Effect of Beverages on Viscoelastic Properties of Resin-Based Dental Composites
Abstract
:1. Introduction
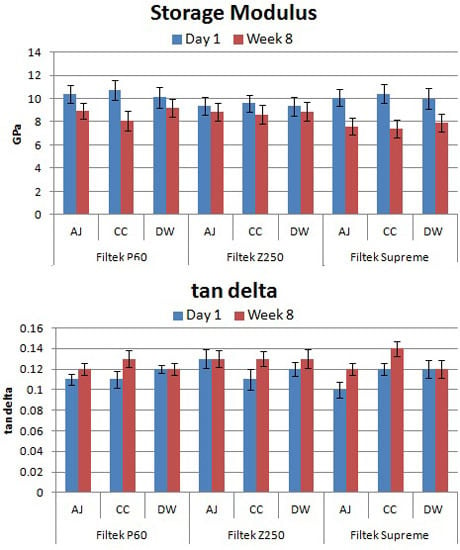
2. Results
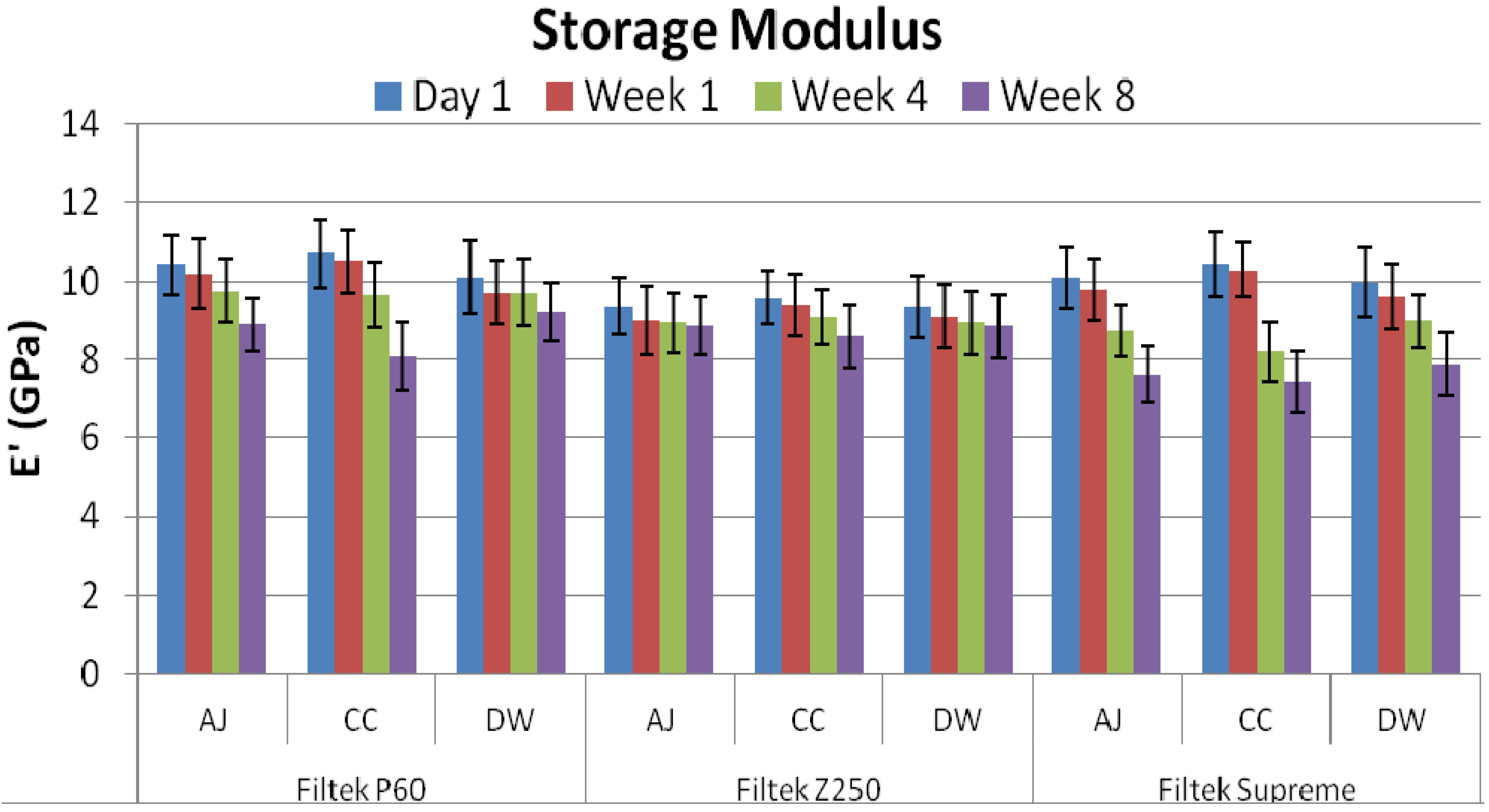

3. Discussion
4. Clinical Significance
5. Materials and Methods
| Material | Shade | Fillers Particle size/Loading by volume | Resin System Ϯ |
|---|---|---|---|
| Filtek™ P60 Batch No. 4720 | A3 | 0.01–3.5 µm/61% vol. | Bis-GMA, UDMA and Bis-EMA |
| Filtek™ Z250 Batch No. 6020 | A2 | 0.01–3.5 µm/60% vol. | |
| Filtek™ Supreme Batch No. 3910 | A2B | 5–75 nm/57%–60% vol. | Bis-GMA, Bis-EMA and TEGDMA |
5.1. Sample Preparation
| Solution | Content | pH |
|---|---|---|
| Coca-Cola |
| 2.5 |
| Apple Juice | Pure Apple juice (nothing added or removed, no flavor and sugar) | 4.0 |
| Deionized Water | 7.4 |
5.2. Dynamic Mechanical Analysis
| Parameter | Conditions |
|---|---|
| Initial Temperature | 26 °C |
| Final Temperature | 50 °C |
| Static Force | 400 mN |
| Dynamic Force | 380 mN |
| Frequency | 1 Hz |
| Heating Rate | 10 °C/min |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dietschi, D.; Magne, P.; Holz, J. Recent trends in esthetic restorations for posterior teeth. Quintessence Int. 1994, 25, 659–677. [Google Scholar] [PubMed]
- Gopferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Lingstrom, P.; Imfeld, T.; Birkhed, D. Comparison of three different methods for measurement of Plaque-pH in humans after consumption of soft bread and potato chips. J. Dent. Res. 1993, 72, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Luiz, B.K.M.; Quintella, C.M.; Friedrich, L.A.; Slva, E.; Veiga, W.; Prates, L.H.M.; Bertolino, J.B.; Pires, A.T.N. Effect of drinks on the surface properties of dental resin composites. Polym. Test. 2007, 26, 855–861. [Google Scholar] [CrossRef]
- Wongkhantee, S.; Patanapiradej, V.; Maneenut, C.; Tantbirojn, D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J. Dent. 2006, 34, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Luiz, B.K.M.; Amboni, R.D.M.C.; Prates, L.H.M.; Roberto, B.J.; Pires, A.T.N. Influence of drinks on resin composite: Evaluation of degree of cure and color change parameters. Polym. Test. 2007, 26, 438–444. [Google Scholar] [CrossRef]
- Sarrett, D.C.; Coletti, D.P.; Peluso, A.R. The effects of alcoholic beverages on composite wear. Dent. Mater. 2000, 16, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Badra, V.V.; Faraoni, J.J.; Ramos, R.P.; Palma-Dibb, R.G. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper. Dent. 2005, 30, 213–219. [Google Scholar] [PubMed]
- Aklonis, J.J.; McKnight, W.J. Introduction to Polymer Viscoelasticity; Wiley: New York, NY, USA, 1983; pp. 10–24. [Google Scholar]
