Polysaccharides for the Delivery of Antitumor Drugs
Abstract
:1. Introduction
2. Polysaccharides
2.1. Polysaccharide Structure

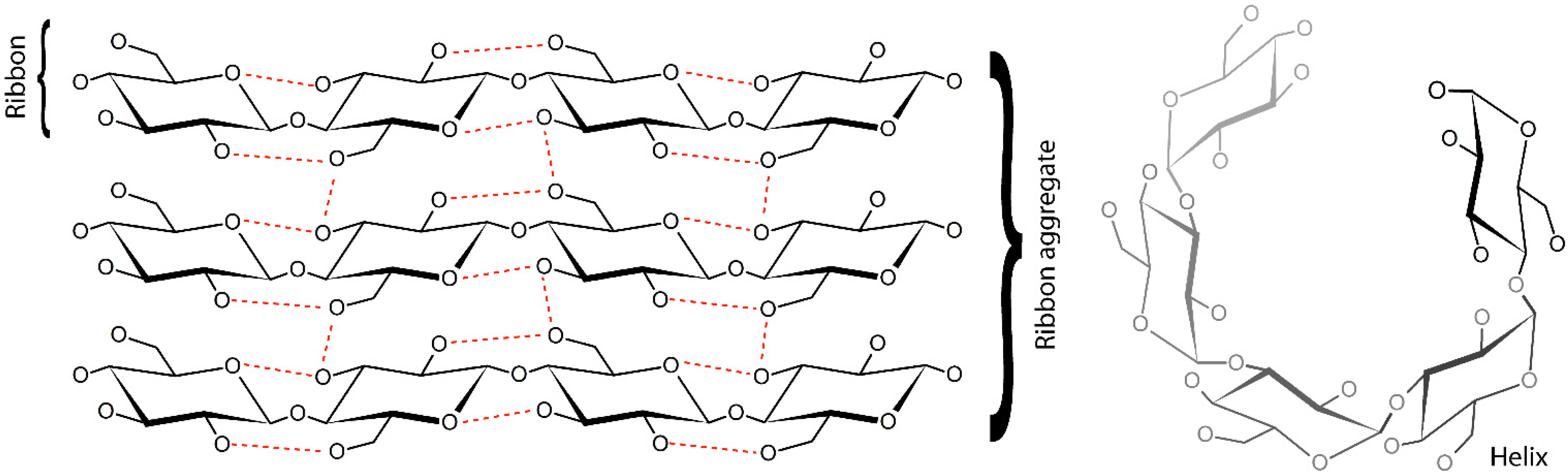
2.2. Polysaccharides Types
| Animal Polysaccharides | |
| Chitin | |
| Chitosan [16,17,18,19,20,21,22,23,24,25,26,27,28] | |
| Glycosamminoglycans [29,30,31,32,33,34,35,36,37,38] | |
| Vegetal Polysaccharides | |
| Land Vegetal | Marine Vegetal |
| Cellulose [39,40,41] | Alginate (Phaeophyceae—brown seaweed) |
| Pectins | Agar (Rhodophyceae—red seaweed) |
| Galactomannans | Carragenans (Rhodophyceae—red seaweed) |
| Acacia gum | – |
| Starch | – |
| Microorganisms/Fungi | |
| Alginate (Pseudomonas aeruginosa and Azotobacter vinelandii) [42,43,44] | |
