Photocatalytic Membrane Reactor for the Removal of C.I. Disperse Red 73
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
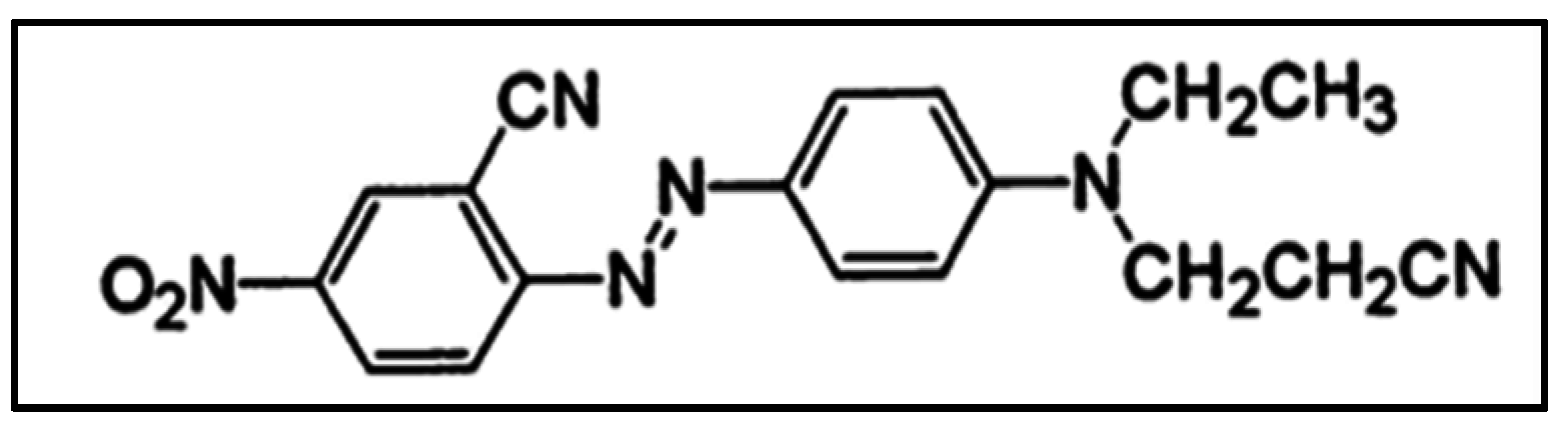
2.2. Photocatalytic Membrane Experiments
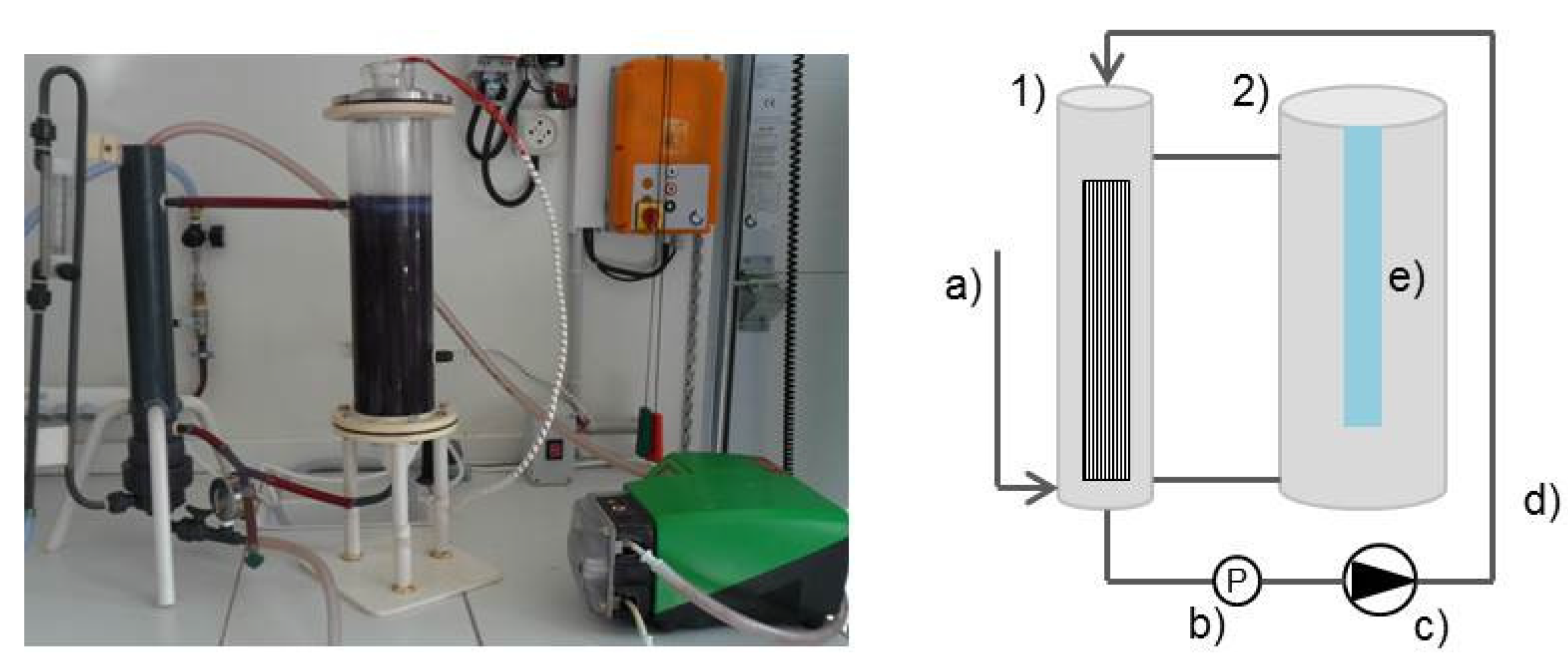
| Material | Polysulfone |
|---|---|
| Nominal pore size (µm) | 0.2 |
| External diameter (mm) | 1.4 |
| Fiber length (cm) | 32 |
| Surface area (m2) | 0.3 |
| Hydraulic resistance (m−1) | 4·1011 |
| Pure water permeability at 20 °C (L·h−1·m−2·bar) | 227 |
2.3. Analytical Methods and Measurements
3. Results and Discussion
3.1. Previous Studies

3.2. Photocatalytic Degradation of DR73
3.2.1. Effect of Initial pH
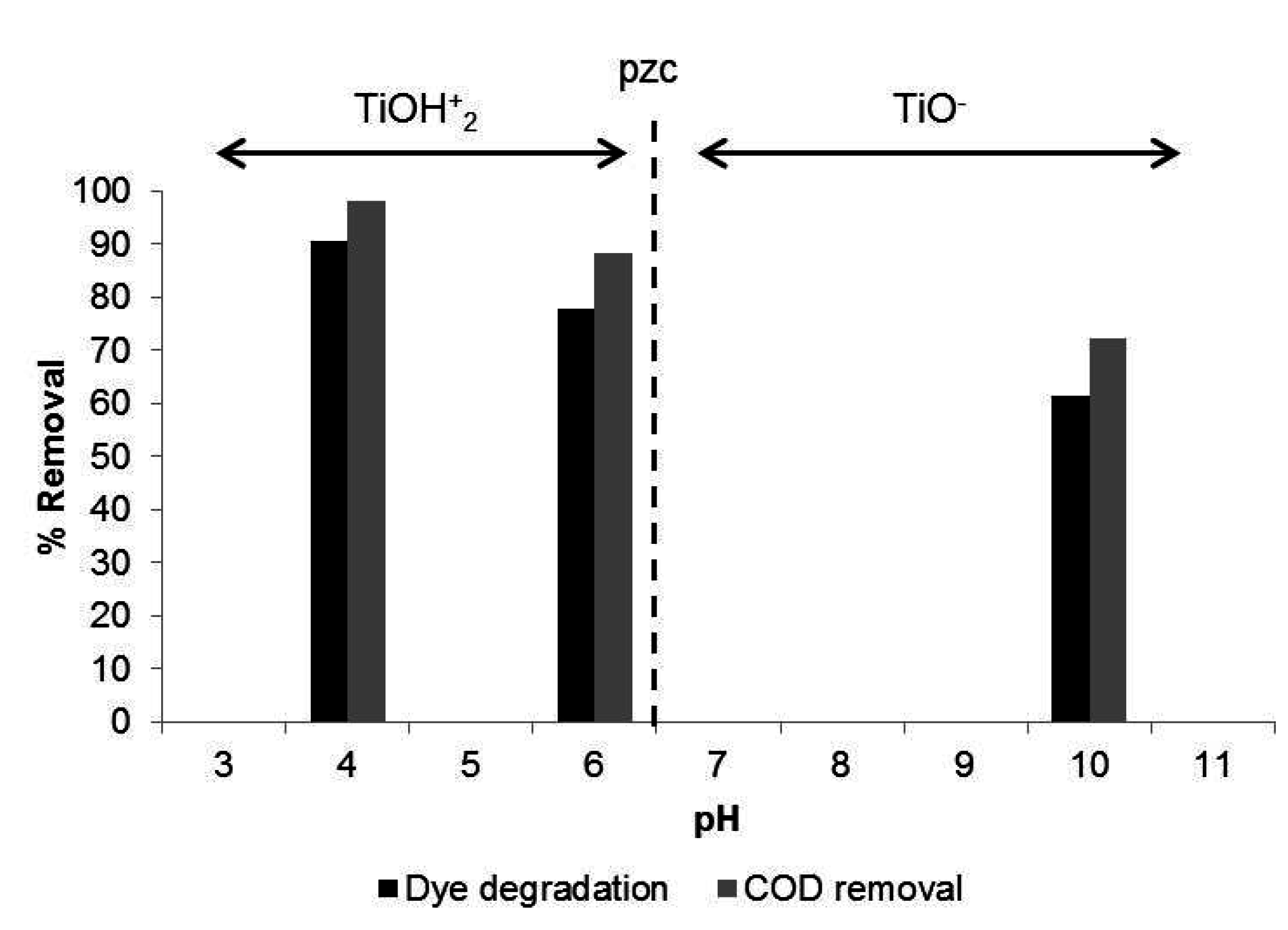
3.2.2. Effect of Photocatalyst Loading
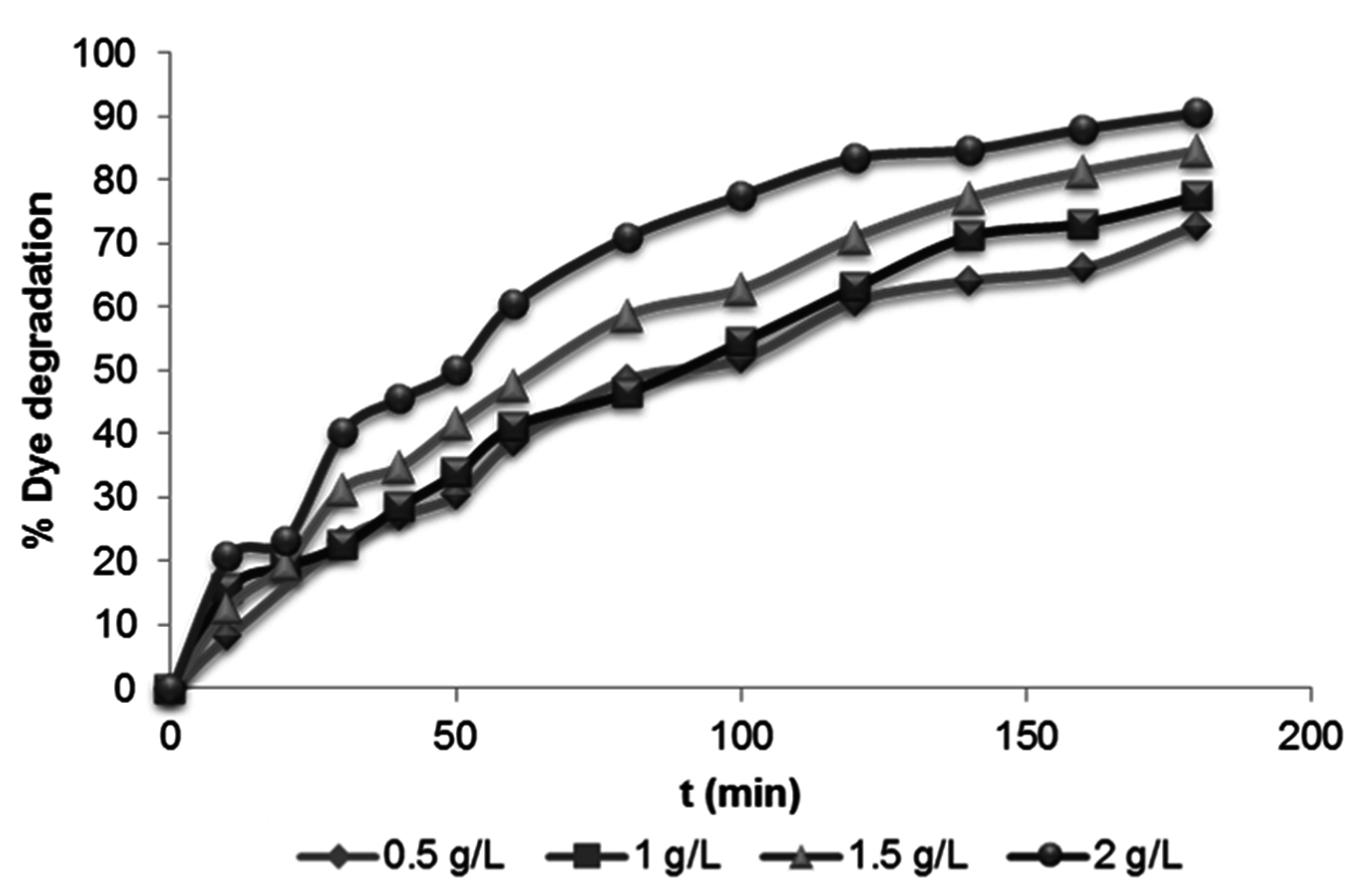
3.2.3. Effect of Initial Dye Concentration
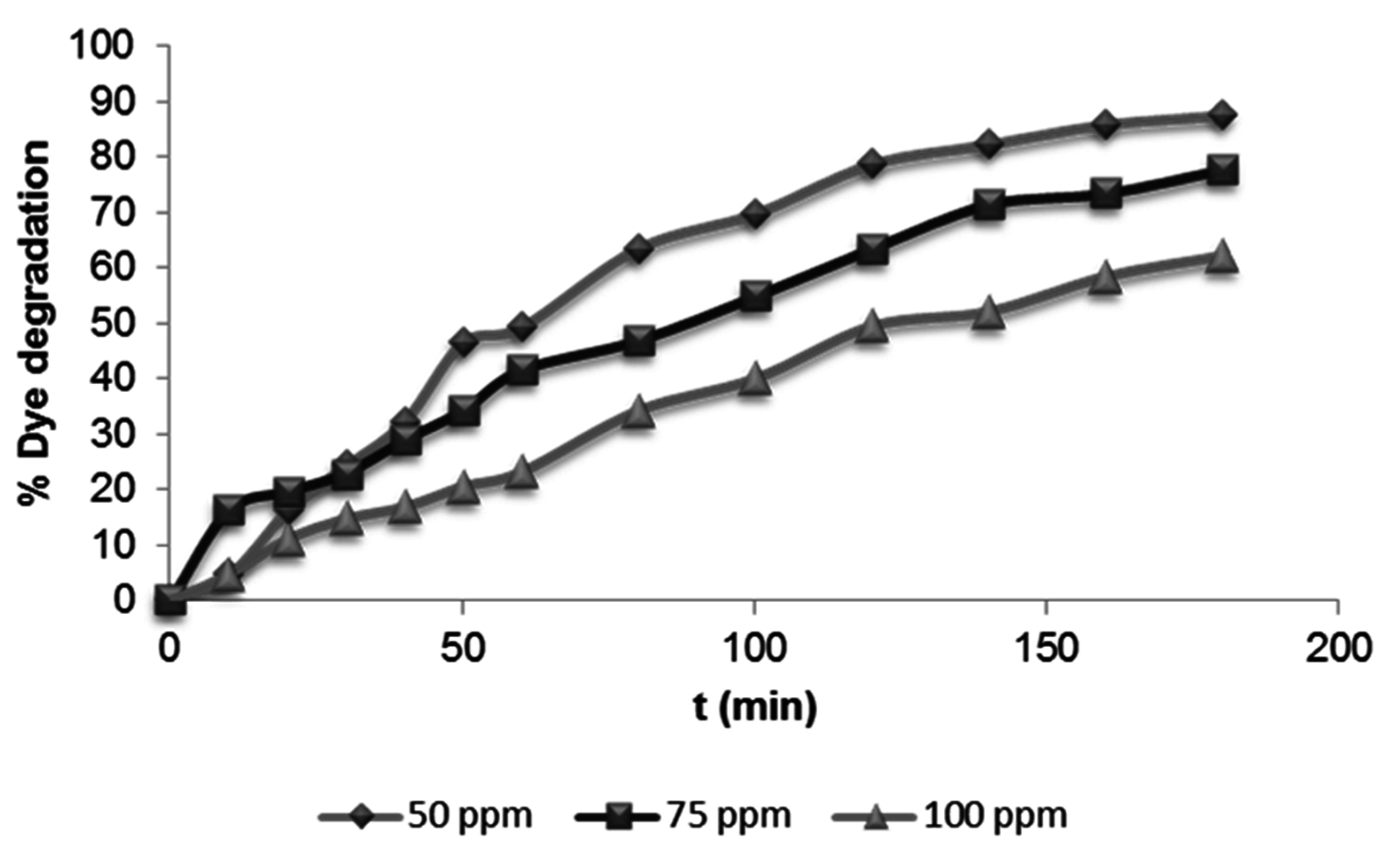
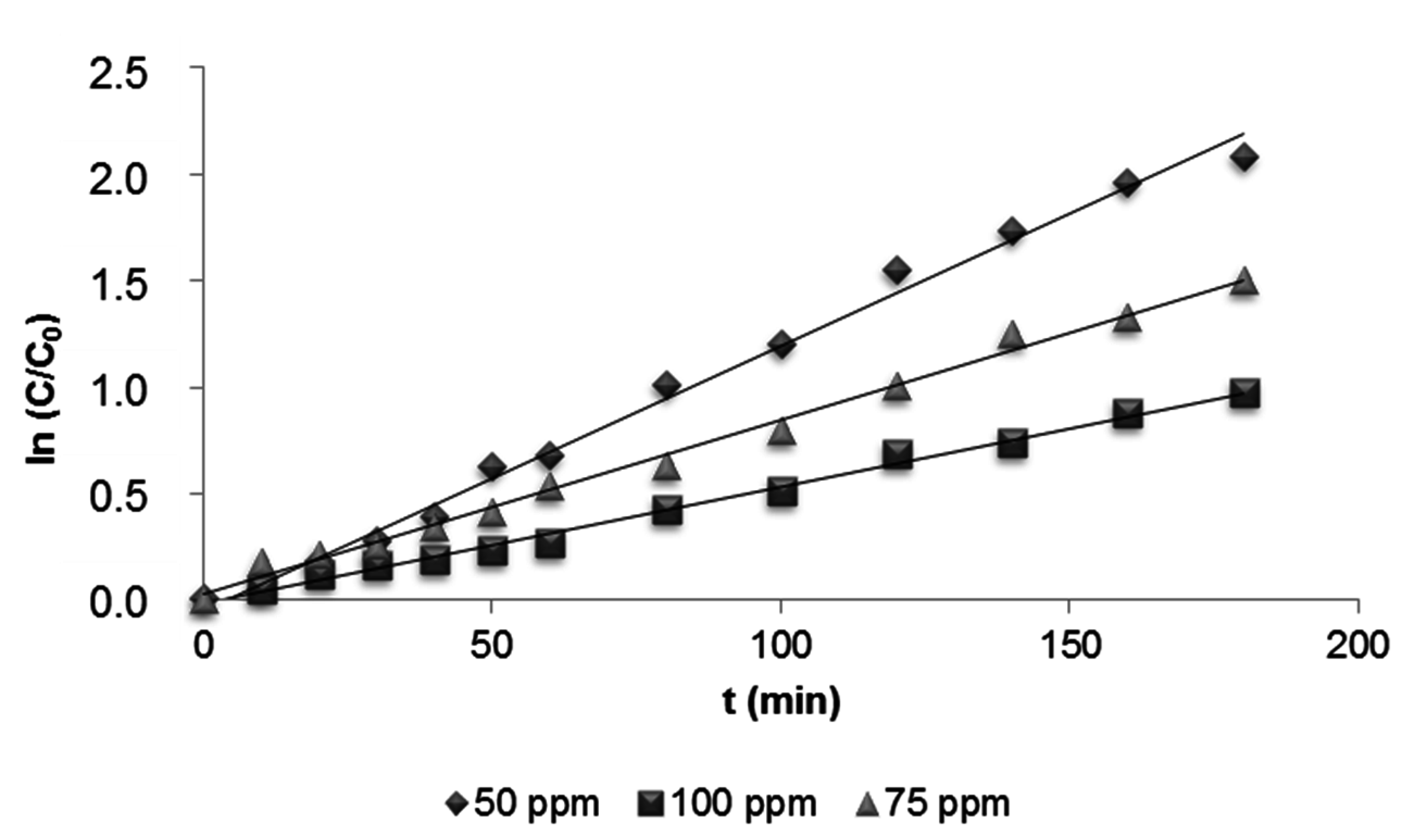
| Dye Concentration (mg·L−1) | Kapp (min−1) | r2 |
|---|---|---|
| 50 | 0.0124 | 0,9937 |
| 75 | 0.0082 | 0,9934 |
| 100 | 0.0055 | 0,9943 |
3.3. Photocatalytic Membrane Treatment
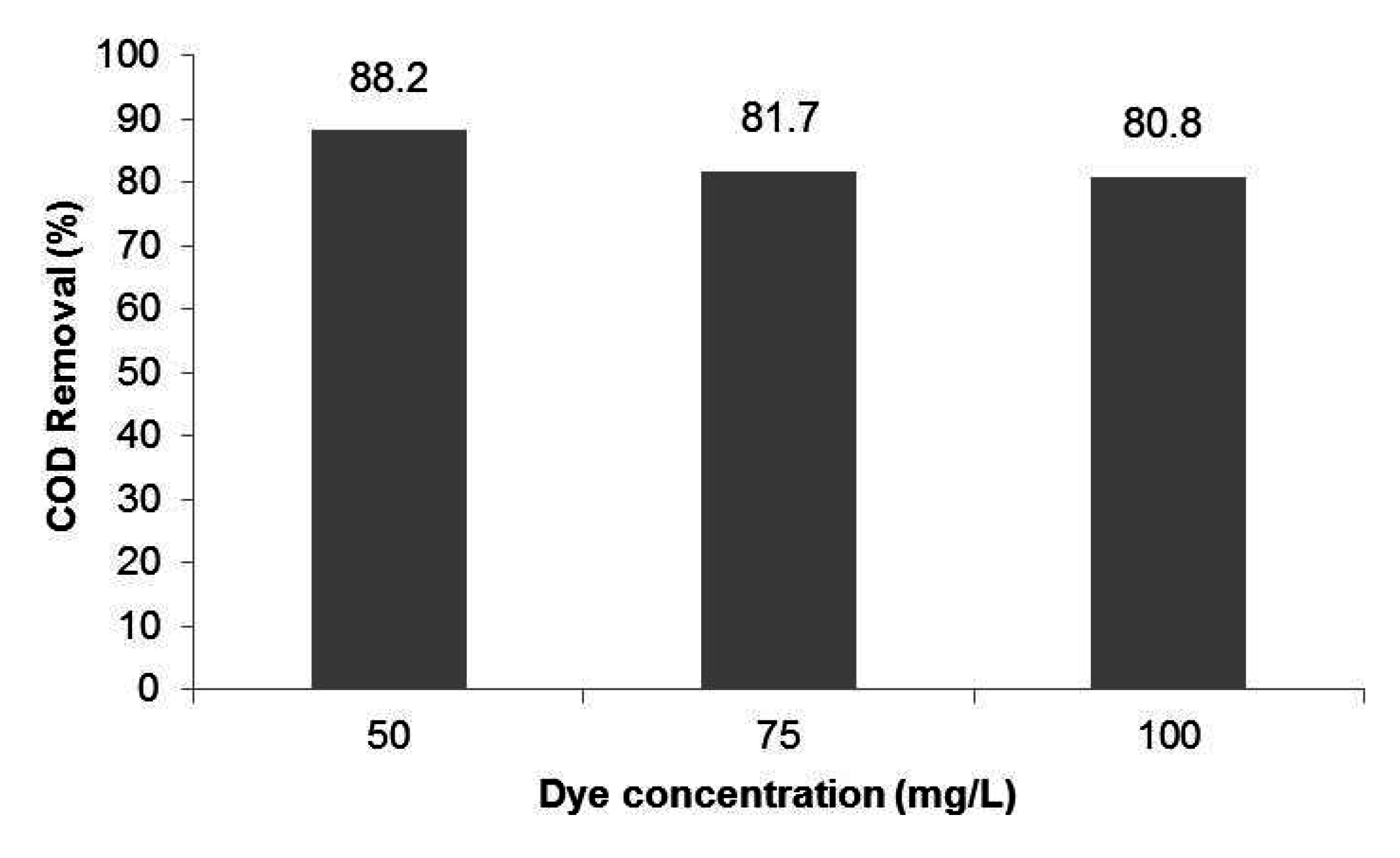
3.3.1. Permeate Quality
3.3.2. Effect of Photocatalytic Treatment on Membrane Fouling
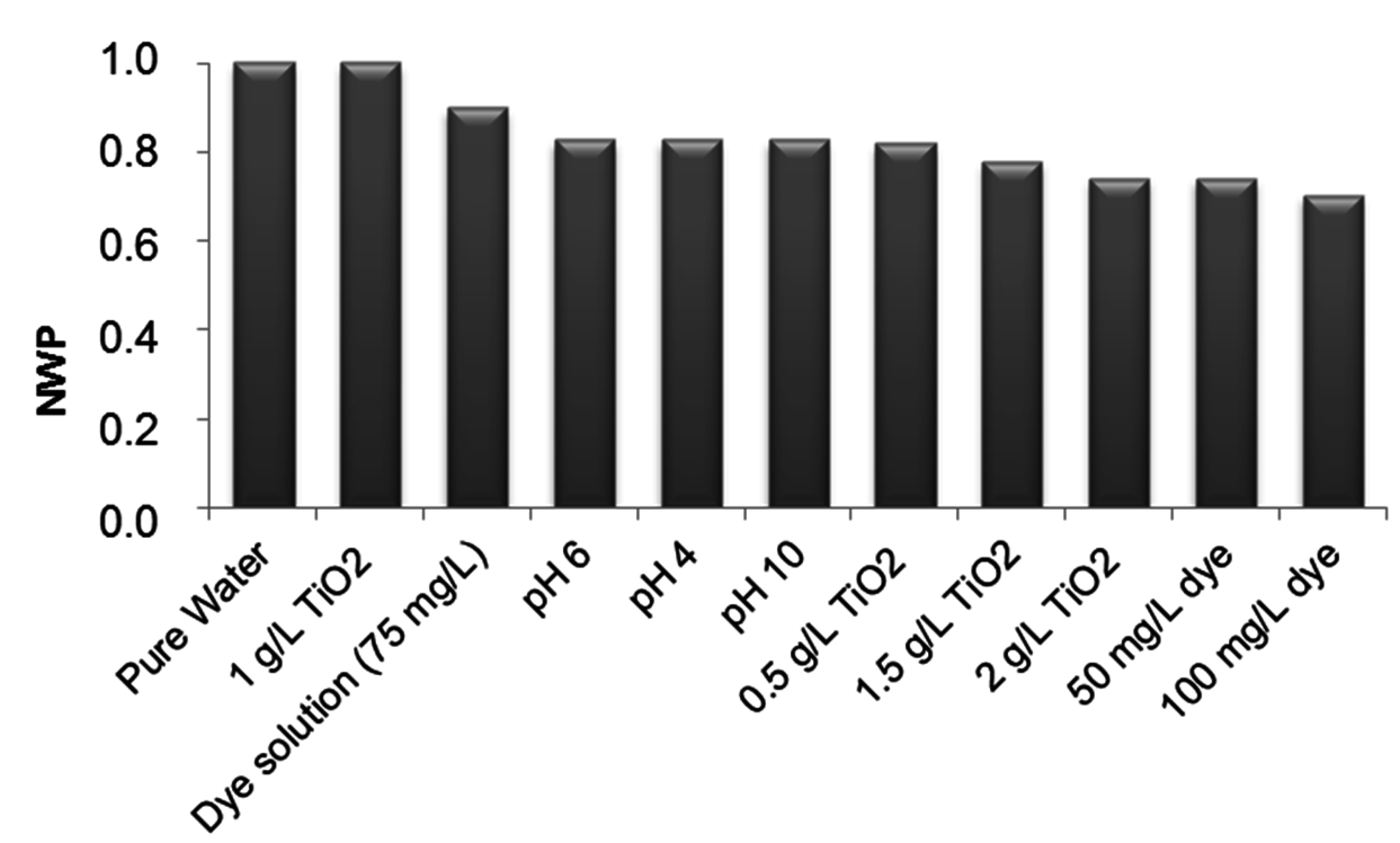
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riera-Torres, M.; Gutiérrez, M.