Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering
Abstract
:1. Introduction
2. Review of Plasmonic Nano-Structures by Bottom-Up Chemical Synthesis Processes
2.1. Plasmonic Nanoparticles
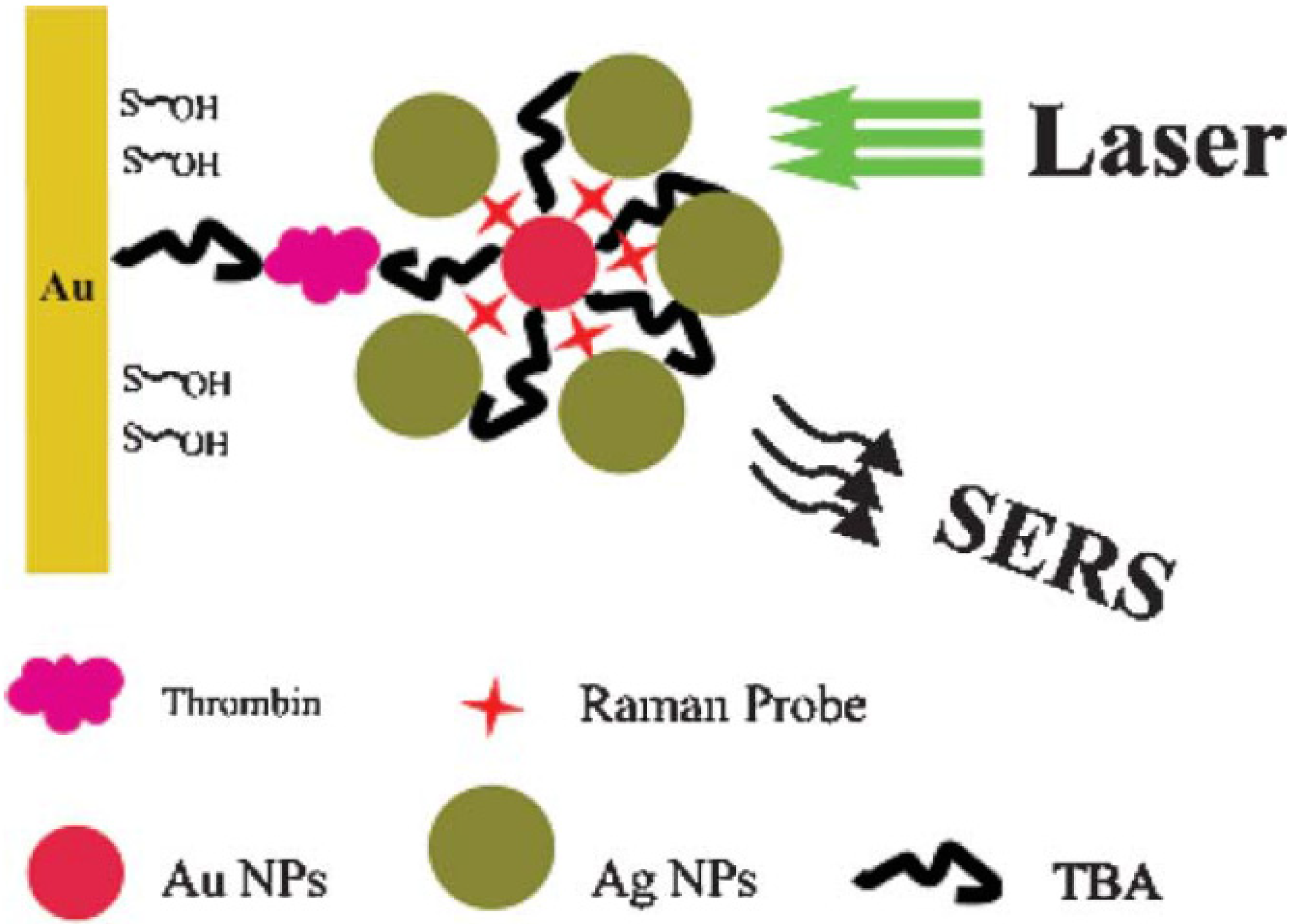
2.2. Core-Shell Nanoparticles

2.3. Plasmonic Nanowires and Nanorods
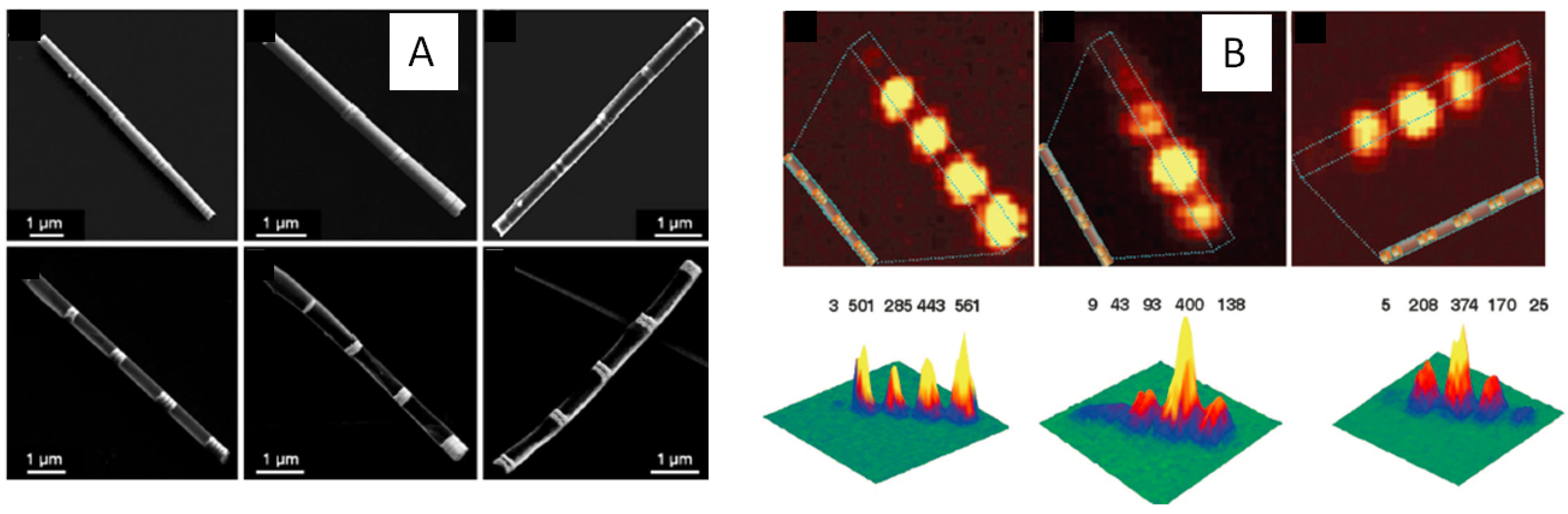
2.4. Preassembled Plasmonic Dimers and Trimers
2.5. Plasmonic Nanoprisms, Nanocubes, Nanostars and Nanosheets

3. Review of Rationally Designed SERS Substrates
3.1. Nanosphere Lithography

3.2. On-Wire Lithography
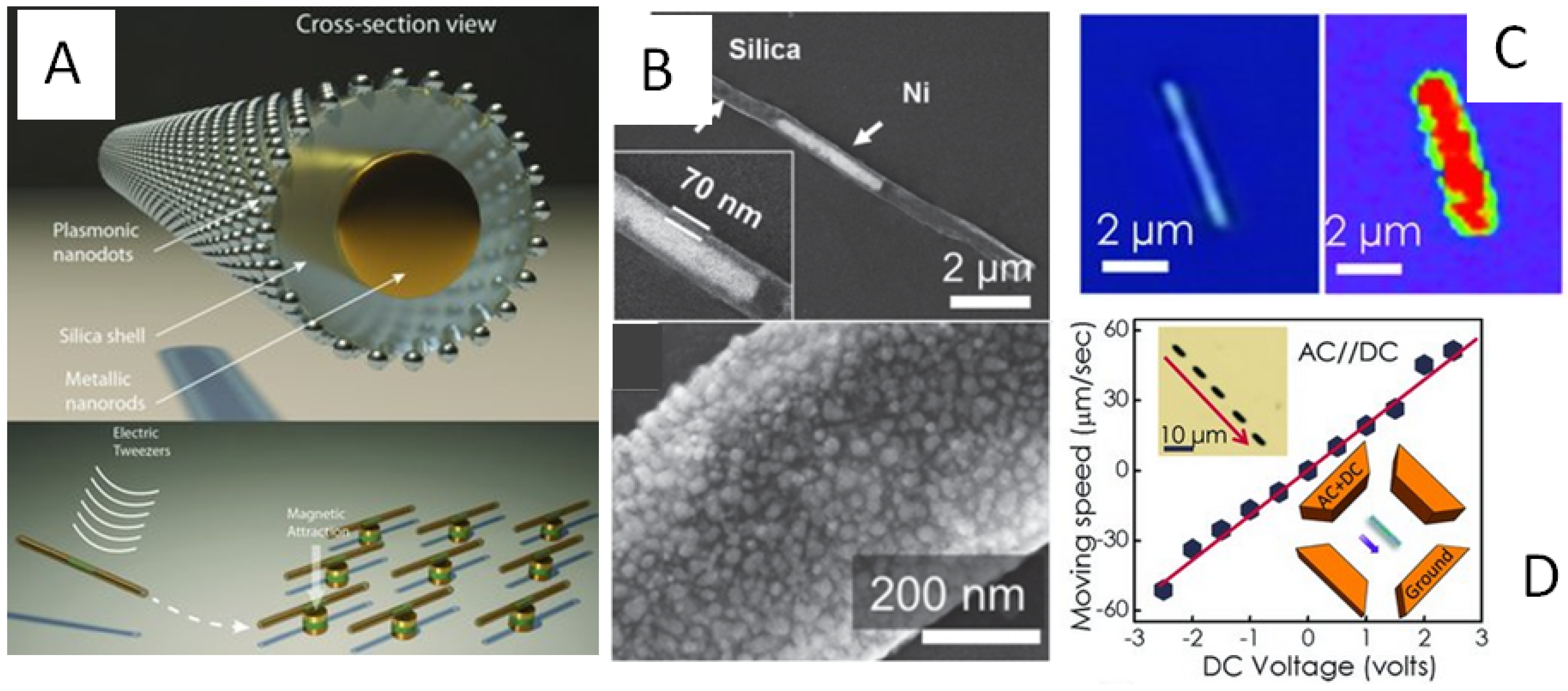
3.3. Plasmonic Nano-Capsules and Electric Tweezers
3.4. Plasmonic Nano-Antennas by E-Beam Lithography
3.5. Nanogaps Created by Non-Traditional Techniques

3.6. Plasmonic Gratings and Other Periodic Metallic Structures
4. Review of Hybrid SERS Substrates on Other Nanophotonic Platforms
4.1. SERS Substrates on Photonic Crystals and Resonant Gratings

4.2. SERS Substrates Using Bio-Enabled Materials

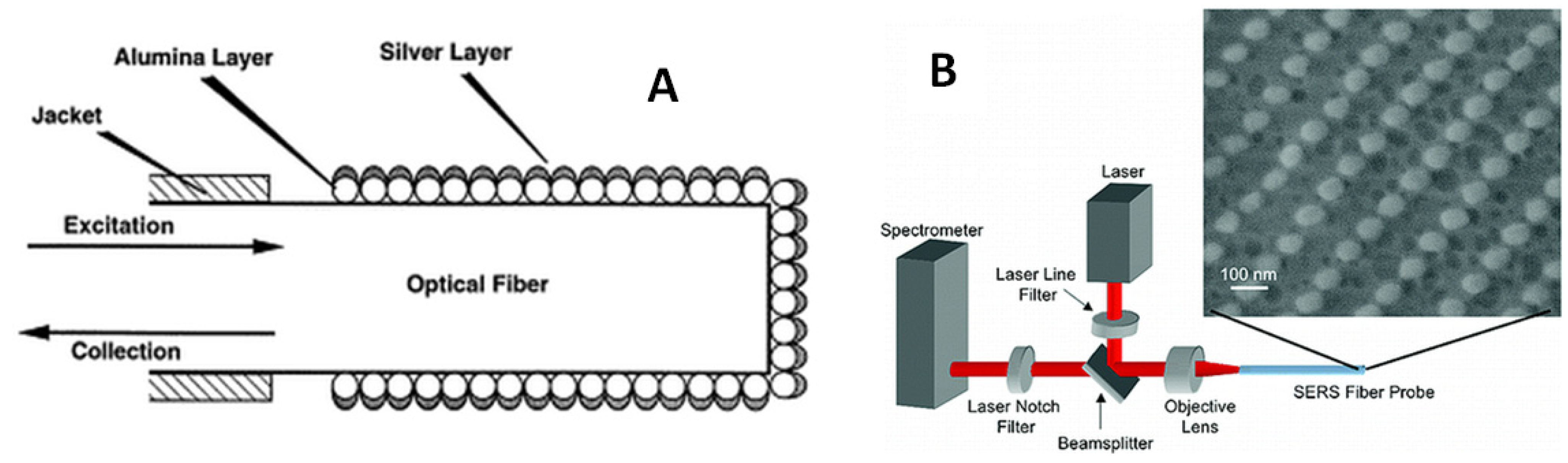
4.3. SERS Sensing Using Optical Fibers
4.4. SERS Sensing in Micro-Fluidics
4.5. SERS Substrates on Graphene
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; McQuillan, A. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Tian, Z. Surface-Enhanced Raman Spectroscopy: Advancements and Applications. J. Raman Spectrosc. 2005, 36, 466–470. [Google Scholar] [CrossRef]
- Qian, X.; Nie, S. Single-Molecule and Single-Nanoparticle SERS: From Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-Enhanced Raman Spectroscopy (SERS): Progress and Trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured Plasmonic Sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef] [PubMed]
- Banholzer, M.J.; Millstone, J.E.; Qin, L.; Mirkin, C.A. Rationally Designed Nanostructures for Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2008, 37, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fernanda Cardinal, M.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-Performance SERS Substrates: Advances and Challenges. MRS Bull 2013, 38, 615–624. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtuluş, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.; Haynes, C.L. Recent Progress in SERS Biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Rae, S.I.; Khan, I. Surface Enhanced Raman Spectroscopy (SERS) Sensors for Gas Analysis. Analyst 2010, 135, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; Van Duyne, R.P. Toward a Glucose Biosensor Based on Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.C.; Phillips, S.T.; Dickey, M.D.; Lu, N.; Suo, Z.; Whitesides, G.M. Foldable Printed Circuit Boards on Paper Substrates. Adv. Funct. Mater. 2010, 20, 28–35. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Ringe, E.; Valley, N.; Wustholz, K.L.; Phillips, E.; Scheidt, K.A.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Surface-Enhanced Raman Spectroscopy of Crystal Violet Isotopologues: Theory and Experiment. J. Am. Chem. Soc. 2011, 133, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.; Meyer, M.; Etchegoin, P. Proof of Single-Molecule Sensitivity in Surface Enhanced Raman Scattering (SERS) by Means of a Two-Analyte Technique. J. Phys. Chem. B 2006, 110, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.; Moula, G.; Aroca, R. SERRS for Single-Molecule Detection of Dye-Labeled Phospholipids in Langmuir−Blodgett Monolayers. Langmuir 2009, 25, 11261–11264. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M. Surface-Enhanced Spectroscopy. Rev. Modern Phys. 1985, 57, 783. [Google Scholar] [CrossRef]
