Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen
Abstract
:1. Introduction
2. Materials and Methods
2.1. PMMA Bone Cement Products
2.2. Preparation of MC Particles
2.3. Addition Methods of the MC
2.4. Injectability of the Modified Bone Cements
2.5. Mechanical Property Tests
2.6. Maximum Temperature and Setting Time Tests
2.7. Processing Times Tests
- Mixing time: time for completely mixing of the powder part and liquid part of the bone cement, as well as the MC particles;
- Waiting time: time from the bone cement being extracted in to the syringe to being suitable for the injection;
- Application time: time from the bone cement being applicable to being hard to inject;
- Setting time: time from the injection of the bone cement to it become hardened.
2.8. In Vitro Cytocompatibility Evaluation
2.9. Statistical Methods
3. Results
3.1. Injectability of the Modified Bone Cements
| Particle size (μm) | Powder part of the bone cement/MC particle (w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100/0 | 100/5 | 100/10 | 100/15 | 100/20 | 95/5 | 90/10 | 85/15 | 80/20 | |
| <200 | ○ | ○ | × | × | × | ○ | × | × | × |
| 200–300 | ○ | ○ | × | × | × | ○ | ○ | ○ | × |
| 300–400 | ○ | ○ | ○ | × | × | ○ | ○ | ○ | ○ |
| 400–500 | ○ | ○ | ○ | × | × | ○ | ○ | ○ | × |
| Particle size (μm) | Powder part of the bone cement/MC particle (w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100/0 | 100/5 | 100/10 | 100/15 | 100/20 | 95/5 | 90/10 | 85/15 | 80/20 | |
| <200 | ○ | ○ | × | × | × | ○ | × | × | × |
| 200–300 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ |
| 300–400 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ |
| 400–500 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ |
| Particle size (μm) | Powder part of the bone cement/MC particle (w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100/0 | 100/5 | 100/10 | 100/15 | 100/20 | 95/5 | 90/10 | 85/15 | 80/20 | |
| <200 | ○ | ○ | × | × | × | ○ | × | × | × |
| 200–300 | ○ | ○ | ○ | × | × | ○ | ○ | ○ | × |
| 300–400 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | × |
| 400–500 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | × |
3.2. The Appearance of the Modified Bone Cements

3.3. Mechanical Properties of the Modified Bone Cements
3.3.1. Mechanical Properties of the Modified Osteopal® V Bone Cement

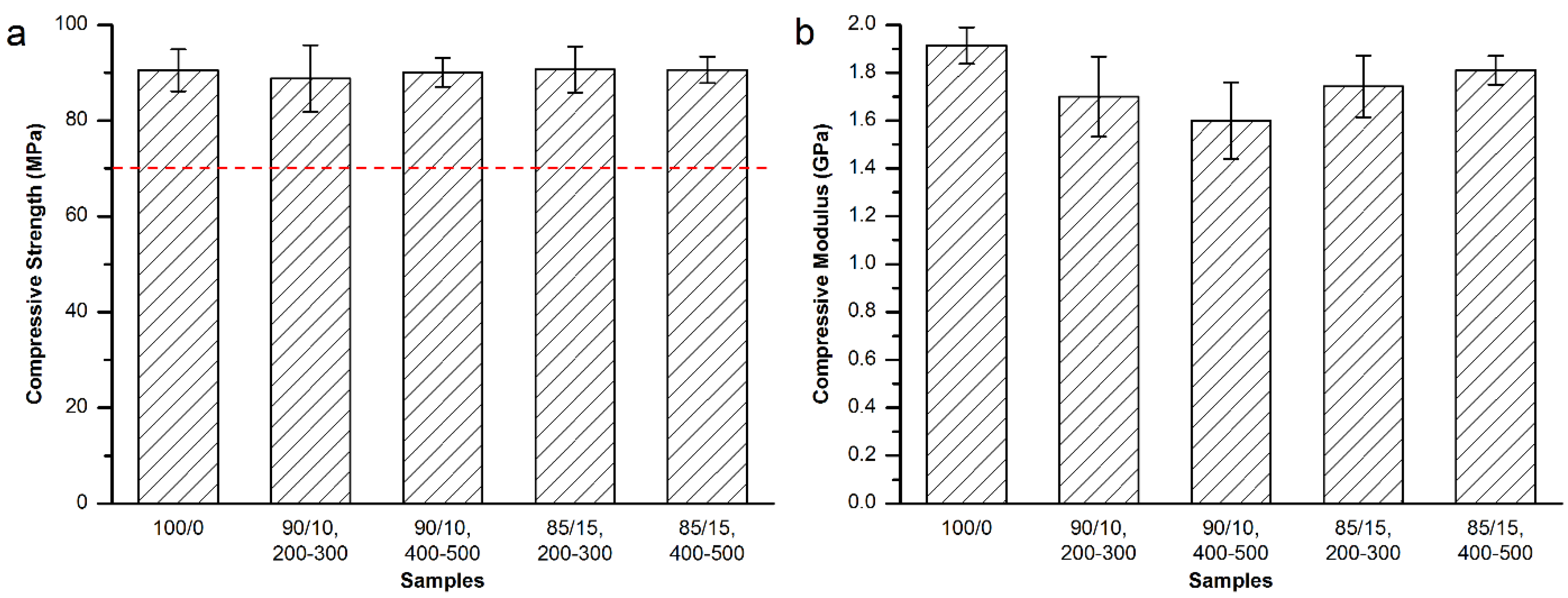

