Graft Remodeling following Transcrestal Sinus Floor Elevation via the Gel-Pressure Technique (GPT) and Pasteous Nano-Crystalline Hydroxyapatite Bone Substitute
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Inclusion and Preoperative Workup
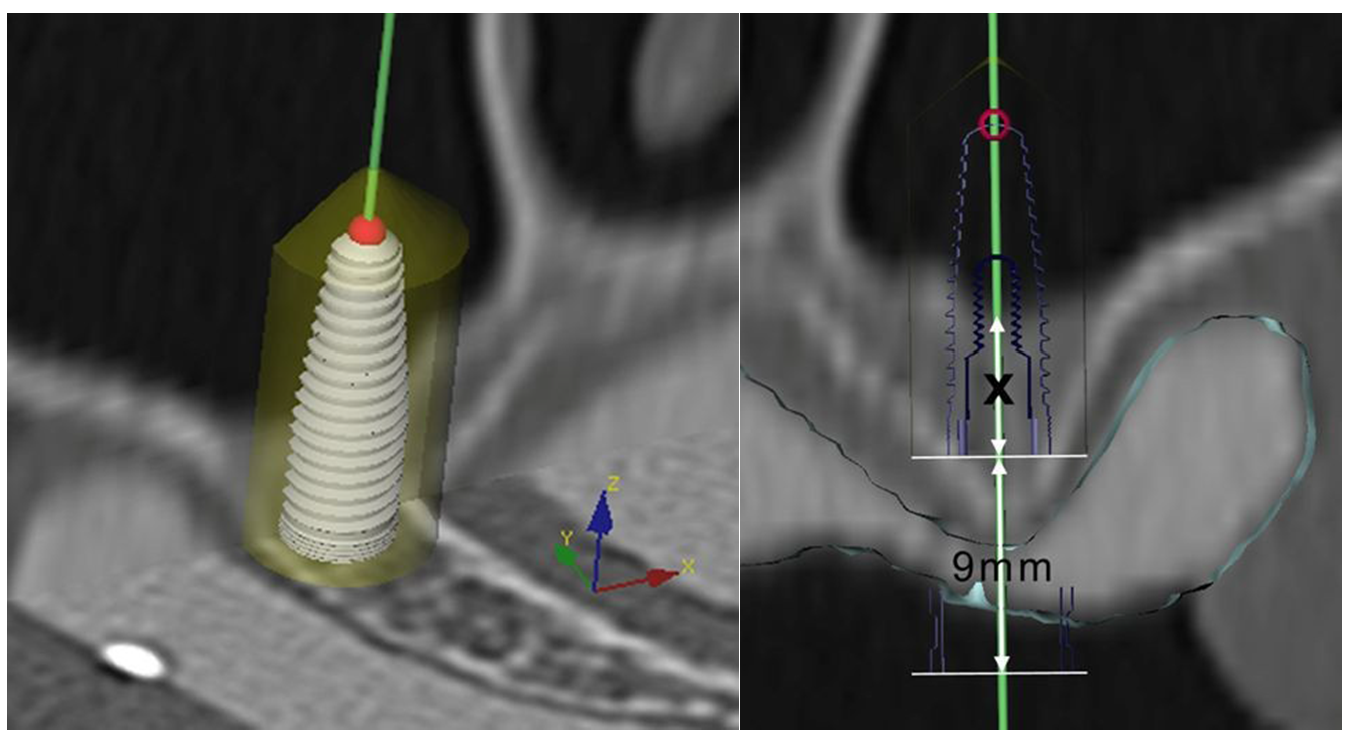
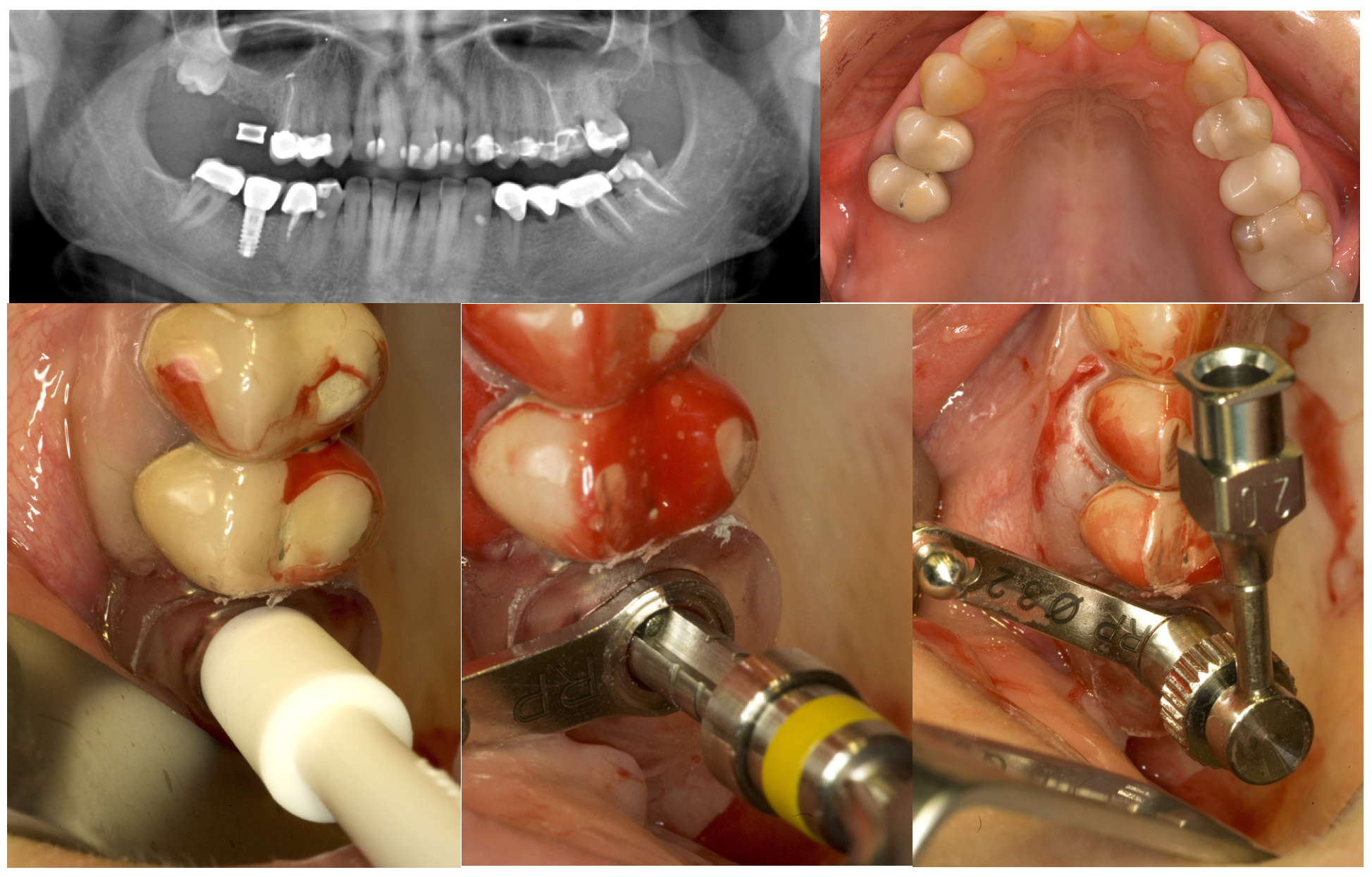
2.2. Bone Graft and Implant Surgery

2.3. Evaluation and Statistical Analysis
3. Results
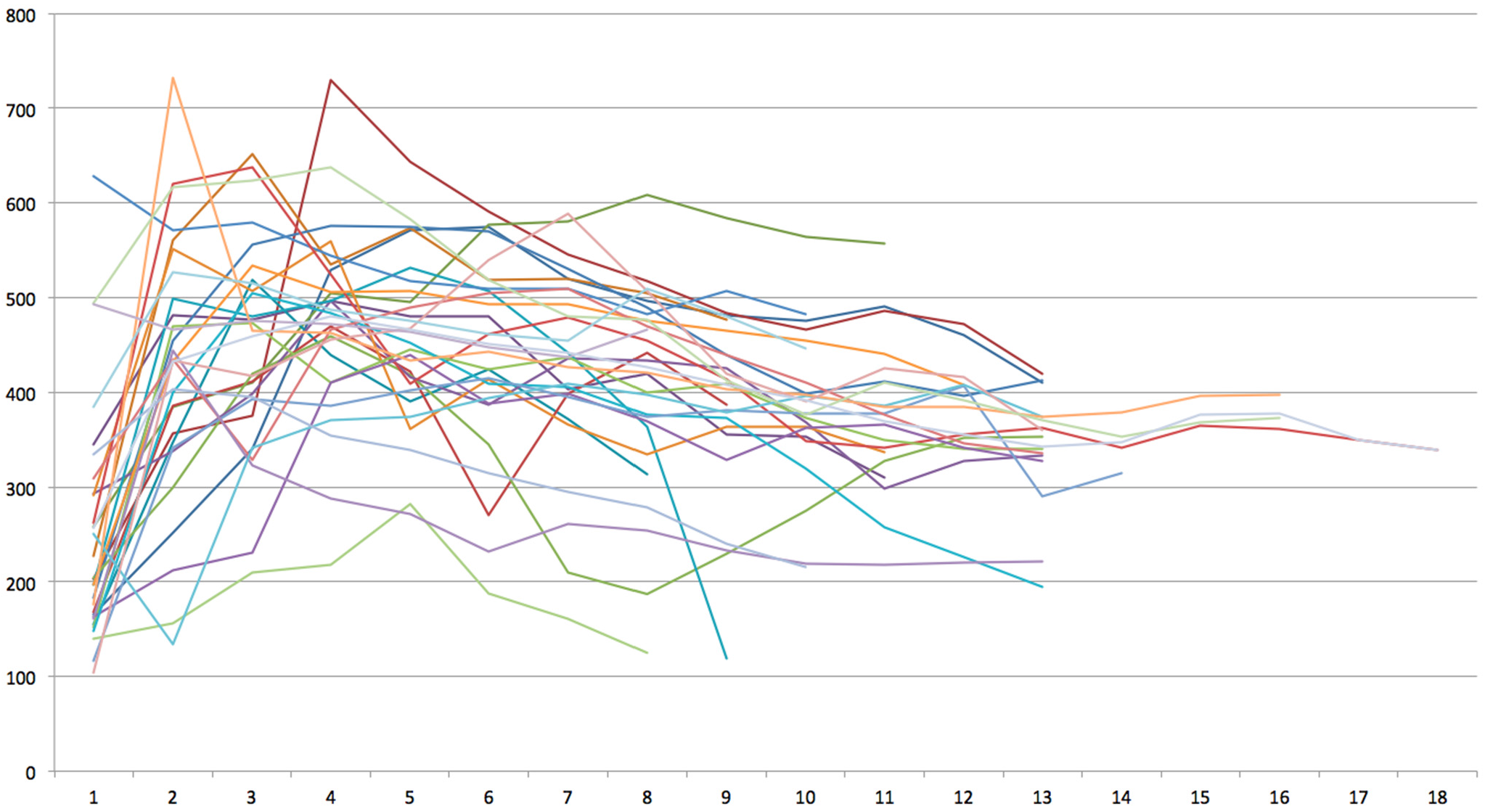
4. Discussion
| Distance to the Sinus Floor | Mean Bone Density | Percentage of Reduction |
|---|---|---|
| 1 to 7 mm | 459.6 HU | |
| 8 mm | 426.3 HU | –7.2% |
| 9 mm | 407.8 HU | –11.3% |
| 10 mm | 391.8 HU | –14.8% |
| 11 mm | 369.5 HU | –19.6% |
| 12 to 18 mm | 355.4 HU | –22.7% |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pommer, B.; Frantal, S.; Willer, J.; Posch, M.; Watzek, G.; Tepper, G. Impact of dental implant length on early failure rates: A meta-analysis of observational studies. J. Clin. Periodontol. 2011, 38, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Corbella, S.; Weinstein, T.; Ceresoli, V.; Taschieri, S. Implant survival rates after osteotome-mediated maxillary sinus augmentation: A systematic review. Clin. Implant. Dent. Relat. Res. 2012, 14, e159–e168. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone—A systematic review and meta-analysis. Eur. J. Oral. Implantol. 2014, 7, S219–S234. [Google Scholar] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, S216–S240. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Froum, S.J. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann. Periodontol. 2003, 8, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Testori, T.; Francetti, L.; Weinstein, R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int. J. Periodontics. Restorative. Dent. 2004, 24, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, D.; Att, W.; Stappert, C. Sinus floor elevation using osteotomes: A systematic review and meta-analysis. J. Periodontol. 