- Schatz, G.C. Theoretical Studies of Surface Enhanced Raman Scattering. Acc. Chem. Res. 1984, 17, 370–376. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman Spectroscopy: A Brief Retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, W. Surface-Enhanced Raman Scattering. J. Phys. Condens. Matter 1992, 4, 1143. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the Structure of Single-Molecule Surface-Enhanced Raman Scattering Hot Spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Campion, A.; Ivanecky, J., III; Child, C.; Foster, M. On the Mechanism of Chemical Enhancement in Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 1995, 117, 11807–11808. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Jensen, L. Understanding the Molecule−Surface Chemical Coupling in SERS. J. Am. Chem. Soc. 2009, 131, 4090–4098. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Haynes, C.L.; McFarland, A.D.; Schatz, G.C.; Van Duyne, R.P.; Zou, S. Plasmonic Materials for Surface-Enhanced Sensing and Spectroscopy. MRS Bull 2005, 30, 368–375. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.; Dieringer, J.A.; Van Duyne, R.P. Creating, Characterizing, and Controlling Chemistry with SERS Hot Spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherland, 2008. [Google Scholar]
- Kleinman, S.L.; Sharma, B.; Blaber, M.G.; Henry, A.; Valley, N.; Freeman, R.G.; Natan, M.J.; Schatz, G.C.; Van Duyne, R.P. Structure Enhancement Factor Relationships in Single Gold Nanoantennas by Surface-Enhanced Raman Excitation Spectroscopy. J. Am. Chem. Soc. 2012, 135, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Greeneltch, N.G.; Blaber, M.G.; Henry, A.; Schatz, G.C.; Van Duyne, R.P. Immobilized Nanorod Assemblies: Fabrication and Understanding of Large Area Surface-Enhanced Raman Spectroscopy Substrates. Anal. Chem. 2013, 85, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Lee, P.; Meisel, D. Adsorption and surface-enhanced raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Xu, H.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of single hemoglobin molecules by surface enhanced raman scattering. Phys. Rev. Lett. 1999, 83, 4357. [Google Scholar] [CrossRef]
- Xu, H.; Aizpurua, J.; Käll, M.; Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced raman scattering. Phys. Rev. E 2000, 62, 4318. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ren, B.; Wu, D. Surface-enhanced raman scattering: From noble to transition metals and from rough surfaces to ordered nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483. [Google Scholar] [CrossRef]
- Lal, S.; Grady, N.K.; Kundu, J.; Levin, C.S.; Lassiter, J.B.; Halas, N.J. Tailoring plasmonic substrates for surface enhanced spectroscopies. Chem. Soc. Rev. 2008, 37, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hasan, D.; Wang, L.; Chakravarty, S.; Chen, R.T.; Fan, D.; Wang, A.X. Guided-mode-resonance-coupled plasmonic-active SiO2 nanotubes for surface enhanced raman spectroscopy. Appl. Phys. Lett. 2012, 100, 191114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhang, W.; Cunningham, B.T. Photonic crystals with SiO2-Ag “post-Cap” nanostructure coatings for surface enhanced Raman spectroscopy. Appl. Phys. Lett. 2008, 93, 143112. [Google Scholar] [CrossRef]
- Yan, H.; Gu, C.; Yang, C.; Liu, J.; Jin, G.; Zhang, J.; Hou, L.; Yao, Y. Hollow core photonic crystal fiber surface-enhanced Raman probe. Appl. Phys. Lett. 2006, 89, 204101. [Google Scholar] [CrossRef]
- Perney, N.; de Abajo, F.G.; Baumberg, J.; Tang, A.; Netti, M.; Charlton, M.; Zoorob, M. Tuning localized plasmon cavities for optimized surface-enhanced Raman scattering. Phys. Rev. B 2007, 76, 035426. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, K.R.; Henkel, T.; Popp, J. Quantitative online detection of low-concentrated drugs via a SERS microfluidic system. Chem. Phys. Chem. 2007, 8, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Van Hyning, D.L.; Zukoski, C.F. Formation Mechanisms and Aggregation Behavior of Borohydride Reduced Silver Particles. Langmuir 1998, 14, 7034–7046. [Google Scholar] [CrossRef]