-C. Colour removal of three reactive dyes by UV light exposure after electrochemical treatment. Chem. Eng. J. 2010, 156, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Sapkal, R.T.; Shinde, S.S.; Mahadik, M.A.; Mohite, V.S.; Waghmode, T.R.; Govindwar, S.P.; Rajpure, K.Y.; Bhosale, C.H. Photoelectrocatalytic decolorization and degradation of textile effluent using ZnO thin films. J. Photochem. Photobiol. B 2012, 114, 102–127. [Google Scholar] [CrossRef]
- Fersi, C.; Gzara, L.; Dhahbi, M. Treatment of textile effluents by membrane technologies. Desalination 2005, 185, 399–409. [Google Scholar] [CrossRef]
- Riera-Torres, M.; Gutiérrez-Bouzán, C.; Crespi, M. Combination of coagulation–flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination 2010, 252, 53–59. [Google Scholar] [CrossRef]
- Nigmet, U.Z.A.L.; Levent Yilmaz, U.Y. Nanofiltration and Reverse Osmosis for Reuse of Indigo Dye Rinsing Waters. Sep. Sci. Technol. 2010, 45, 331–338. [Google Scholar]
- Körbahti, B.K.; Tanyolaç, A. Continuous electrochemical treatment of simulated industrial textile wastewater from industrial components in a tubular reactor. J. Hazard. Mater. 2009, 170, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pía, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Polgumhang, S.; Tongdaung, W.; Karakat, B.; Nuyut, T. Electrocoagulation of blue reactive, red disperse and mixed dyes, and application in treating textile effluent. J. Environ. Manag. 2010, 91, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Schrank, S.G.; dos Santos, J.N.R.; Souza, D.S.; Souza, E.E.S. Decolourisation effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. J. Photochem. Photobiol. A Chem. 2007, 186, 125–129. [Google Scholar] [CrossRef]