- Emami, N.; Soderholm, K.J.M. Dynamic mechanical thermal analysis of two light-cured dental composites. Dent. Mater. 2005, 21, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Whiting, R.; Jacobsen, P.H. Dynamic mechanical properties of resin-based filling materials. J. Dent. Res. 1980, 59, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Turner, D.T. Characterization of polydimethacrylates and their composites by dynamic mechanical analysis. J. Dent. Res. 1987, 66, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Papadogiannis, Y.; Lakes, R.S.; Petrou-Americanos, A.; Theothoridou-Pahini, S. Temperature dependence of the dynamic viscoelastic behavior of chemical- and light-cured composites. Dent. Mater. 1993, 9, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.V.; Geis-Gerstorfer, J. Influence of temperature on the visco-elastic properties of direct and indirect dental composite resins. Dent. Mater. 2008, 24, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater. 2008, 24, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Aframian, D.J.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Neamat, A.B.; Han, L.; Okamoto, A.; Iwaku, M. Effect of alcoholic and low pH soft drinks on fluoride release from compomer. J. Esthet. Dent. 2000, 12, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C.; Thompson, J.Y.; Swift, E.J., Jr.; Stamatiades, P.; Wilkerson, M. A characterization of first generation flowable composites. J. Am. Dent. Assoc. 1998, 129, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, M.; Owens, B.M. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J. Clin. Pediatr. Dent. 2007, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Surface characteristics of aesthetic restorative materials—An SEM study. J. Oral Rehabil. 2007, 34, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.J.; Tan, S.H.L.; Wee, S.S.C.; Lee, C.W.; Lim, E.L.C.; Zeng, K.Y. Chemical degradation of composite restoratives. J. Oral Rehabil. 2001, 28, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Papadogiannis, D.Y.; Lakes, R.S.; Papadogiannis, Y.; Palaghias, G.; Helvatjoglu-Antoniades, M. The effect of temperature on the viscoelastic properties of nano-hybrid composites. Dent. Mater. 2008, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, E.; Güler, A.U.; Yücel, A.C.; Köprülü, H.; Güler, E. Color stability of resin composites after immersion in different drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Hamada, T.; McCabe, J.F. The effects of cross-linking agents on some properties of HEMA-based resins. J. Dent. Res. 1995, 74, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Douglas, W.H.; Craig, R.G. Resistance to extrinsic stains by hydrophobic composite resin systems. J. Dent. Res. 1982, 61, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; von Fraunhofer, J.A.; Razavi, R. The staining characteristics, transverse strength and microhardness of a visible light cured tenture base material. J. Prosthet. Dent. 1987, 57, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Aliping-Mckenzie, M.; Linden, R.W.A.; Nicholson, J.W. The effect of Coca-Cola and fruit juices on the surface hardness of glass ionomers and compomers. J. Oral Rehabil. 2004, 31, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of staining and bleaching on color change of dental composite resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.A.; Garcia, P.P.; de Oliveira, A.L.; Chinelatti, M.A.; Palma-Dibb, R.G. Chemical and morphological features of dental composite resin: Influence of light curing units and immersion media. Microsc. Res. Tech. 2010, 73, 176–181. [Google Scholar] [PubMed]