- Ahern, A.M.; Garrell, R.L. In situ photoreduced silver nitrate as a substrate for surface-enhanced raman spectroscopy. Anal. Chem. 1987, 59, 2813–2816. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nature 1972, 20–22. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and Characterization of Au Colloid Monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Exploring How to Increase the Brightness of Surface-Enhanced Raman Spectroscopy Nanolabels: The Effect of the Raman-Active Molecules and of the Label Size. Adv. Funct. Mater. 2012, 22, 353–360. [Google Scholar] [CrossRef]
- Shaw, C.P.; Fan, M.K.; Lane, C.; Barry, G.; Jirasek, A.I.; Brolo, A.G. Statistical correlation between SERS intensity and nanoparticle cluster size. J. Phys. Chem. C. 2013, 117, 16596–16605. [Google Scholar] [CrossRef]
- Sant’Ana, A.C.; Rocha, T.C. R.; Santos, P.S.; Zanchetand, D.; Temperini, M.L. A. Size-dependent SERS enhancement of colloidal silver nanoplates: the case of 2-amino-5-nitropyridine. J. Raman Spectrosc. 2009, 40, 183–190. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Lipert, R.J.; Dawson, G.B.; Porter, M.D. Immunoassay Readout Method using Extrinsic Raman Labels Adsorbed on Immunogold Colloids. Anal. Chem. 1999, 71, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman Spectroscopic Fingerprints for DNA and RNA Detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, H.; Li, B.; Ren, W.; Guo, S.; Dong, S.; Wang, E. SERS Opens a New Way in Aptasensor for Protein Recognition with High Sensitivity and Selectivity. Chem. Commun. 2007, 5220–5222. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Agnoli, S.; Polizzi, S.; Meneghetti, M. Strong dependence of surface plasmon resonance and surface enhanced Raman scattering on the composition of Au–Fe nanoalloys. Nanoscale 2014, 6, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.H.; Zhang, G.L.; Jiang, X.D.; Huang, J.L.; Jing, X.L.; Zheng, Y.M.; He, J.; Li, Q.B. Biogenic flower-shaped Au–Pd nanoparticles: Synthesis, SERS detection and catalysis towards benzyl alcohol oxidation. J. Mater. Chem. A 2014, 2, 1767–1773. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, N.H.; Lee, K.Y.; Kwon, K.; Kim, M.J.; Han, S.W. Synthesis and Characterization of Flower-Shaped Porous Au-Pd Alloy Nanoparticles. J. Phys. Chem. C. 2008, 112, 6717–6722. [Google Scholar] [CrossRef]
- Li, J.M.; Yang, Y.; Qin, D. Hollow nanocubes made of Ag–Au alloys for SERS detection with sensitivity of 10−8 M for melamine. J. Mater. Chem. C 2014, 2, 9934–9940. [Google Scholar] [CrossRef]
- Kong, X.; Yu, Q.; Zhang, X.; Du, X.; Gong, H.; Jiang, H. Synthesis and Application of Surface Enhanced Raman Scattering (SERS) Tags of Ag@SiO2 Core/Shell Nanoparticles in Protein Detection. J. Mater. Chem. 2012, 22, 7767–7774. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold-Silica Core-Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Álvarez-Puebla, R.A.; Contreras-Cáceres, R.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Au@pNIPAM Colloids as Molecular Traps for Surface-Enhanced, Spectroscopic, Ultra-Sensitive Analysis. Angew. Chem. Inter. Ed. 2009, 48, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Doering, W.E.; Nie, S. Spectroscopic tags using dye-embedded nanoparticles and surface-enhanced raman scattering. Anal. Chem. 2003, 75, 6171–6176. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, S.P.; Musick, M.D.; Keating, C.D.; Natan, M.J. Glass-coated, analyte-tagged nanoparticles: A ‘new tagging system based on detection with surface-enhanced raman scattering. Langmuir 2003, 19, 4784–4790. [Google Scholar] [CrossRef]