- Qu, D.; Qiang, Z.; Xiao, S.; Liu, Q.; Lei, Y.; Zhou, T. Degradation of Reactive Black 5 in a submerged photocatalytic membrane distillation reactor with microwave electrodeless lamps as light source. Sep. Purif. Technol. 2014, 122, 54–59. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, D.; Gao, H.; Deng, L. Mechanism of enhanced photocatalysis of TiO2 by Fe3+ in suspensions. Appl. Surf. Sci. 2011, 258, 1294–1299. [Google Scholar] [CrossRef]
- Garza-Campos, B.R.; Guzmán-Mar, J.L.; Reyes, L.H.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.J.; Brillas, E. Coupling of solar photoelectro-Fenton with a BDD anode and solar heterogeneous photocatalysis for the mineralization of the herbicide atrazine. Chemosphere 2014, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Liang, R.; Zhang, X.; Kurdi, S.; Luong, D.; Huang, H.; Penga, P.; Marzbanrada, E.; Zhou, Y.; Oakesb, K.D.; et al. Enhanced Photocatalytic Degradation of dyes by TiO2 Nanobelts with Hierarchical Structures. J. Photochem. Photobiol. A Chem. 2013, 256, 7–15. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Kertèsz, S.; Cakl, J.; Jiránková, H. Submerged hollow fiber microfiltration as a part of hybrid photocatalytic process for dye wastewater treatment. Desalination 2014, 343, 106–112. [Google Scholar] [CrossRef]
