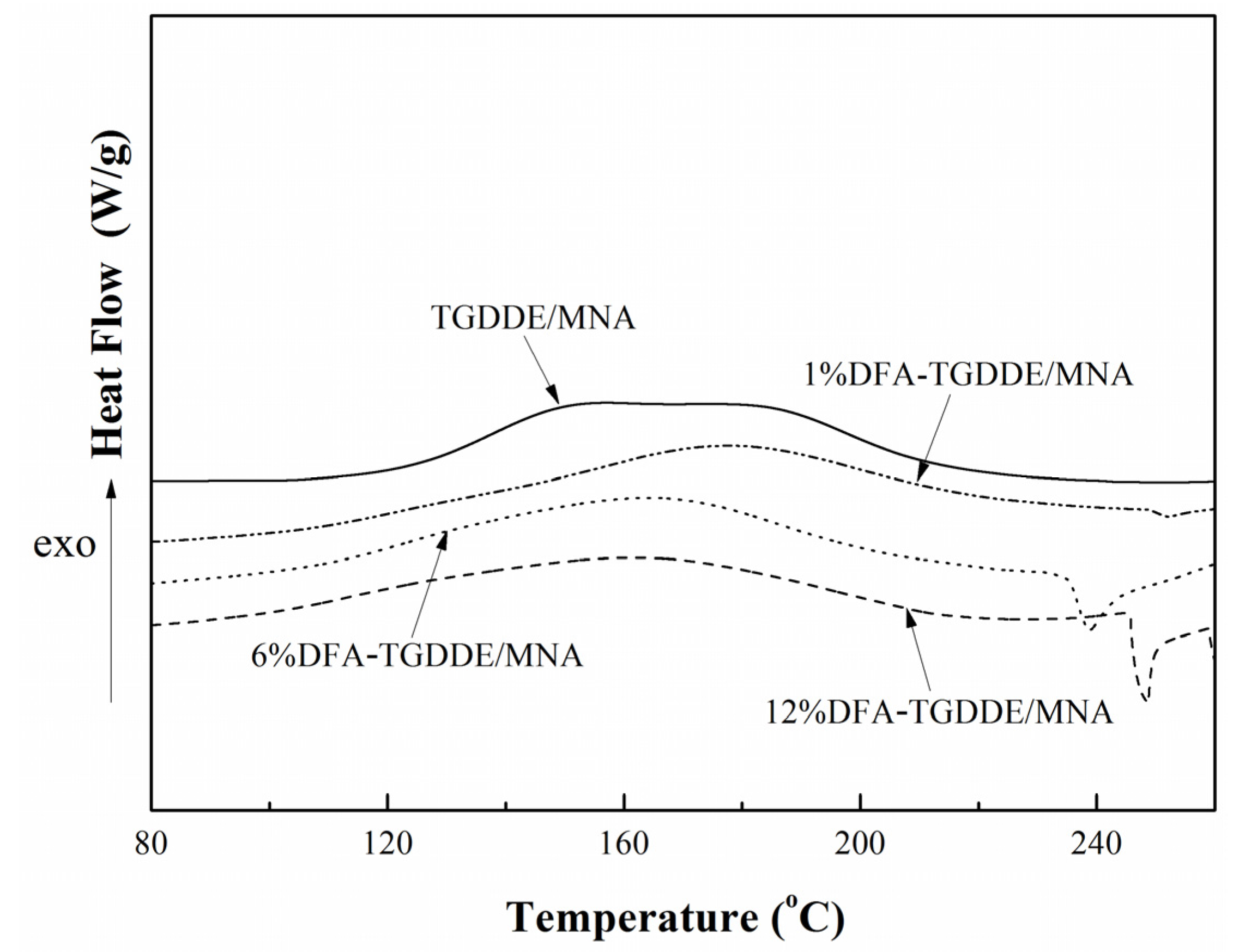
| Systems | Ti (°C) | Tp (°C) | Tf (°C) | ΔH (J·g−1) |
|---|---|---|---|---|
| TGDDE/MNA | 122 | 187 | 216 | 109.5 |
| 1%DFA-TGDDE/MNA | 105 | 177 | 219 | 155.0 |
| 6%DFA-TGDDE/MNA | 98 | 165 | 213 | 189.3 |
| 12%DFA-TGDDE/MNA | 89 | 162 | 213 | 199.4 |
2.2.2. Curing Kinetics
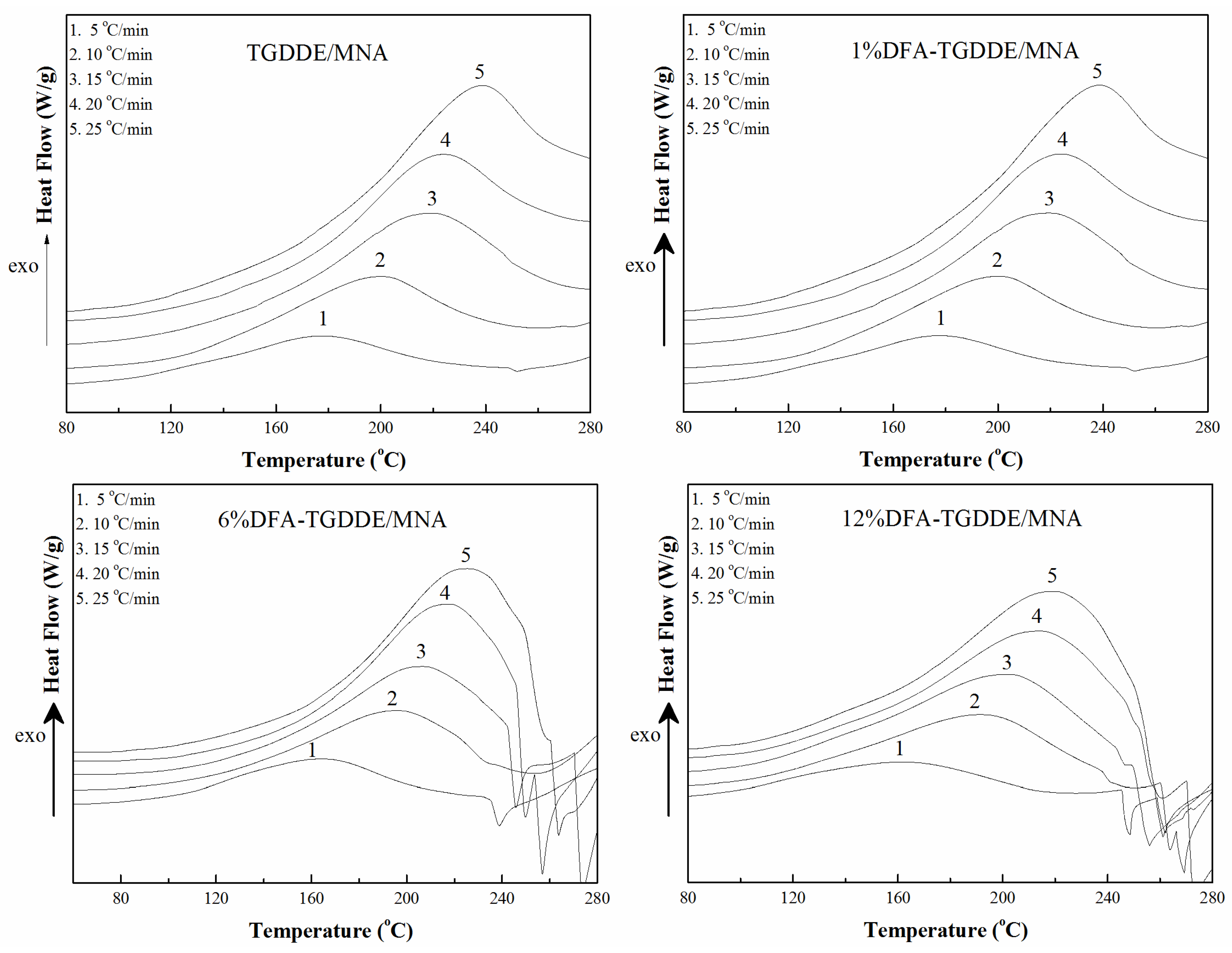

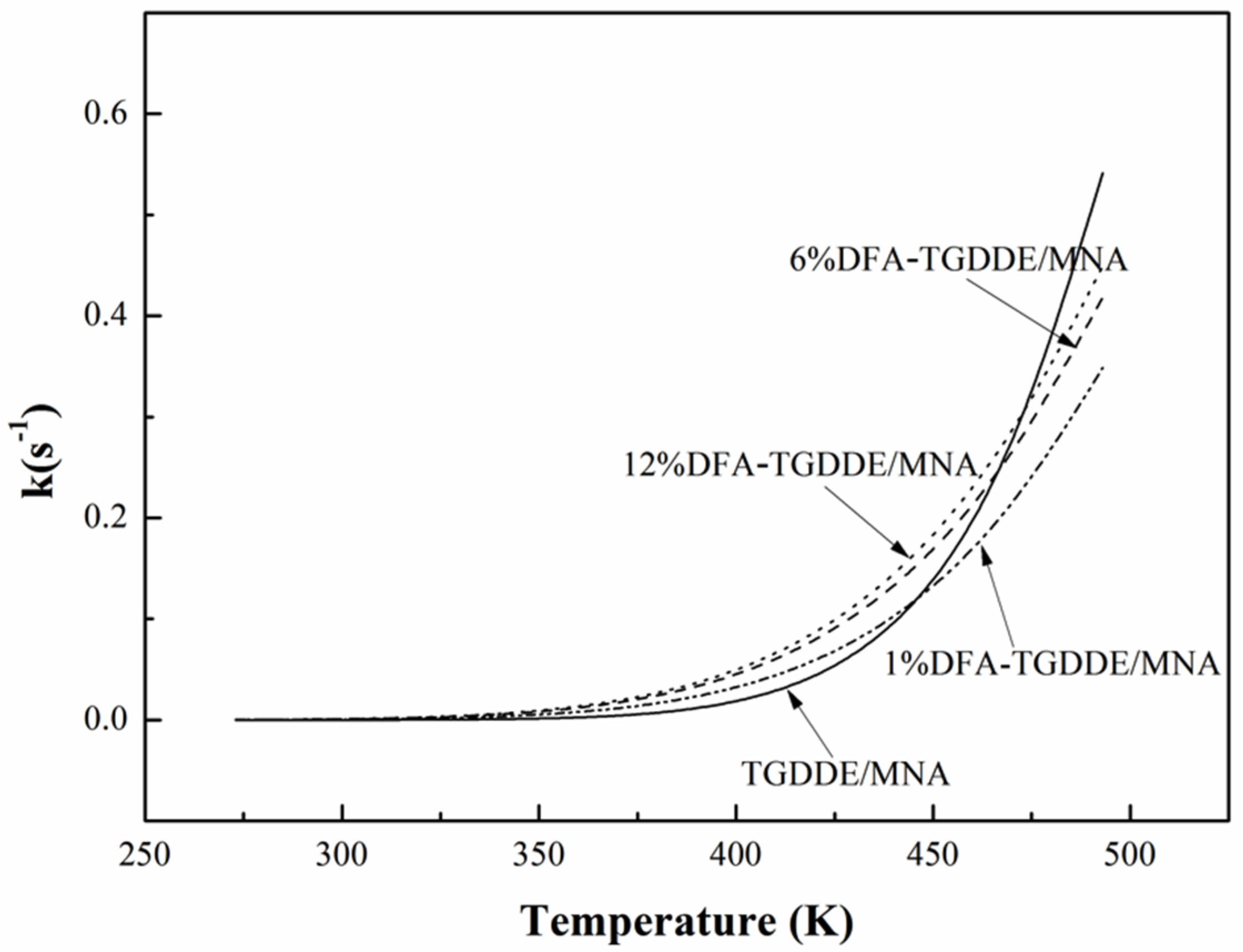
| Systems | Ek (kJ/mol) | Eo (kJ/mol) | E (kJ/mol) | A (s−1) | n |
|---|---|---|---|---|---|
| TGDDE/MNA | 61.57 | 66.13 | 63.85 | 1.81 × 106 | 0.918 |
| 1%DFA-TGDDE/MNA | 42.88 | 48.32 | 45.60 | 1.22 × 104 | 0.897 |
| 6%DFA-TGDDE/MNA | 40.09 | 45.47 | 42.78 | 7.39 × 103 | 0.894 |