- Kong, X.; Yu, Q.; Lv, Z.; Du, X. Tandem assays of protein and glucose with functionalized core/shell particles based on magnetic separation and surface-enhanced raman scattering. Small 2013, 9, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Ren, B. Shell-isolated nanoparticle-enhanced raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Tian, X.D.; Li, S.B.; Anema, J.R.; Yang, Z.L.; Ding, Y.; Wu, Y.F.; Zeng, Y.M.; Chen, Q.Z.; Ren, B. Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Protoc. 2013, 8, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.; Noh, M.S.; Kim, J.; Kim, G.; Kang, H.; Kim, M.; Seo, Y.; Baek, J.; Kim, J.; Park, J. Multifunctional silver-embedded magnetic nanoparticles as SERS nanoprobes and their applications. Small 2010, 6, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.G.; Hommer, M.B.; Grabar, K.C.; Jackson, M.A.; Natan, M.J. Ag-clad Au nanoparticles: Novel aggregation, optical, and surface-enhanced raman scattering properties. J. Phys. Chem. 1996, 100, 718–724. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag Shell nanoparticles using Neem (Azadirachta Indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Akimov, A.; Mukherjee, A.; Yu, C.; Chang, D.; Zibrov, A.; Hemmer, P.; Park, H.; Lukin, M. Generation of single optical plasmons in metallic nanowires coupled to quantum dots. Nature 2007, 450, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P. Plasmonic nano-architectures for surface enhanced raman scattering: A review. J. Nanophotonics 2012, 6, 064503. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-Blodgett Silver Nanowire Monolayers for Molecular Sensing using Surface-Enhanced Raman Spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Lee, S.J.; Morrill, A.R.; Moskovits, M. Hot Spots in Silver Nanowire Bundles for Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2006, 128, 2200–2201. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Centrone, A.; Park, J.; Ramanathan, R.; Sailor, M.J.; Hatton, T.A.; Bhatia, S.N. SERS-coded Gold Nanorods as a Multifunctional Platform for Densely Multiplexed Near-infrared Imaging and Photothermal Heating. Adv. Mater. 2009, 21, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Chen, L. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2012, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Camargo, P.H.; Lu, X.; Xia, Y. Dimers of Silver Nanospheres: Facile Synthesis and their use as Hot Spots for Surface-Enhanced Raman Scattering. Nano Lett. 2008, 9, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Wustholz, K.L.; Henry, A.; McMahon, J.M.; Freeman, R.G.; Valley, N.; Piotti, M.E.; Natan, M.J.; Schatz, G.C.; Duyne, R.P.V. Structure−Activity Relationships in Gold Nanoparticle Dimers and Trimers for Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2010, 132, 10903–10910. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Yang, M.; Xu, J.; Goh, S.J.; Pan, M.; Chen, H. Measuring Ensemble-Averaged Surface-Enhanced Raman Scattering in the Hotspots of Colloidal Nanoparticle Dimers and Trimers. J. Am. Chem. Soc. 2010, 132, 3644–3645. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Chen, Z.; Lu, X.; Dai, G.; Ni, W.; Wang, Q. DNA-Directed Gold Nanodimers with Tailored Ensemble Surface-Enhanced Raman Scattering Properties. ACS Appl. Mater. Interfaces 2013, 5, 10423–10427. [Google Scholar] [CrossRef] [PubMed]
- Thacker, V.V.; Herrmann, L.O.; Sigle, D.O.; Zhang, T.; Liedl, T.; Baumberg, J.J.; Keyser, U.F. DNA Origami Based Assembly of Gold Nanoparticle Dimers for Surface-Enhanced Raman Scattering. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.P.; Feldheim, D.L. Assembly of Phenylacetylene-Bridged Silver and Gold Nanoparticle Arrays. J. Am. Chem. Soc. 2000, 122, 3979–3980. [Google Scholar] [CrossRef]