- Choo, K.H.; Chang, D.I.; Park, K.W.; Kim, M.H. Use of an integrated photocatalysis/hollow fiber microfiltration system for the removal of trichloroethylene in water. J. Hazard. Mater. 2008, 152, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Choo, K.-H.; Kim, M.-H. A hybridized photocatalysis-microfiltration system with iron oxide-coated membranes for the removal of natural organic matter in water treatment: Effects of iron oxide layers and colloids. Water Res. 2009, 43, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Horng, R.Y.; Huang, C.; Chang, M.-C.; Shao, H.; Shiau, B.-L.; Hu, Y.-J. Application of TiO2 photocatalytic oxidation and non-woven membrane filtration hybrid system for degradation of 4-chlorophenol. Desalination 2009, 245, 169–182. [Google Scholar] [CrossRef]
- Reguero, V.; López-Fernández, R.; Fermoso, J.; Prieto, O.; Pocostales, P.; González, R.; Irusta, R.; Villaverde, S. Comparison of conventional technologies and a Submerged Membrane Photocatalytic Reactor (SMPR) for removing trihalomethanes (THM) precursors in drinking water treatment plants. Desalination 2013, 330, 28–34. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.; Huang, T.; Chen, J.; Wang, Q.; Meng, Q. Photocatalytic membrane reactor for degradation of acid red B wastewater. Chem. Eng. J. 2010, 156, 571–577. [Google Scholar] [CrossRef]
- Patsios, S.I.; Sarasidis, V.C.; Karabelas, A.J. A hybrid photocatalysis–ultrafiltration continuous process for humic acids degradation. Sep. Purif. Technol. 2013, 104, 333–341. [Google Scholar] [CrossRef]