| 12%DFA-TGDDE/MNA | 39.65 | 45.01 | 42.33 | 7.14 × 103 | 0.894 |
2.3. Viscoelasticity of the Cured Resins
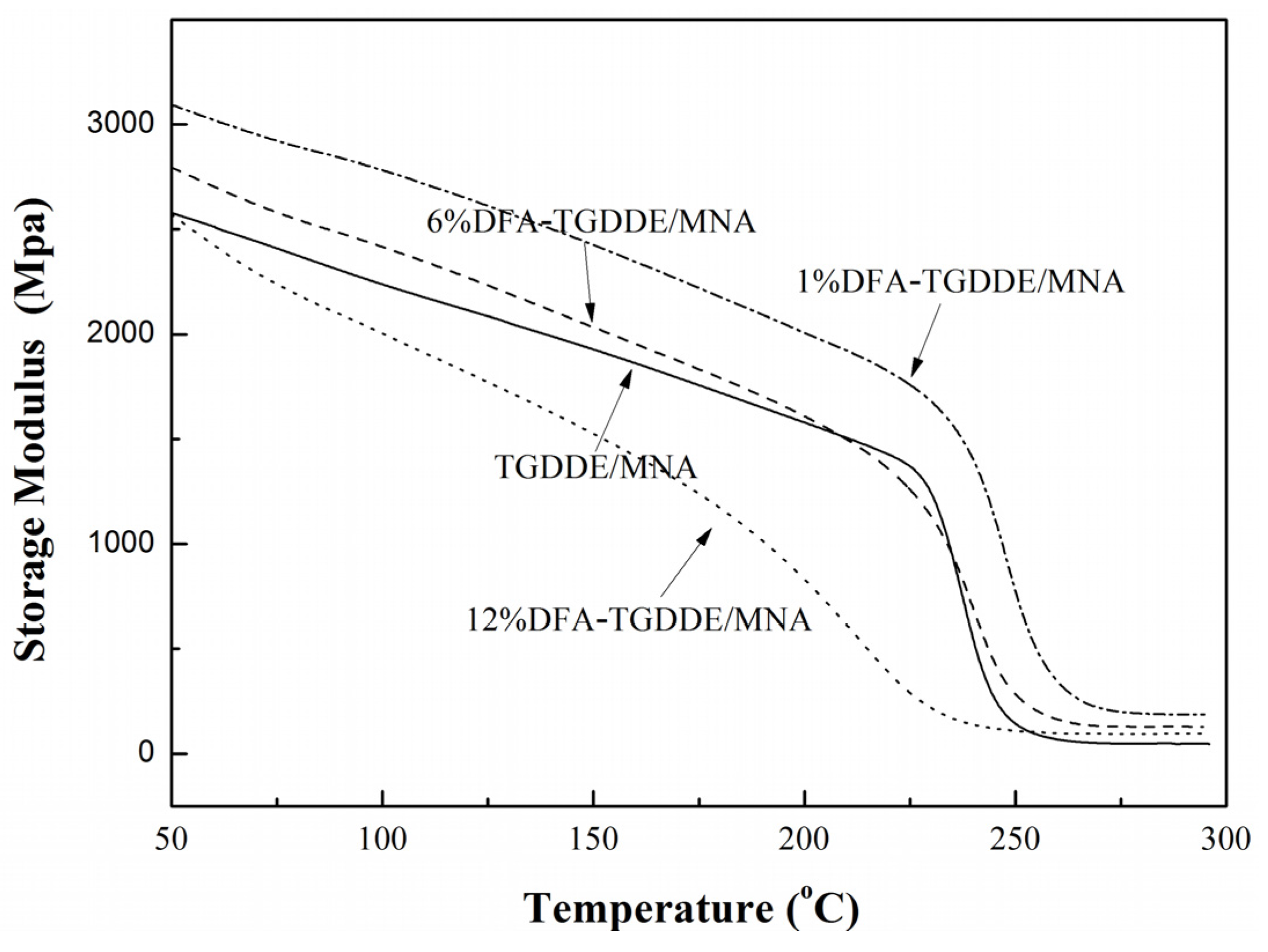
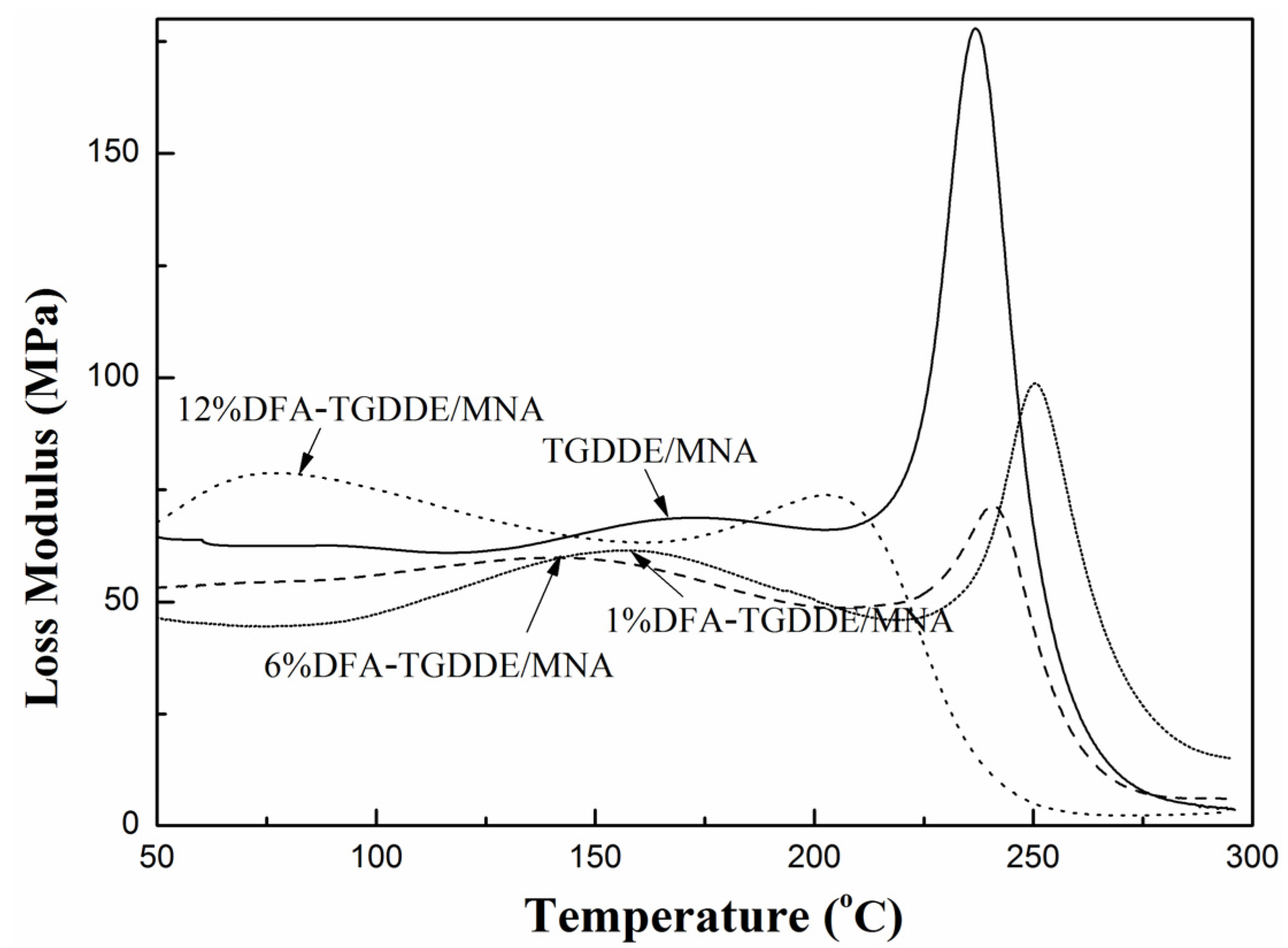
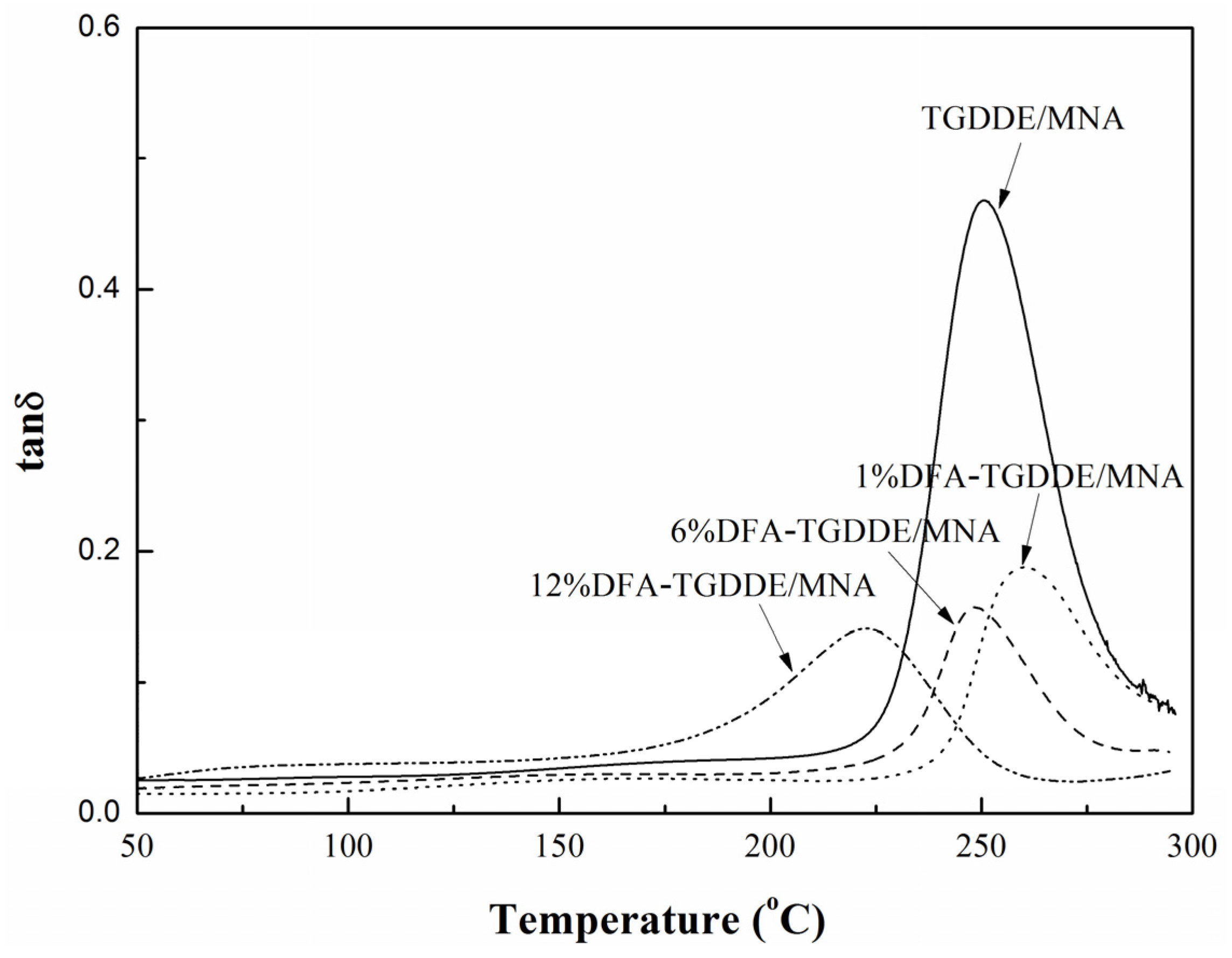
| Systems | DFA (g) | TGDDE (g) | Tg (°C) | Impact Strength (kJ/m2) |
|---|---|---|---|---|
| TGDDE/MNA | – | 100 | 230 | 4.14 |
| 1%DFA-TGDDE/MNA | 2 | 100 | 237 | 5.59 |
| 6%DFA-TGDDE/MNA | 14 | 100 | 228 | 6.31 |
| 12%DFA-TGDDE/MNA | 30 | 100 | 188 | 7.13 |
3. Experimental Section
3.1. Materials
3.2. Preparation of DFA-TGDDE
| Systems | DFA (g) | TGDDE (g) | EEW (g/mol) |
|---|---|---|---|
| 1%DFA-TGDDE | 2 | 100 | 121 |
| 6%DFA-TGDDE | 14 | 100 | 128 |