3.3.2. Mechanical Properties of the Modified Mendec® Spine Bone Cement
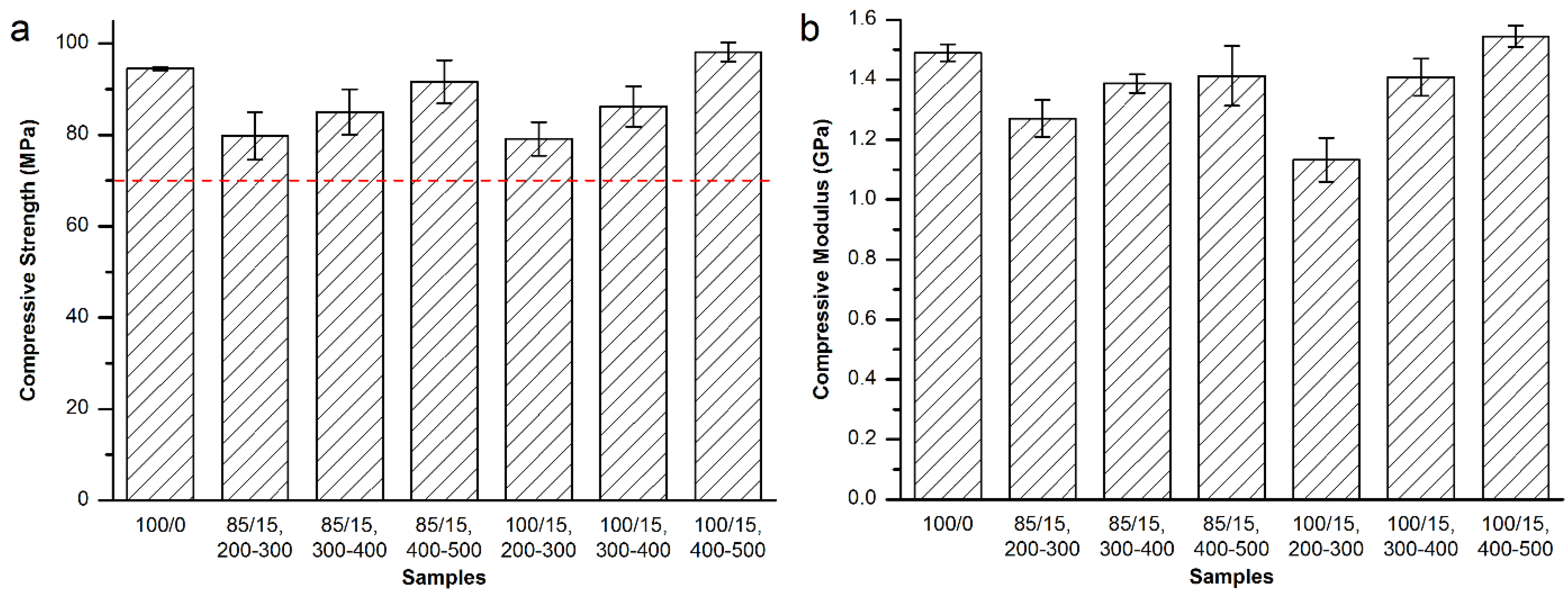
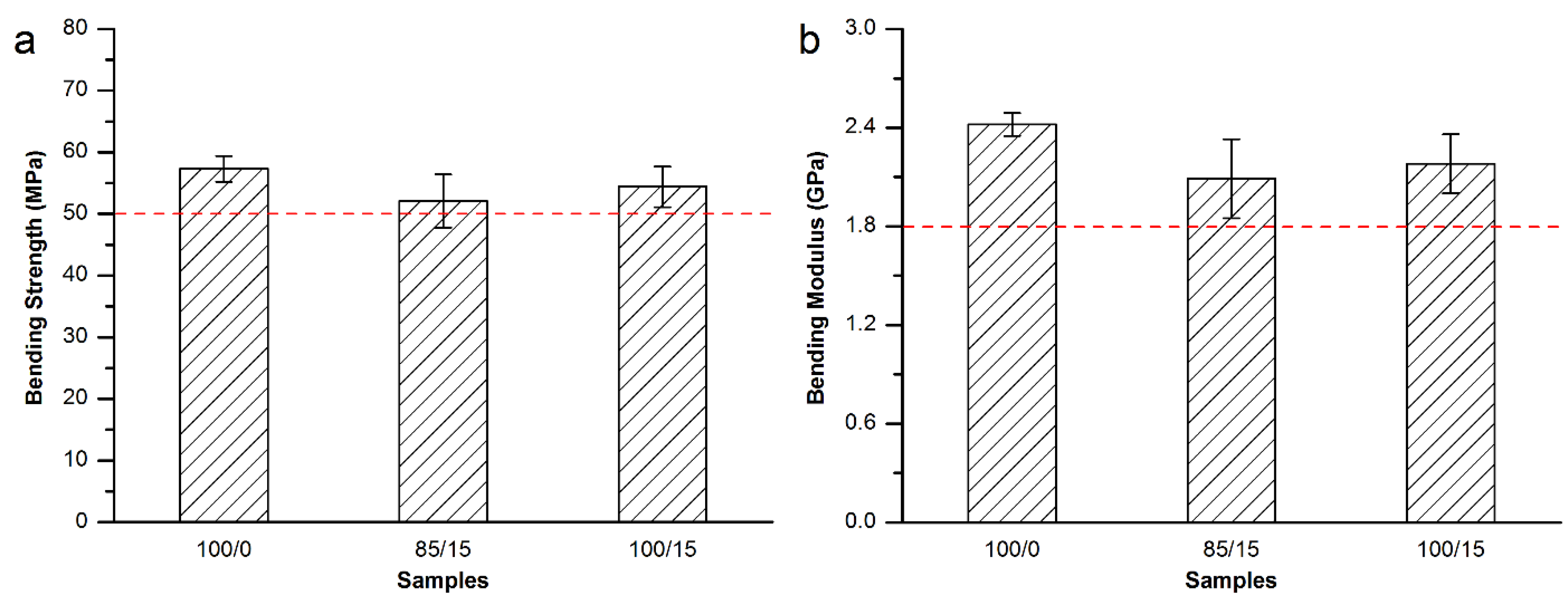
3.3.3. Mechanical Properties of the Modified Spineplex™ Bone Cement
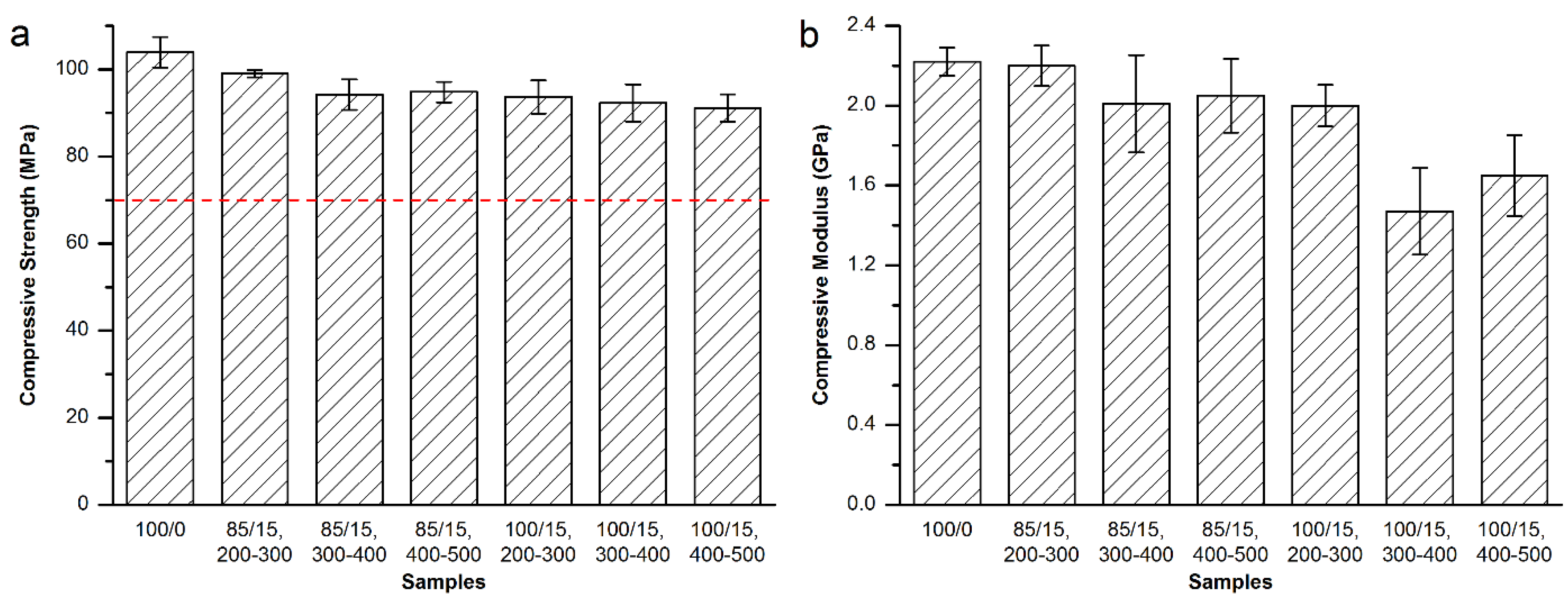

3.4. Maximum Temperature and Setting Time
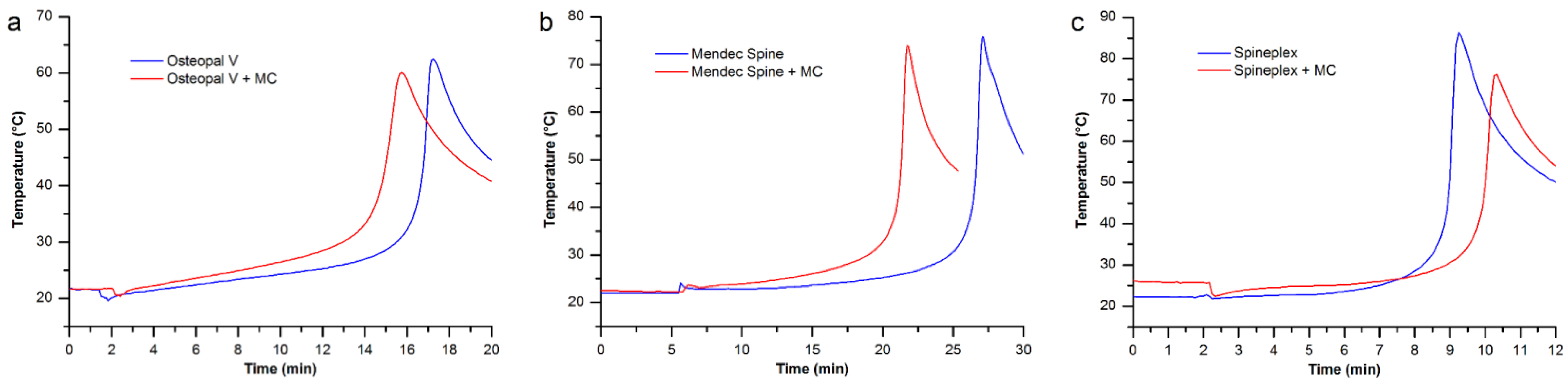
| Bone cements | Osteopal® V | Mendec® Spine | Spineplex™ |
|---|---|---|---|
| Original product | 16’44” | 26’36” | 9’02” |
| Modified by MC particles | 14’51” | 21’18” | 10’01” |
3.5. Processing Times for the Modified Bone Cements
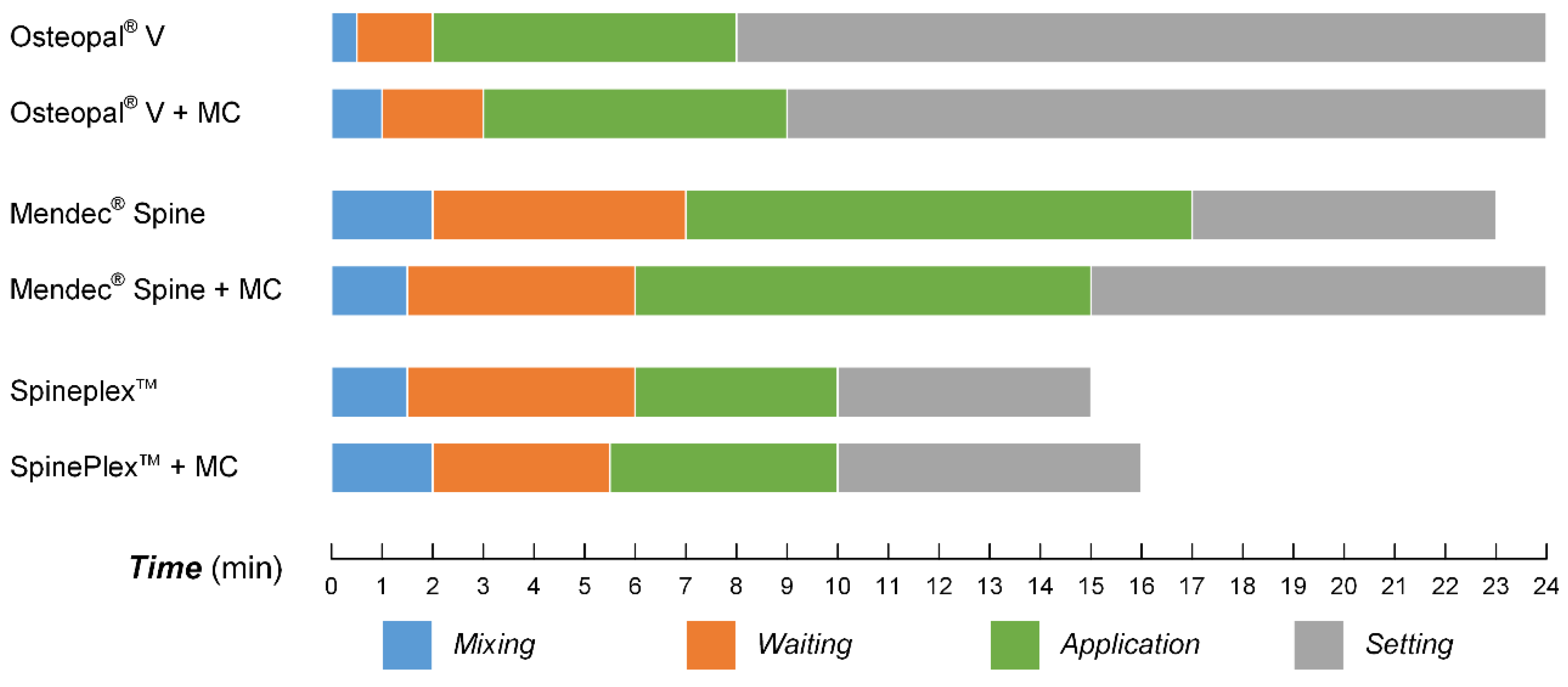

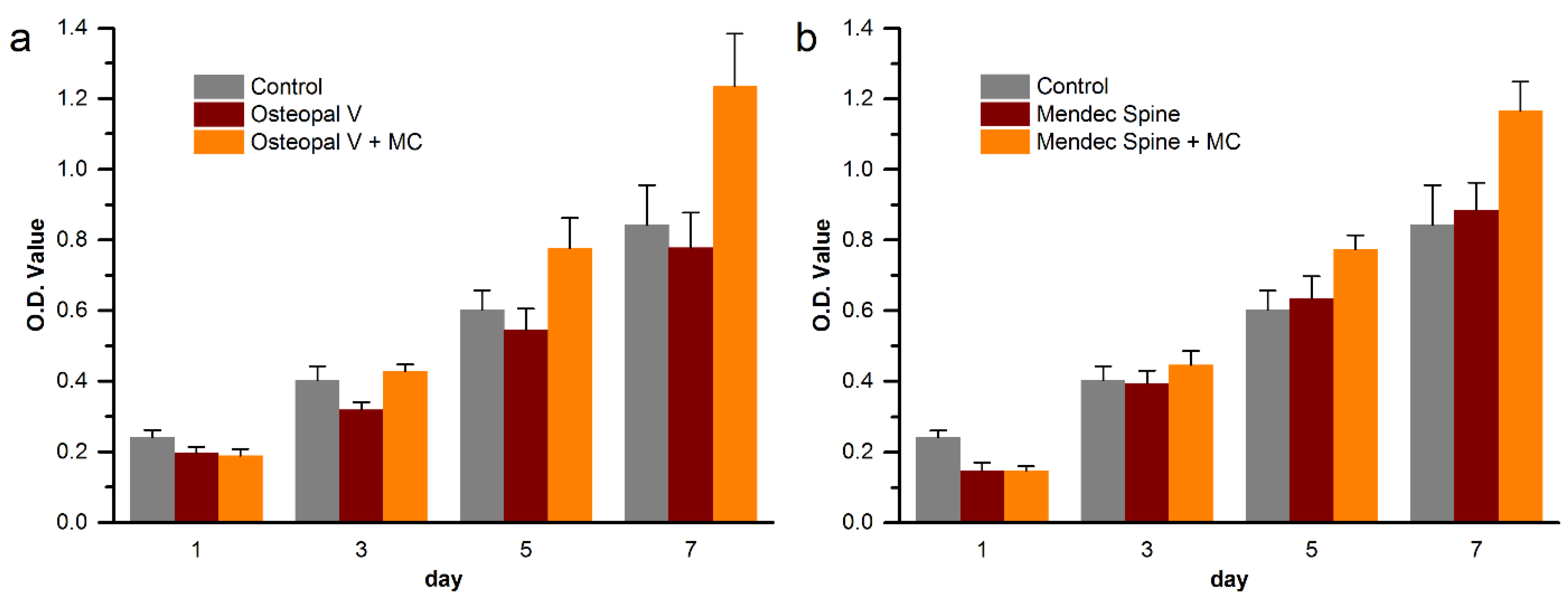
3.6. Cytocompatibility Improvement of the Modified Bone Cements
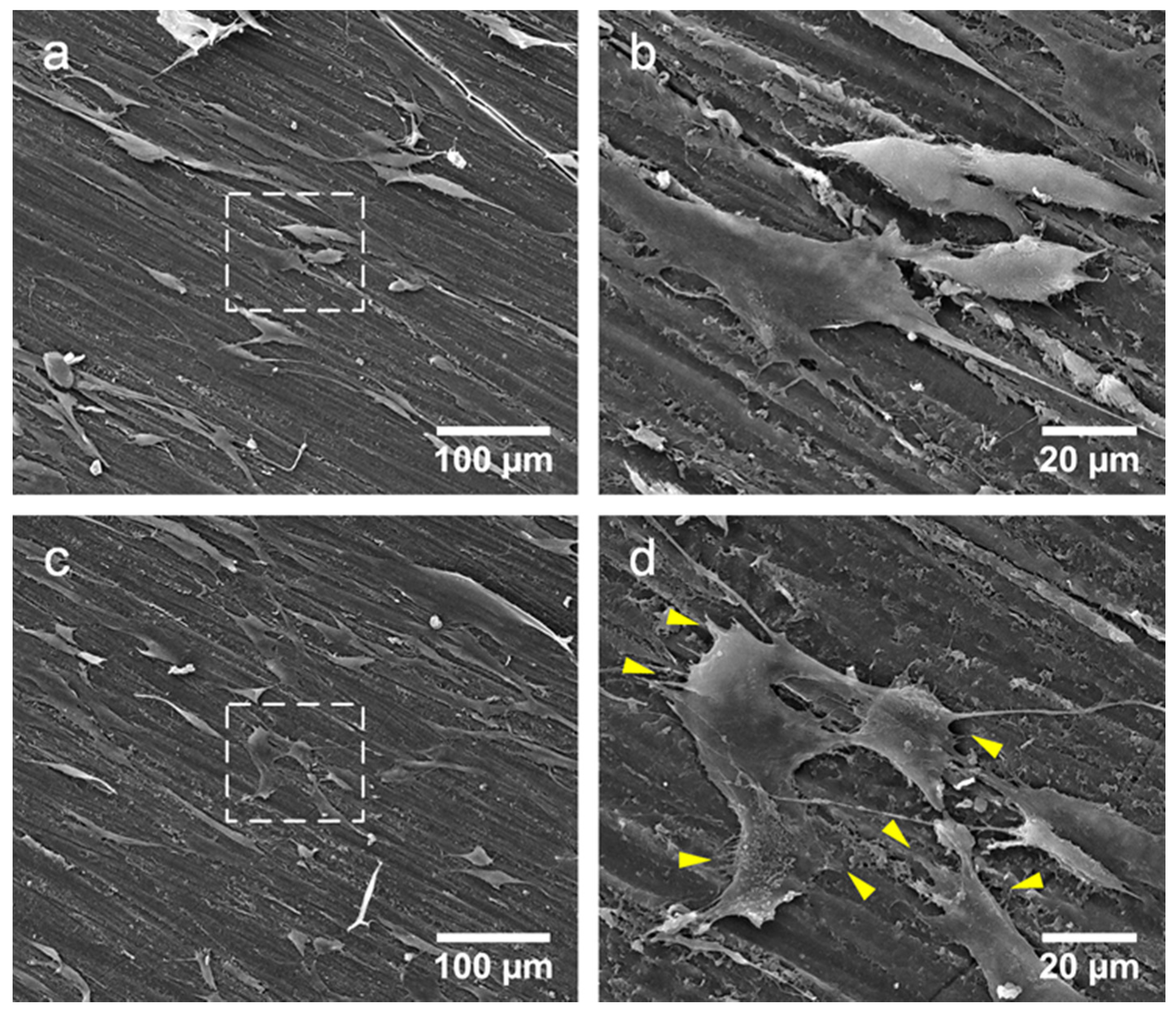
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Old, J.L.; Calvert, M. Vertebral compression fractures in the elderly. Am. Fam. Physician 2004, 69, 111–116. [Google Scholar] [PubMed]
- Furstenberg, C.H.; Grieser, T.; Wiedenhofer, B.; Gerner, H.J.; Putz, C.M. The role of kyphoplasty in the management of osteogenesis imperfecta: Risk or benefit? Eur. Spine J. 2010, 19 (Suppl. 2), S144–S148. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Li, Y.D.; Wu, C.G.; Sun, Z.K.; He, C.J. Safety and efficacy of percutaneous vertebroplasty and interventional tumor removal for metastatic spinal tumors and malignant vertebral compression fractures. AJR Am. J. Roentgenol. 2014, 202, W298–W305. [Google Scholar] [CrossRef] [PubMed]
- Banse, X.; Sims, T.J.; Bailey, A.J. Mechanical properties of adult vertebral cancellous bone: Correlation with collagen intermolecular cross-links. J. Bone Miner. Res. 2002, 17, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.J.; Lang, S.M.; Hoshaw, S.J.; Reimann, D.A.; Fyhrie, D.P. Human vertebral body apparent and hard tissue stiffness. J. Biomech. 1998, 31, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Villarraga, M.L.; Zhao, K.; Edidin, A.A. Static and fatigue mechanical behavior of bone cement with elevated barium sulfate content for treatment of vertebral compression fractures. Biomaterials 2005, 26, 3699–3712. [Google Scholar] [CrossRef] [PubMed]
- Jasper, L.E.; Deramond, H.; Mathis, J.M.; Belkoff, S.M. Material properties of various cements for use with vertebroplasty. J. Mater. Sci. Mater. Med. 2002, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Grados, F.; Depriester, C.; Cayrolle, G.; Hardy, N.; Deramond, H.; Fardellone, P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000, 39, 1410–1414. [Google Scholar] [CrossRef]