- Berberidou, C.; Avlonitis, S.; Poulios, I. Dyestuff effluent treatment by integrated sequential photocatalytic oxidation and membrane filtration. Desalination 2009, 249, 1099–1106. [Google Scholar] [CrossRef]
- Martínez, F.; López-Muñoz, M.J.; Aguado, J.; Melero, J.A.; Arsuaga, J.; Sotto, A.; Molinaa, R.; Seguraa, Y.; Parientea, M.I.; Revillaa, A.; et al. Coupling membrane separation and photocatalytic oxidation processes for the degradation of pharmaceutical pollutants. Water Res. 2013, 47, 5647–5658. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S.; Darowna, D.; Szymański, K.; Grondzewska, S.; Borchert, K.; Wróbel, R.; Morawski, A.W. Performance of two photocatalytic membrane reactors for treatment of primary and secondary effluents. Catal. Today 2014, 236, 135–145. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Inagaki, M. Effectiveness of photodecomposition of an azo dye on a novel anatase-phase TiO2 and two commercial photocatalysts in a photocatalytic membrane reactor (PMR). Sep. Purif. Technol. 2008, 63, 386–391. [Google Scholar] [CrossRef]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Grzechulska-Damszel, J.; Mozia, S.; Morawski, A.W. Integration of photocatalysis with membrane processes for purification of water contaminated with organic dyes. Catal. Today 2010, 156, 295–300. [Google Scholar] [CrossRef]
- Damodar, R.; You, S.-J.; Ou, S.-H. Coupling of membrane separation with photocatalytic slurry reactor for advanced dye wastewater treatment. Sep. Purif. Technol. 2010, 76, 64–71. [Google Scholar] [CrossRef]