- Sardar, R.; Heap, T.B.; Shumaker-Parry, J.S. Versatile Solid Phase Synthesis of Gold Nanoparticle Dimers using an Asymmetric Functionalization Approach. J. Am. Chem. Soc. 2007, 129, 5356–5357. [Google Scholar] [CrossRef] [PubMed]
- Ciou, S.; Cao, Y.; Huang, H.; Su, D.; Huang, C. SERS Enhancement Factors Studies of Silver Nanoprism and Spherical Nanoparticle Colloids in the Presence of Bromide Ions. J. Phys. Chem. C 2009, 113, 9520–9525. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, X.; Zhang, Q.; Blackstad, L.G.; Fu, Z.; Li, Z.; Qin, D. The Role of Etching in the Formation of Ag Nanoplates with Straight, Curved and Wavy Edges and Comparison of their SERS Properties. Small 2014, 10, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.M.; Li, Z.; Siekkinen, A.R.; Xia, Y. The SERS Activity of a Supported Ag Nanocube Strongly Depends on its Orientation Relative to Laser Polarization. Nano Lett. 2007, 7, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Agrawal, A.; Rodríguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzán, L.M. Tuning Size and Sensing Properties in Colloidal Gold Nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef] [PubMed]
- Hrelescu, C.; Sau, T.K.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Single Gold Nanostars Enhance Raman Scattering. Appl. Phys. Lett. 2009, 94, 153113. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Y.; Fales, A.M.; Li, Y.L.; Liu, J.; Vo-Dinh, T. Quantitative Surface-Enhanced Resonant Raman Scattering Multiplexing of Biocompatible Gold Nanostars for in Vitro and Ex Vivo Detection. Anal. Chem. 2012, 85, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, B.; Mack, N.H.; Doorn, S.K.; Han, X.; Wang, H. Synthesis of Homogeneous Silver Nanosheet Assemblies for Surface Enhanced Raman Scattering Applications. J. Mater. Chem. 2010, 20, 7222–7226. [Google Scholar] [CrossRef]
- Yan, J.; Han, X.; He, J.; Kang, L.; Zhang, B.; Du, Y.; Zhao, H.; Dong, C.; Wang, H.; Xu, P. Highly Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Platforms Based on Silver Nanostructures Fabricated on Polyaniline Membrane Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.L.; Van Duyne, R.P. Nanosphere Lithography: A Versatile Nanofabrication Tool for Studies of Size-Dependent Nanoparticle Optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Treichel, D.A.; Smith, M.T.; Duval, M.L.; Jensen, T.R.; Van Duyne, R.P. Nanosphere Lithography: Size-Tunable Silver Nanoparticle and Surface Cluster Arrays. J. Phys. Chem. B 1999, 103, 3854–3863. [Google Scholar] [CrossRef]
- Zhang, X.; Yonzon, C.R.; Van Duyne, R.P. Nanosphere Lithography Fabricated Plasmonic Materials and Their Applications. J. Mater. Res. 2006, 21, 1083–1092. [Google Scholar] [CrossRef]
- Maxwell, D.J.; Emory, S.R.; Nie, S. Nanostructured Thin-Film Materials with Surface-Enhanced Optical Properties. Chem. Mater. 2001, 13, 1082–1088. [Google Scholar] [CrossRef]
- Dick, L.A.; McFarland, A.D.; Haynes, C.L.; Van Duyne, R.P. Metal Film Over Nanosphere (MFON) Electrodes for Surface-Enhanced Raman Spectroscopy (SERS): Improvements in Surface Nanostructure Stability and Suppression of Irreversible Loss. J. Phys. Chem. B 2002, 106, 853–860. [Google Scholar] [CrossRef]
- Wang, H.; Levin, C.S.; Halas, N.J. Nanosphere Arrays with Controlled Sub-10-Nm Gaps as Surface-Enhanced Raman Spectroscopy Substrates. J. Am. Chem. Soc. 2005, 127, 14992–14993. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Park, S.; Huang, L.; Mirkin, C.A. On-Wire Lithography. Science 2005, 309, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zou, S.; Xue, C.; Atkinson, A.; Schatz, G.C.; Mirkin, C.A. Designing, Fabricating, and Imaging Raman Hot Spots. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 13300–13303. [Google Scholar] [CrossRef] [PubMed]