| 12%DFA-TGDDE | 30 | 100 | 137 |
3.3. Preparation of Cured Epoxy Resins
| Systems | Epoxy (g) | Curing Agent (g) |
|---|---|---|
| TGDDE/MNA | 100 | 126 |
| 1%DFA-TGDDE/MNA | 100 | 125 |
| 6%DFA-TGDDE/MNA | 100 | 118 |
| 12%DFA-TGDDE/MNA | 100 | 110 |
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.B. Handbook of Composites Based on Polymer; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Toldy, A.; Szolnoki, B.; Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 2011, 96, 371–376. [Google Scholar] [CrossRef]
- Edwards, E.R.; Kostov, K.G.; Botelho, E.C. Evaluation of the chemical interaction between carbon nanotubes functionalized with TGDDM tetrafunctional resin and hardener DDS. Compos. B Eng. 2013, 51, 197–203. [Google Scholar] [CrossRef]
- Chen, W.M.; Tao, Z.Q.; Fan, L.; Yang, S.Y.; Jiang, W.G.; Wang, J.F.; Xiong, Y.L. Effect of poly(etherimide) chemical structures on the properties of epoxy/poly(etherimide) blends and their carbon fiber-reinforced composites. J. Appl. Polym. Sci. 2011, 119, 3162–3169. [Google Scholar] [CrossRef]
- Charalampos, K.; Everson, K.; Baljinder, K.K. The effect of nanoparticles on structural morphology, thermal and flammability properties of two epoxy resins with different functionalities. Polym. Degrad. Stab. 2011, 96, 529–540. [Google Scholar]