- Ma, S.; Meng, J.; Li, J.; Zhang, Y.; Ni, L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J. Membr. Sci. 2014, 453, 221–229. [Google Scholar] [CrossRef]
- Paschoal, F.M.M.; Anderson, M.A.; Zanoni, M.V.B. The photoelectrocatalytic oxidative treatment of textile wastewater containing disperse dyes. Desalination 2009, 249, 1350–1355. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Koh, J. Chapter 10: Dyeing with Disperse Dyes. In Textile Dyeing, 1st ed.; Hauser, P.J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 195–220. [Google Scholar]
- Boyjoo, Y.; Ang, M.; Pareek, V. Photocatalytic Treatment of Shower Water Using a Pilot Scale Reactor. Int. J. Photoenergy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Bizani, E.; Fytianos, K.; Poulios, I.; Tsiridis, V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J. Hazard. Mater. 2006, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Visa, T.; Sánchez, M.; López-Grimau, V.; Navarro, R.; Reche, S.; Gutiérrez-Bouzán, M.C. Photocatalysis with titanium dioxide to remove colour of exhausted reactive dyebaths without pH modification. Desalin. Water Treat. 2012, 45, 91–99. [Google Scholar] [CrossRef]
- Muhammad, U.; Hamidi Abdul, A. Chapter 8: Photocatalytic Degradation of Organic Pollutants in Water. In Organic Pollutants - Monitoring, Risk and Treatment; InTech: Rijeka, Croatia, 2013; pp. 198–208. [Google Scholar]