2005, 76, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral. Implants Res. 2006, 17, S136–S159. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Hof, M.; Fädler, A.; Gahleitner, A.; Watzek, G.; Watzak, G. Primary implant stability in the atrophic sinus floor of human cadaver maxillae: Impact of residual ridge height, bone density, and implant diameter. Clin. Oral. Implants Res. 2014, 25, e109–e113. [Google Scholar] [CrossRef] [PubMed]
- Engelke, W.; Schwarzwäller, W.; Behnsen, A.; Jacobs, H.G. Subantroscopic laterobasal sinus floor augmentation (SALSA): An up-to-5-year clinical study. Int. J. Oral. Maxillofac. Implants 2003, 18, 135–143. [Google Scholar] [PubMed]
- Summers, R.B. The osteotome technique: Part 3—Less invasive methods of elevating the sinus floor. Compendium 1994, 15, 698–704. [Google Scholar] [PubMed]
- Soltan, M.; Smiler, D.G. Antral membrane balloon elevation. J. Oral. Implantol. 2005, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kfir, E.; Kfir, V.; Mijiritsky, E.; Rafaeloff, R.; Kaluski, E. Minimally invasive antral membrane balloon elevation followed by maxillary bone augmentation and implant fixation. J. Oral. Implantol. 2006, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cha, J. An 8-year retrospective study: 1,100 patients receiving 1,557 implants using the minimally invasive hydraulic sinus condensing technique. J. Periodontol. 2005, 76, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Sotirakis, E.G.; Gonshor, A. Elevation of the maxillary sinus floor with hydraulic pressure. J. Oral. Implantol. 2005, 31, 197–204. [Google Scholar] [CrossRef]
- Vitkov, L.; Gellrich, N.C.; Hannig, M. Sinus floor elevation via hydraulic detachment and elevation of the Schneiderian membrane. Clin. Oral. Implants Res. 2005, 16, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Suguimoto, R.M.; Trindade, I.K.; Carvalho, R.M. The use of negative pressure for the sinus lift procedure: A technical note. Int. J. Oral. Maxillofac. Implants 2006, 21, 455–458. [Google Scholar] [PubMed]