| Dextran (Leuconostoc spp. and Lactobacillus spp.) [45,46,47,48,49,50] | |
| Gellan (Pseudomonas elodea) | |
| Pullulan (Aureobasidium pullulans) [51,52,53] | |
| Scleroglucan (Sclerotium glucanicum) Xanthomonas campestris | |
| Xanthan (Xanthomonas campestris) | |
2.2.1. Animal Polysaccharides
2.2.2. Vegetal Polysaccharides
2.2.3. Microorganisms/Fungi Polysaccharides
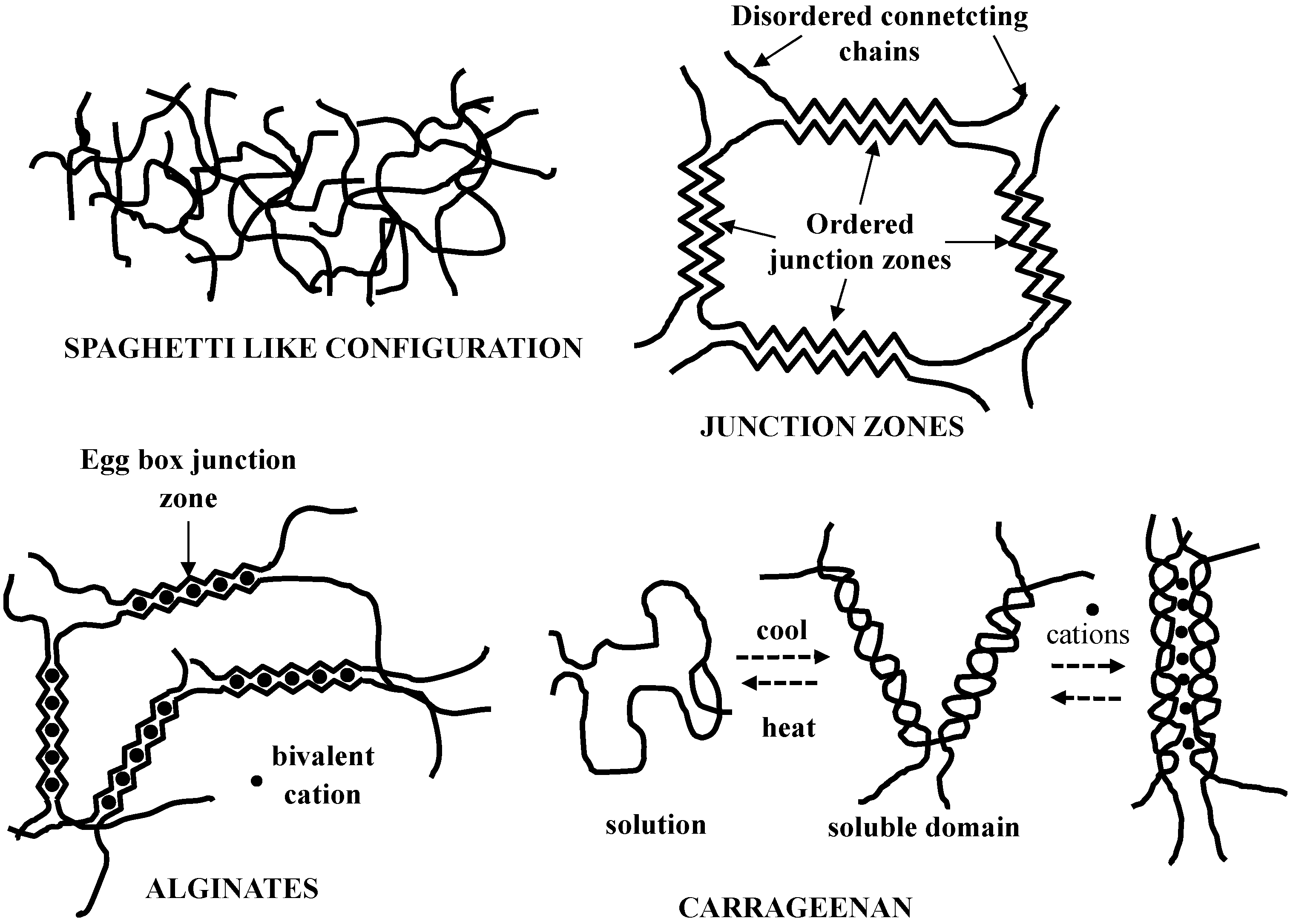
2.3. Polysaccharide Gels
2.4. Polysaccharide Micro and Nanoparticles
3. Polysaccharide-Based Delivery Systems for Anti-Cancer Drugs
3.1. Polysaccharides for the Delivery of Clinically Relevant Anti-Cancer Drugs
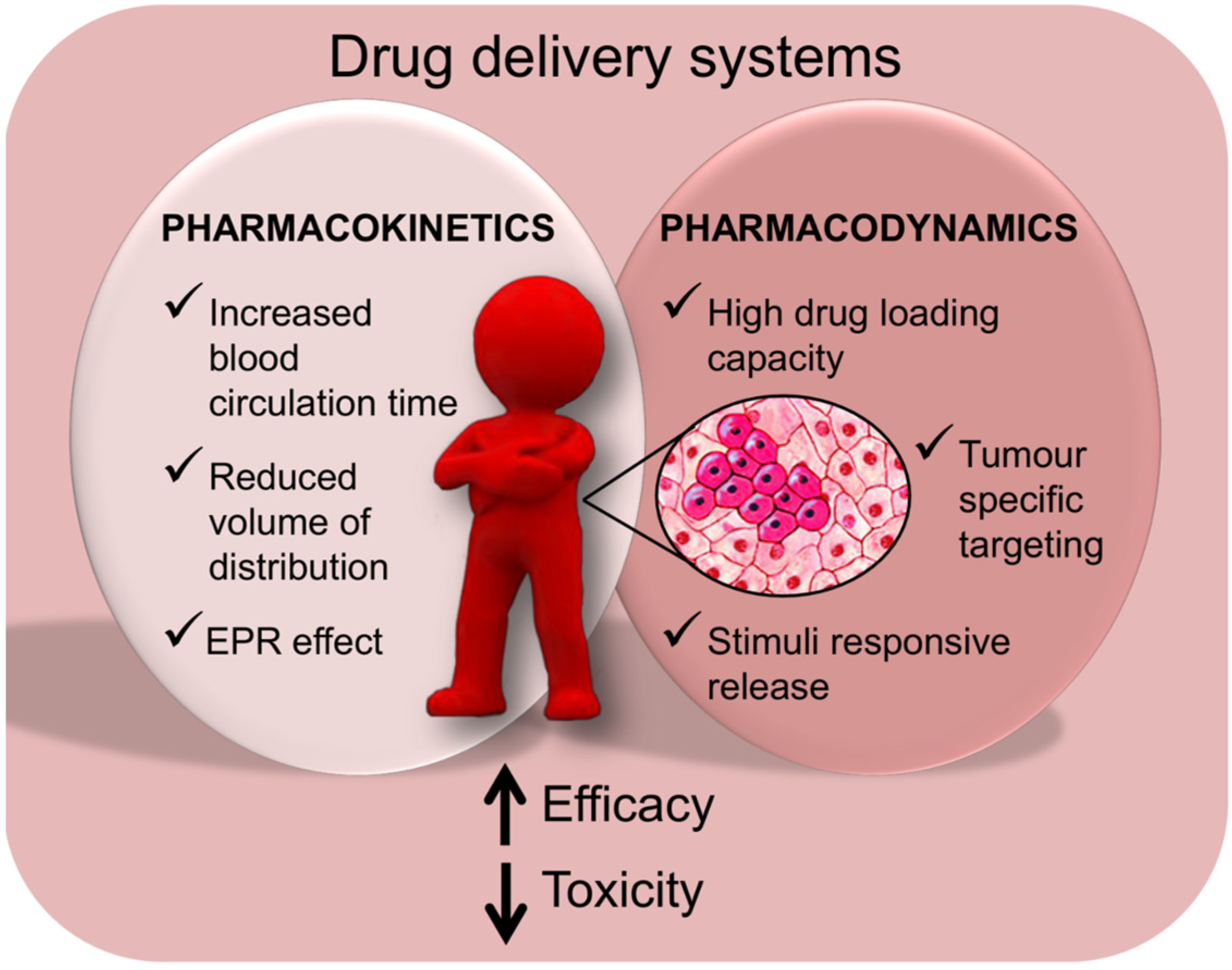
3.1.1. Critical Aspects of Systemic Drug Administration
3.1.2. Anticancer Drugs