- Lu, C.S.; Mai, F.D.; Wu, C.W.; Wu, R.J.; Chen, C.C. Titanium dioxide-mediated photocatalytic degradation of Acridine Orange in aqueous suspensions under UV irradiation. Dyes Pigments 2008, 76, 706–713. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, L.; Li, S. Photocatalytic degradation of Reactive Brilliant Blue KN-R by TiO2/UV process. Desalination 2010, 258, 48–53. [Google Scholar] [CrossRef]
- Toor, A.P.; Verma, A.; Jotshi, C.K.; Bajpai, P.K.; Singh, V. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes Pigments 2006, 68, 53–60. [Google Scholar] [CrossRef]
- Habib, A.; Ismail, I.M.I.; Mahmood, A.J.; Ullah, R. Photocatalytic decolorization of Brilliant Golden Yellow in TiO2 and ZnO suspensions. J. Saudi Chem. Soc. 2012, 16, 423–429. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Wenjuan, L.; Zhou, Y. Enhanced degradation of persistent pharmaceuticals found in wastewater treatment effluents using TiO2 nanobelt photocatalysts. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Martínez, C.; Vilariño, S.; Fernández, M.I.; Faria, J.; Canle, M.; Santaballa, J.A. Mechanism of degradation of ketoprofen by heterogeneousphotocatalysis in aqueous solution. Appl. Catal. B Environ. 2013, 142–143, 633–646. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Wang, Q.; Wang, X.; Jiang, W.; Zhang, Z.; Yang, Y. A novel double-cylindrical-shell photoreactor immobilized with monolayer TiO2-coated silica gel beads for photocatalytic degradation of Rhodamine B and Methyl Orange in aqueous solution. Sep. Purif. Technol. 2014, 123, 130–138. [Google Scholar] [CrossRef]