- Banholzer, M.J.; Qin, L.; Millstone, J.E.; Osberg, K.D.; Mirkin, C.A. On-Wire Lithography: Synthesis, Encoding and Biological Applications. Nat. Protoc. 2009, 4, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jeon, Y.; Jang, J.; Qin, L.; Huo, F.; Wei, W.; Mirkin, C.A. On-Wire Lithography-Generated Molecule-Based Transport Junctions: A New Testbed for Molecular Electronics. J. Am. Chem. Soc. 2008, 130, 8166–8168. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jang, J.; Huang, L.; Mirkin, C.A. Sub-5-nm Gaps Prepared by On-Wire Lithography: Correlating Gap Size with Electrical Transport. Small 2007, 3, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kwanoh, K.; Li, H.; Fan, D. Ordered arrays of Raman nanosensors for ultrasensitive and location predictable biochemical detection. Adv. Mater. 2012, 24, 5457–5463. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Hasan, D.; Ruoff, R.S.; Wang, A.X.; Fan, D. Near-Field Enhanced Plasmonic-Magnetic Bifunctional Nanotubes for Single Cell Bioanalysis. Adv. Funct. Mater. 2013, 23, 4332–4338. [Google Scholar] [CrossRef]
- Fan, D.; Zhu, F.; Cammarata, R.; Chien, C. Electric Tweezers. Nano Today 2011, 6, 339–354. [Google Scholar] [CrossRef]
- Muhlschlegel, P.; Eisler, H.J.; Martin, O.J.; Hecht, B.; Pohl, D.W. Resonant Optical Antennas. Science 2005, 308, 1607–1609. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Deutsch, B.; Novotny, L. Optical Antennas. Advances in Optics and Photonics 2009, 1, 438–483. [Google Scholar] [CrossRef]
- Novotny, L.; Van Hulst, N. Antennas for Light. Nat. Photonics 2011, 5, 83–90. [Google Scholar] [CrossRef]
- Taminiau, T.; Stefani, F.; Segerink, F.; Van Hulst, N. Optical Antennas Direct Single-Molecule Emission. Nature Photonics 2008, 2, 234–237. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; Le Ru, E.C.; Fainstein, A. Bi-Analyte Single Molecule SERS Technique with Simultaneous Spatial Resolution. Phys. Chem. Chem. Phys. 2011, 13, 4500–4506. [Google Scholar] [CrossRef] [PubMed]
- Fromm, D.P.; Sundaramurthy, A.; Kinkhabwala, A.; Schuck, P.J.; Kino, G.S.; Moerner, W. Exploring the Chemical Enhancement for Surface-Enhanced Raman Scattering with Au Bowtie Nanoantennas. J. Chem. Phys. 2006, 124, 061101. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, F.; Kinkhabwala, A.; Moerner, W. Gold Bowtie Nanoantennas for Surface-Enhanced Raman Scattering Under Controlled Electrochemical Potential. Chem. Phys. Lett. 2007, 446, 339–343. [Google Scholar] [CrossRef]
- Uda, S. On the Wireless Beam of Short Electric Waves, by Shintaro Uda; Institute of Electrical Engineers of Japan: Tokyo, Japan, 1928. [Google Scholar]
- Yagi, H. Beam Transmission of Ultra Short Waves. Proc. Inst. Radio Eng. 1928, 16, 715–740. [Google Scholar] [CrossRef]
- Crozier, K.B.; Zhu, W.; Wang, D.; Lin, S.; Best, M.D.; Camden, J.P. Plasmonics for Surface Enhanced Raman Scattering: Nanoantennas for Single Molecules. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 152–162. [Google Scholar] [CrossRef]
- D’Andrea, C.; Bochterle, J.; Toma, A.; Huck, C.; Neubrech, F.; Messina, E.; Fazio, B.; Marago, O.M.; Di Fabrizio, E.; de La Chapelle, L. Marc Optical Nanoantennas for Multiband Surface-Enhanced Infrared and Raman Spectroscopy. ACS Nano 2013, 7, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.R.; Grady, N.K.; Levin, C.S.; Halas, N.J.; Wu, Y.; Nordlander, P.; Natelson, D. Electromigrated nanoscale gaps for surface-enhanced raman spectroscopy. Nano Lett. 2007, 7, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Diebold, E.D.; Peng, P.; Mazur, E. Isolating Surface-Enhanced Raman Scattering Hot Spots using Multiphoton Lithography. J. Am. Chem. Soc. 2009, 131, 16356–16357. [Google Scholar] [CrossRef] [PubMed]