- Pommer, B.; Watzek, G. Gel-pressure technique for flapless transcrestal maxillary sinus floor elevation: A preliminary cadaveric study of a new surgical technique. Int. J. Oral. Maxillofac. Implants 2009, 24, 817–822. [Google Scholar] [PubMed]
- Toffler, M. Osteotome-mediated sinus floor elevation: A clinical report. Int. J. Oral. Maxillofac. Implants 2004, 19, 266–273. [Google Scholar] [PubMed]
- Nkenke, E.; Schlegel, A.; Schultze-Mosgau, S.; Neukam, F.W.; Wiltfang, J. The endoscopically controlled osteotome sinus floor elevation: A preliminary prospective study. Int. J. Oral. Maxillofac. Implants 2002, 17, 557–566. [Google Scholar] [PubMed]
- Pommer, B.; Ulm, C.; Lorenzoni, M.; Palmer, R.; Watzek, G.; Zechner, W. Prevalence, location and morphology of maxillary sinus septa: Systematic review and meta-analysis. J. Clin. Periodontol. 2012, 39, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Tepper, G.; Haas, R.; Schneider, B.; Watzak, G.; Mailath, G.; Jovanovic, S.A.; Busenlechner, D.; Zechner, W.; Watzek, G. Effects of sinus lifting on voice quality. A prospective study and risk assessment. Clin. Oral. Implants Res. 2003, 14, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Engelke, W.; Capobianco, M. Flapless sinus floor augmentation using endoscopy combined with CT scan-designed surgical templates: Method and report of 6 consecutive cases. Int. J. Oral. Maxillofac. Implants 2005, 20, 891–897. [Google Scholar] [PubMed]
- Berengo, M.; Sivolella, S.; Majzoub, Z.; Cordioli, G. Endoscopic evaluation of the bone-added osteotome sinus floor elevation procedure. Int. J. Oral. Maxillofac. Surg. 2004, 33, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B. Techniques to preserve keratinized peri-implant mucosa in CT-guided oral implant surgery. Surg. Tech. Dev. 2012, 2, 25–27. [Google Scholar] [CrossRef]