- Gohring, T.N.; Gallo, L.; Luthy, H. Effect of water storage, thermocycling, the incorporation and site of placement of glass-fibers on the flexural strength of veneering composite. Dent. Mater. 2005, 21, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Musanje, L.; Shu, M.; Darvell, B.W. Water sorption and mechanical behavior of cosmetic direct restorative materials in artificial saliva. Dent. Mater. 2001, 17, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil, A.; Lassila, L.V.J.; Vallitu, P.K. The effect of fiber orientation on the thermal expansion co-efficient of fiber-reinforced composite. Dent. Mater. 2003, 19, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Phillips, M.J.; Tanner, K.E.; Wong, F.S.L. Comparison of the visco-elastic behavior of a pre-impregnated reinforced glass fiber composite with resin-based composite. Dent. Mater. 2008, 24, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Tahir, M.T.; Khan, M.; Mian, S.A.; Rehman, I.U. An update on glass fiber dental restorative composites: A systematic review. Mater. Sci. Eng. C 2015, 47, 26–39. [Google Scholar] [CrossRef]
- Trengrove, H.G.; Carter, G.M.; Hood, J.A.A. Stress relaxation properties of human dentin. Dent. Mater. 1995, 11, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Gent, A.N. Relaxation processes in vulcanized rubber. I. Relation among stress relaxation, creep, and hysterisis. J. Appl. Polym. Sci. 1962, 6, 433–441. [Google Scholar] [CrossRef]
- Braden, M.; Wilson, A.D. Relationship between stress relaxation and viscoelastic properties of dental materials. J. Dent. 1982, 10, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Renson, C.E.; Braden, M. Experimental determination of the rigidity modulus Poisson’s ratio and elastic limit in shear of human dentin. Arch. Oral Biol. 1975, 20, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Balooch, M.; Marshall, G.W.; Marshall, S.J.; Warren, O.L.; Asif, S.A.S. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J. Biomech. 2004, 37, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem, M.; Khan, A.S.; Rehman, I.U.; Wong, F.S. Effect of Beverages on Viscoelastic Properties of Resin-Based Dental Composites. Materials 2015, 8, 2863-2872. https://doi.org/10.3390/ma8062863
Kaleem M, Khan AS, Rehman IU, Wong FS. Effect of Beverages on Viscoelastic Properties of Resin-Based Dental Composites. Materials. 2015; 8(6):2863-2872. https://doi.org/10.3390/ma8062863
Chicago/Turabian StyleKaleem, Muhammad, Abdul S. Khan, Ihtesham U. Rehman, and Ferranti S. Wong. 2015. "Effect of Beverages on Viscoelastic Properties of Resin-Based Dental Composites" Materials 8, no. 6: 2863-2872. https://doi.org/10.3390/ma8062863