- Trout, A.T.; Kallmes, D.F.; Layton, K.F.; Thielen, K.R.; Hentz, J.G. Vertebral endplate fractures: An indicator of the abnormal forces generated in the spine after vertebroplasty. J. Bone Miner. Res. 2006, 21, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.W.; Mendoza, T.; Gebhardt, R.; Hamid, B.; Nouri, K.; Perez-Toro, M.; Ting, J.; Koyyalagunta, D. Vertebral compression fracture treatment with vertebroplasty and kyphoplasty: Experience in 407 patients with 1,156 fractures in a tertiary cancer center. Pain Med. 2011, 12, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Kallmes, D.F.; Kaufmann, T.J. New fractures after vertebroplasty: Adjacent fractures occur significantly sooner. AJNR Am. J. Neuroradiol. 2006, 27, 217–223. [Google Scholar] [PubMed]
- Sugino, A.; Miyazaki, T.; Kawachi, G.; Kikuta, K.; Ohtsuki, C. Relationship between apatite-forming ability and mechanical properties of bioactive PMMA-based bone cement modified with calcium salts and alkoxysilane. J. Mater. Sci. Mater. Med. 2008, 19, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Portigliatti-Barbos, M.; Rossi, P.; Salvadori, L.; Carando, S.; Gallinaro, M. Bone-cement interface: A histological study of aseptic loosening in twelve prosthetic implants. Ital. J. Orthop. Traumatol. 1986, 12, 499–505. [Google Scholar] [PubMed]
- Mann, K.A.; Miller, M.A.; Cleary, R.J.; Janssen, D.; Verdonschot, N. Experimental micromechanics of the cement-bone interface. J. Orthop. Res. 2008, 26, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng.: R: Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.S.; Cui, F.Z. Hierarchical Self-Assembly of Nano-Fibrils in Mineralized Collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: Nanohydroxyapatite/collagen/poly(L-lactide). Tissue Eng. 2004, 10, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.S.; Guan, K.; Cui, F.Z.; Shi, S.S.; Sun, T.S. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine (Phila Pa 1976) 2003, 28, 1954–1960. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically biomimetic bone scaffold materials: Nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization, Implants for Surgery—Acrylic Resin Cements; ISO 5833:2002(E); International Organization for Standardization: Geneva, Switzerland, 2002.