| System | Structure | Drug | Disease | In vitro Model | In vivo Model | Ref. | |
|---|---|---|---|---|---|---|---|
| Chitosan | |||||||
| 1 | LMWC-PTX | LMWC | PTX | Melanoma, NSCLC | – | Mouse | [16] |
| 2 | LMWC-DTX | LMWC | DTX | NSCLC, GBM | NCI-H358, U87MG | Mouse | [17] |
| 3 | PTX-Cy5.5-CS | NPs | PTX | SCC | SCC7 | Mouse | [18] |
| 4 | Cy5-CS | NPs | DOX | Sarcoma | HT1080 | – | [19] |
| 5 | HGCS-Ce6 | NPs | Ce6 | SCC, adenocarcinoma | SCC-7 | Mouse | [20] |
| GCS-Ce6 | HT-29 | ||||||
| 6 | FLT-CS | MPs | FLT | – | – | – | [21] |
| 7 | CS/GP | Hydrogel | CDDP | Oral cancer | – | – | [22] |
| CS/GP/GE | |||||||
| Hyaluronic Acid | |||||||
| 8 | MSP-HA-DOX | Nano-conjugate | DOX | Breast cancer | MDA-MB-231 | – | [29] |
| 9 | HA-DOX | Nano-conjugate | DOX | Breast cancer | MDA-MB-468NL | Rat | [30] |
| 10 | HA-CDDP | Nano-conjugate | CDDP | Lung cancer | A549 | Rat | [31] |
| 11 | HA-CA-PTX | Nano-conjugate | PTX | Head and neck cancer | SCC7, NIH3T3 | Mouse | [32] |
| 12 | HACD-AuNPs | Gold nanocluster | several | Breast cancer | MCF7, NIH3T3 | – | [33] |
| Dextran | |||||||
| 13 | CMD-g-β-CD/(PAD-g-AD&AD-DOX))4 | Microsystem | DOX | – | HeLa | – | [45] |
| 14 | DEX-PDP | Nanosystem | DOX, CPT11 | Breast cancer | MCF7, DLD1 | – | [46] |
| Colon cancer | |||||||
| Pullulan | |||||||
| 15 | P-PTX | NPs | PTX | Colon cancer | HCT116 | Mouse | [51] |
| Cellulose | |||||||
| 16 | HPC-DNR | Conjugate | DNR | – | HeLa | Mouse | [39] |