- Gahleitner, A.; Watzek, G.; Imhof, H. Dental CT: Imaging technique, anatomy, and pathologic conditions of the jaws. Eur. Radiol. 2003, 13, 366–376. [Google Scholar] [PubMed]
- Verstreken, K.; Van Cleynenbreugel, J.; Marchal, G.; Naert, I.; Suetens, P.; van Steenberghe, D. Computer-assisted planning of oral implant surgery: A three-dimensional approach. Int. J. Oral. Maxillofac. Implants 1996, 11, 806–810. [Google Scholar] [PubMed]
- Marchack, C.B.; Moy, P.K. The use of a custom template for immediate loading with the definitive prosthesis: A clinical report. J. Calif Dent. Assoc. 2003, 31, 925–929. [Google Scholar] [PubMed]
- Van Steenberghe, D.; Naert, I.; Andersson, M.; Brajnovic, I.; Van Cleynenbreugel, J.; Suetens, P. A custom template and definitive prosthesis allowing immediate implant loading in the maxilla: A clinical report. Int. J. Oral. Maxillofac. Implants 2002, 17, 663–670. [Google Scholar] [PubMed]
- Haider, R.; Watzek, G.; Plenk, H. Effects of drill cooling and bone structure on IMZ implant fixation. Int. J. Oral. Maxillofac. Implants 1993, 8, 83–91. [Google Scholar] [PubMed]
- Brägger, U.; Gerber, C.; Joss, A.; Haenni, S.; Meier, A.; Hashorva, E.; Lang, N.P. Patterns of tissue remodeling after placement of ITI dental implants using an osteotome technique: A longitudinal radiographic case cohort study. Clin. Oral. Implants Res. 2004, 15, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kirmeier, R.; Payer, M.; Wehrschütz, M.; Jakse, N.; Platzer, S.; Lorenzoni, M. Evaluation of three-dimensional changes after sinus floor augmentation with different grafting materials. Clin. Oral. Implants Res. 2008, 19, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, N.; Laureti, M.; Fanali, S. Dental implants placement in conjunction with osteotome sinus floor elevation: A 12-year life-table analysis from a prospective study on 588 ITI implants. Clin. Oral. Implants Res. 2006, 17, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nedir, R.; Bischof, M.; Vazquez, L.; Nurdin, N.; Szmukler-Moncler, S.; Bernard, J.P. Osteotome sinus floor elevation technique without grafting material: 3-year results of a prospective pilot study. Clin. Oral. Implants Res. 2009, 20, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Lin, Y.H.; Yang, Y.C.; Wang, H.L. The influence of sinus membrane thickness upon membrane perforation during transcrestal sinus lift procedure. Clin. Oral. Implants Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Schiebel, V.; Hof, M.; Ulm, C.; Watzek, G.; Pommer, B. Risk factors of membrane perforation and postoperative complications in sinus floor elevation surgery: Review of 407 augmentation procedures. J. Oral. Maxillofac. Surg. 2015. [Google Scholar] [CrossRef] [PubMed]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral. Implants Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pommer, B.; Unger, E.; Busenlechner, D.; Haas, R.; Mailath-Pokorny, G.; Fürhauser, R.; Watzek, G. Graft Remodeling following Transcrestal Sinus Floor Elevation via the Gel-Pressure Technique (GPT) and Pasteous Nano-Crystalline Hydroxyapatite Bone Substitute. Materials 2015, 8, 3210-3220. https://doi.org/10.3390/ma8063210
Pommer B, Unger E, Busenlechner D, Haas R, Mailath-Pokorny G, Fürhauser R, Watzek G. Graft Remodeling following Transcrestal Sinus Floor Elevation via the Gel-Pressure Technique (GPT) and Pasteous Nano-Crystalline Hydroxyapatite Bone Substitute. Materials. 2015; 8(6):3210-3220. https://doi.org/10.3390/ma8063210
Chicago/Turabian StylePommer, Bernhard, Ewald Unger, Dieter Busenlechner, Robert Haas, Georg Mailath-Pokorny, Rudolf Fürhauser, and Georg Watzek. 2015. "Graft Remodeling following Transcrestal Sinus Floor Elevation via the Gel-Pressure Technique (GPT) and Pasteous Nano-Crystalline Hydroxyapatite Bone Substitute" Materials 8, no. 6: 3210-3220. https://doi.org/10.3390/ma8063210