- Buscio, V.; Marín, M.J.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of textile wastewater after homogenization–decantation treatment coupled to PVDF ultrafiltration membranes. Chem. Eng. J. 2015, 265, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Tsumura, T. Effect of process parameters on photodegradation of Acid Yellow 36 in a hybrid photocatalysis–membrane distillation system. Chem. Eng. J. 2009, 150, 152–159. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Tsumura, T. Integration of photocatalysis and membrane distillation for removal of mono- and poly-azo dyes from water. Desalination 2010, 250, 666–672. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buscio, V.; Brosillon, S.; Mendret, J.; Crespi, M.; Gutiérrez-Bouzán, C. Photocatalytic Membrane Reactor for the Removal of C.I. Disperse Red 73. Materials 2015, 8, 3633-3647. https://doi.org/10.3390/ma8063633
Buscio V, Brosillon S, Mendret J, Crespi M, Gutiérrez-Bouzán C. Photocatalytic Membrane Reactor for the Removal of C.I. Disperse Red 73. Materials. 2015; 8(6):3633-3647. https://doi.org/10.3390/ma8063633
Chicago/Turabian StyleBuscio, Valentina, Stephan Brosillon, Julie Mendret, Martí Crespi, and Carmen Gutiérrez-Bouzán. 2015. "Photocatalytic Membrane Reactor for the Removal of C.I. Disperse Red 73" Materials 8, no. 6: 3633-3647. https://doi.org/10.3390/ma8063633