- Kinloch, A.J.; Lee, S.H.; Taylor, A.C. Improving the fracture toughness and the cyclic-fatigue resistance of epoxy-polymer blends. Polymer 2014, 55, 6325–6334. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, H.T.; Zhou, J.G.; Wang, H.G.; Lin, T. Synergistic effects of PEK-C/VGCNF composite nanofibres on a trifunctional epoxy resin. Compos. Sci. Technol. 2011, 71, 1060–1067. [Google Scholar] [CrossRef]
- Li, G.; Huang, Z.B.; Xin, C.L.; Jia, X.L.; Yang, X.P. Curing kinetics and mechanisms of polysulfone nanofibrous membranes toughened epoxy/amine systems using isothermal DSC and NIR. Thermochim. Acta 2010, 497, 27–34. [Google Scholar] [CrossRef]
- Wang, Y.S. Solvent Resistance of Epoxy Resins Toughened with Polyethersulfone. US8686069 B2, 12 October 2010. [Google Scholar]
- Yu, M.M.; Feng, B.; Yang, M.; Liu, L.Q.; Li, H.; Ren, M.S.; Sun, J.L.; Zhang, J.B. Toughness and thermal properties of a dimer acid modified tetrafunctional epoxy resin. Appl. Mech. Mater. 2015, 764, 107–110. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.F.; Yang, S.G.; Zhao, N.; Zhang, X.L.; Xu, J. Kinetics and thermal properties of epoxy resins based on bisphenol fluorine. Eur. Polym. J. 2009, 45, 1941–1948. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Crane, L.W.; Dynes, P.J.; Kaelble, D.H. Analysis of curing kinetics in polymer composites. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 533–540. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Feng, B.; Xie, W.; Fang, L.; Li, H.; Liu, L.; Ren, M.; Sun, J.; Zhang, J.; Hu, H. The Modification of a Tetrafunctional Epoxy and Its Curing Reaction. Materials 2015, 8, 3671-3684. https://doi.org/10.3390/ma8063671
Yu M, Feng B, Xie W, Fang L, Li H, Liu L, Ren M, Sun J, Zhang J, Hu H. The Modification of a Tetrafunctional Epoxy and Its Curing Reaction. Materials. 2015; 8(6):3671-3684. https://doi.org/10.3390/ma8063671
Chicago/Turabian StyleYu, Mingming, Bin Feng, Wang Xie, Lin Fang, Hong Li, Liqi Liu, Musu Ren, Jinliang Sun, Jiabao Zhang, and Hefeng Hu. 2015. "The Modification of a Tetrafunctional Epoxy and Its Curing Reaction" Materials 8, no. 6: 3671-3684. https://doi.org/10.3390/ma8063671