- Tsai, T.T.; Chen, W.J.; Lai, P.L.; Chen, L.H.; Niu, C.C.; Fu, T.S.; Wong, C.B. Polymethylmethacrylate cement dislodgment following percutaneous vertebroplasty: A case report. Spine (Phila Pa 1976) 2003, 28, E457–E460. [Google Scholar] [CrossRef]
- Lam, W.; Pan, H.B.; Fong, M.K.; Cheung, W.S.; Wong, K.L.; Li, Z.Y.; Luk, K.D.; Chan, W.K.; Wong, C.T.; Yang, C.; Lu, W.W. In vitro characterization of low modulus linoleic acid coated strontium-substituted hydroxyapatite containing PMMA bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Gurruchaga, M.; Goni, I. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Formulation and rheological behaviour. J. Mater. Sci. Mater. Med. 2009, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Parra, J.; Vazquez, B.; Bravo, A.L.; Collia, F.; Goni, I.; Gurruchaga, M.; San Roman, J. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Bioactivity and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, Y.J.; Yoon, T.L.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.W. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Boger, A.; Bohner, M.; Heini, P.; Verrier, S.; Schneider, E. Properties of an injectable low modulus PMMA bone cement for osteoporotic bone. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Boger, A.; Wheeler, K.; Montali, A.; Gruskin, E. NMP-modified PMMA bone cement with adapted mechanical and hardening properties for the use in cancellous bone augmentation. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Mestres, G.; Karlsson Ott, M.; Engqvist, H.; Ferguson, S.J.; Persson, C.; Helgason, B. Compressive mechanical properties and cytocompatibility of bone-compliant, linoleic acid-modified bone cement in a bovine model. J .Mech. Behav. Biomed. Mater. 2014, 32, 245–256. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616-2634. https://doi.org/10.3390/ma8052616
Jiang H-J, Xu J, Qiu Z-Y, Ma X-L, Zhang Z-Q, Tan X-X, Cui Y, Cui F-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials. 2015; 8(5):2616-2634. https://doi.org/10.3390/ma8052616
Chicago/Turabian StyleJiang, Hong-Jiang, Jin Xu, Zhi-Ye Qiu, Xin-Long Ma, Zi-Qiang Zhang, Xun-Xiang Tan, Yun Cui, and Fu-Zhai Cui. 2015. "Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen" Materials 8, no. 5: 2616-2634. https://doi.org/10.3390/ma8052616