- Buividas, R.; Stoddart, P.R.; Juodkazis, S. Laser fabricated ripple substrates for surface-enhanced Raman scattering. Annalen Phys. 2012, 524, L5–L10. [Google Scholar] [CrossRef]
- Hu, M.; Ou, F.S.; Wu, W.; Naumov, I.; Li, X.; Bratkovsky, A.M.; Williams, R.S.; Li, Z. Gold Nanofingers for Molecule Trapping and Detection. J. Am. Chem. Soc. 2010, 132, 12820–12822. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.S.; Hu, M.; Naumov, I.; Kim, A.; Wu, W.; Bratkovsky, A.M.; Li, X.; Williams, R.S.; Li, Z. Hot-Spot Engineering in Polygonal Nanofinger Assemblies for Surface Enhanced Raman Spectroscopy. Nano Lett. 2011, 11, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ou, F.S.; Ohlberg, D.A.; Hu, M.; Williams, R.S.; Li, Z. Study of Molecular Trapping Inside Gold Nanofinger Arrays on Surface-Enhanced Raman Substrates. J. Am. Chem. Soc. 2011, 133, 8234–8239. [Google Scholar] [CrossRef] [PubMed]
- Kahl, M.; Voges, E.; Kostrewa, S.; Viets, C.; Hill, W. Periodically Structured Metallic Substrates for SERS. Sens. Actuators B Chem. 1998, 51, 285–291. [Google Scholar] [CrossRef]
- Kahl, M.; Voges, E. Analysis of Plasmon Resonance and Surface-Enhanced Raman Scattering on Periodic Silver Structures. Phys. Rev. B 2000, 61, 14078. [Google Scholar] [CrossRef]
- Dhawan, A.; Canva, M.; Vo-Dinh, T. Narrow Groove Plasmonic Nano-Gratings for Surface Plasmon Resonance Sensing. Optics Express 2011, 19, 787–813. [Google Scholar] [CrossRef] [PubMed]
- Baltog, I.; Primeau, N.; Reinisch, R.; Coutaz, J. Surface Enhanced Raman Scattering on Silver Grating: Optimized Antennalike Gain of the Stokes Signal of 104. Appl. Phys. Lett. 1995, 66, 1187–1189. [Google Scholar] [CrossRef]
- Grande, M.; Bianco, G.; Vincenti, M.; Stomeo, T.; De Ceglia, D.; De Vittorio, M.; Petruzzelli, V.; Scalora, M.; Bruno, G.; D’Orazio, A. Experimental Surface-Enhanced Raman Scattering Response of Two-Dimensional Finite Arrays of Gold Nanopatches. Appl. Phys. Lett. 2012, 101, 111606. [Google Scholar] [CrossRef]
- Jiao, Y.; Ryckman, J.D.; Ciesielski, P.N.; Escobar, C.A.; Jennings, G.K.; Weiss, S.M. Patterned Nanoporous Gold as an Effective SERS Template. Nanotechnology 2011, 22, 295302. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, J.D.; Liscidini, M.; Sipe, J.; Weiss, S. Direct Imprinting of Porous Substrates: A Rapid and Low-Cost Approach for Patterning Porous Nanomaterials. Nano Lett. 2010, 11, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Joannopoulos, J. Analysis of Guided Resonances in Photonic Crystal Slabs. Phys. Rev. B 2002, 65, 235112. [Google Scholar] [CrossRef]
- Wang, S.; Magnusson, R.; Bagby, J.S.; Moharam, M. Guided-Mode Resonances in Planar Dielectric-Layer Diffraction Gratings. JOSA A 1990, 7, 1470–1474. [Google Scholar] [CrossRef]
- Hu, M.; Fattal, D.; Li, J.; Li, X.; Li, Z.; Williams, R.S. Optical properties of sub-wavelength dielectric gratings and their application for surface-enhanced raman scattering. Appl. Phys. A 2011, 105, 261–266. [Google Scholar] [CrossRef]
- Li, J.; Fattal, D.; Li, Z. Plasmonic optical antennas on dielectric gratings with high field enhancement for surface enhanced raman spectroscopy. Appl. Phys. Lett. 2009, 94, 263114. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, W.; Cunningham, B.T. Coupling discrete metal nanoparticles to photonic crystal surface resonant modes and application to raman spectroscopy. Optics Express 2010, 18, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, F.; Chong, X.; Fan, D.; Chakravarty, S.; Wang, Z.; Chen, R.T.; Wang, A.X. Guided-Mode Resonance Grating with Self-Assembled Silver Nanoparticles for Surface-Enhanced Raman Scattering Spectroscopy. Photonics 2014, 1, 380–389. [Google Scholar] [CrossRef]
- Deep Blue Home. Available online: http://deepbluehome.blogspot.com/2011/01/psychedelic-diatoms.html (accessed on 27 May 2015).
- Jeffryes, C.; Campbell, J.; Li, H.; Jiao, J.; Rorrer, G. The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Environ. Sci. 2011, 4, 3930–3941. [Google Scholar] [CrossRef]
- Song, M.; Park, S.; Alamgir, F.M.; Cho, J.; Liu, M. Nanostructured electrodes for lithium-ion and lithium-air batteries: The latest developments, challenges, and perspectives. Mater. Sci. Eng. R Rep. 2011, 72, 203–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Li, H. Development of a micro swimming robot using optimised giant magnetostrictive thin films. Appl. Bionics Biomech. 2006, 3, 161–170. [Google Scholar] [CrossRef]
- Jeffryes, C.; Solanki, R.; Rangineni, Y.; Wang, W.; Chang, C.; Rorrer, G.L. Electroluminescence and photoluminescence from nanostructured diatom frustules containing metabolically inserted germanium. Adv. Mater. 2008, 20, 2633–2637. [Google Scholar] [CrossRef]
- Qin, T.; Gutu, T.; Jiao, J.; Chang, C.; Rorrer, G.L. Photoluminescence of silica nanostructures from bioreactor culture of marine diatom nitzschia frustulum. J. Nanosci. Nanotechnol. 2008, 8, 2392–2398. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. Rapid Fabrication of Micro- and Nanoscale Patterns by Replica Molding from Diatom Biosilica. Adv. Funct. Mater. 2007, 17, 2439–2446. [Google Scholar] [CrossRef]
- Losic, D.; Rosengarten, G.; Mitchell, J.G.; Voelcker, N.H. Pore Architecture of Diatom Frustules: Potential Nanostructured Membranes for Molecular and Particle Separations. J. Nanosci. Nanotechnol. 2006, 6, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Campbell, J.; Wang, X.; Rorrer, G.L.; Wang, A.X. Enhancing Surface Plasmon Resonances of Metallic Nanoparticles by Diatom Biosilica. Opt. Express 2013, 21, 15308–15313. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Campbell, J.; Rorrer, G.L.; Wang, A.X. Surface-Enhanced Raman Spectroscopy Sensors from Nanobiosilica with Self-Assembled Plasmonic Nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 127–132. [Google Scholar]
- Yang, J.; Zhen, L.; Ren, F.; Campbell, J.; Rorrer, G.L.; Wang, A.X. Ultra-sensitive Immunoassay Biosensors using Hybrid Plasmonic-biosilica Nanostructured Materials. J. Biophotonics 2014, 9999. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.; White, D. Optical Fibre SERS Sensors. Anal. Bioanal. Chem. 2009, 394, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Y.; Gu, C.; Chen, B.; Seballos, L.; Olson, T.; Zhang, J.Z. Molecular Fiber Sensors Based on Surface Enhanced Raman Scattering (SERS). J. Nanosci. Nanotechnol. 2009, 9, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.L.; Vo-Dinh, T. Development of an Integrated Single-Fiber SERS Sensor. Sens. Actuators B Chem. 2000, 69, 28–36. [Google Scholar] [CrossRef]
- Smythe, E.J.; Dickey, M.D.; Bao, J.; Whitesides, G.M.; Capasso, F. Optical Antenna Arrays on a Fiber Facet for in Situ Surface-Enhanced Raman Scattering Detection. Nano Lett. 2009, 9, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Kostovski, G.; Chinnasamy, U.; Jayawardhana, S.; Stoddart, P.R.; Mitchell, A. Sub-15 nm Optical Fiber Nanoimprint Lithography: A Parallel, Self-aligned and Portable Approach. Adv. Mater. 2011, 23, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, S.; Lü, Z.; Chen, L.; Zhao, B.; Ozaki, Y. Ultrasensitive Detection of 1, 4-Bis(4-Vinylpyridyl) Phenylene in a Small Volume of Low Refractive Index Liquid by Surface-Enhanced Raman Scattering-Active Light Waveguide. Appl. Spectrosc. 2004, 58, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, C.; Schwartzberg, A.; Zhang, J. Surface-Enhanced Raman Scattering Sensor Based on D-Shaped Fiber. Appl. Phys. Lett. 2005, 87, 123105. [Google Scholar] [CrossRef]
- Yin, D.; Deamer, D.; Schmidt, H.; Barber, J.; Hawkins, A. Integrated Optical Waveguides with Liquid Cores. Appl. Phys. Lett. 2004, 85, 3477–3479. [Google Scholar] [CrossRef]
- Measor, P.; Seballos, L.; Yin, D.; Zhang, J.Z.; Lunt, E.J.; Hawkins, A.R.; Schmidt, H. On-Chip Surface-Enhanced Raman Scattering Detection using Integrated Liquid-Core Waveguides. Appl. Phys. Lett. 2007, 90, 211107. [Google Scholar] [CrossRef]
- März, A.; Ackermann, K.R.; Malsch, D.; Bocklitz, T.; Henkel, T.; Popp, J. Towards a Quantitative SERS Approach–online Monitoring of Analytes in a Microfluidic System with Isotope—edited Internal Standards. J. Biophotonics 2009, 2, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lim, C.; Chen, L.; Chon, H.; Choo, J.; Hong, J. Surface-Enhanced Raman Scattering in Nanoliter Droplets: Towards High-Sensitivity Detection of Mercury (II) Ions. Anal. Bioanal. Chem. 2009, 394, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Choo, J. Recent Advances in Surface—Enhanced Raman Scattering Detection Technology for Microfluidic Chips. Electrophoresis 2008, 29, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Quang, L.X.; Lim, C.; Seong, G.H.; Choo, J.; Do, K.J.; Yoo, S.K. A Portable Surface-Enhanced Raman Scattering Sensor Integrated with a Lab-on-a-Chip for Field Analysis. Lab Chip 2008, 8, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, Z.; Hu, H.; Hao, Y.; Wong, C.P.; Yu, T.; Thong, J.T.; Shen, Z.X. Gold on Graphene as a Substrate for Surface Enhanced Raman Scattering Study. Appl. Phys. Lett. 2010, 97, 163111. [Google Scholar] [CrossRef]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A Platform for Surface—Enhanced Raman Spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef] [PubMed]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for Better Plasmonic Materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative Plasmonic Materials: Beyond Gold and Silver. Adv Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.X.; Kong, X. Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering. Materials 2015, 8, 3024-3052. https://doi.org/10.3390/ma8063024
Wang AX, Kong X. Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering. Materials. 2015; 8(6):3024-3052. https://doi.org/10.3390/ma8063024
Chicago/Turabian StyleWang, Alan X., and Xianming Kong. 2015. "Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering" Materials 8, no. 6: 3024-3052. https://doi.org/10.3390/ma8063024