| 17 | CMC-PABA, CMC-PAH | Nano-conjugate | Curcumin and derivates | Colon cancer | HT29, CRL1790 | – | [40] |
| 18 | HPC-PAA-CdSe | Nanogel | TMZ | Melanoma | B16F10 | – | [41] |
| Other | |||||||
| 19 | MR-5-FU | NPs | 5-FU | Breast cancer | MCF7 | – | [80] |
| 20 | FA-AG-MTX | NPs | MTX | – | AA8 | – | [81] |
3.1.3. Chitosan Based Delivery
3.1.4. Hyaluronic Acid Based Delivery
3.1.5. Dextran Based Delivery
3.1.6. Pullulan Based Delivery
3.1.7. Cellulose Based Delivery
3.1.8. Other Polysaccharide Based Delivery
3.2. Polysaccharides for the Delivery of Nucleic Acid Based Drugs
3.2.1. Small and Micro Interfering RNA Molecules
3.2.2. The Delivery Problems
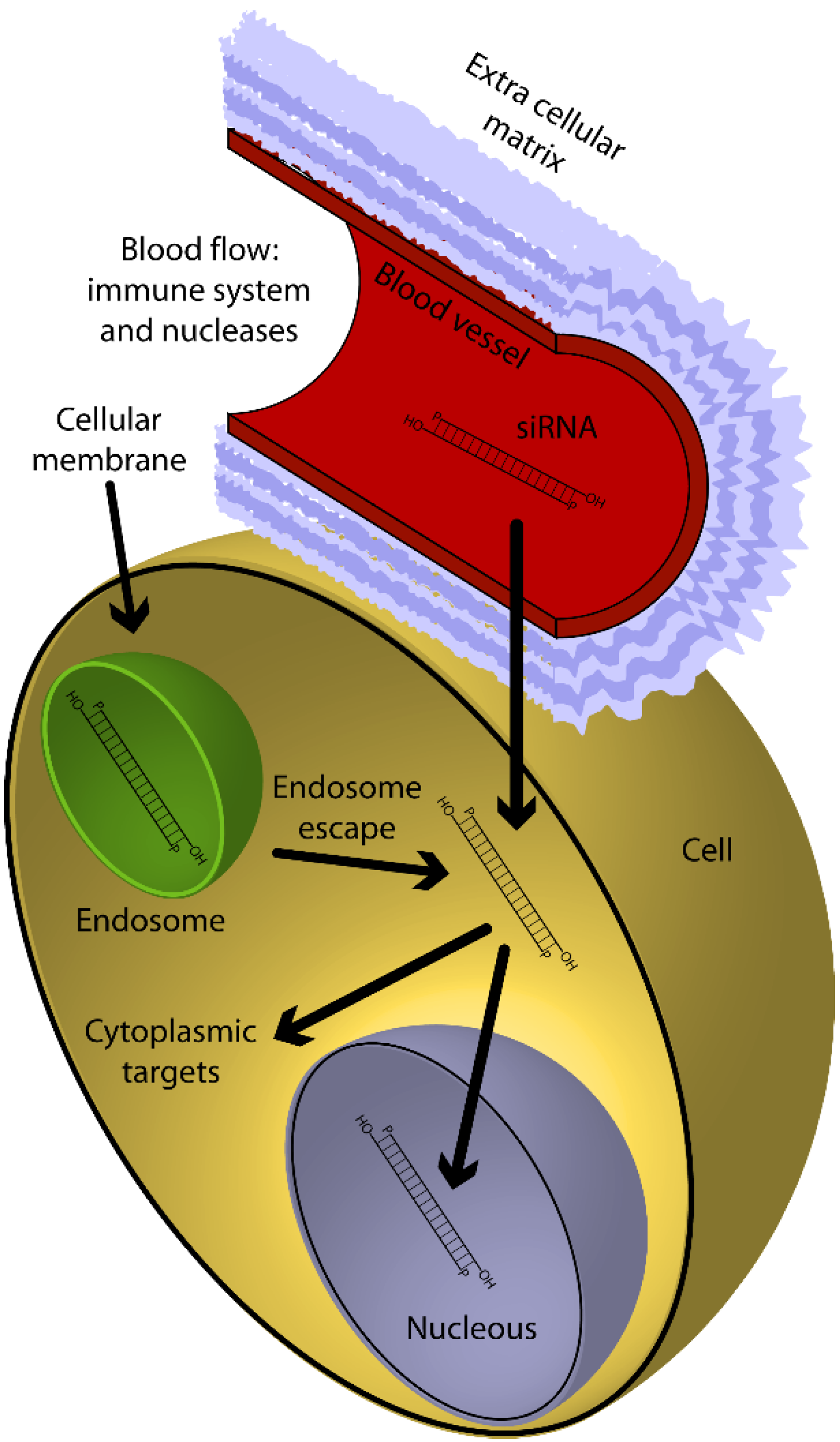
| System | Structure | Drug | Disease | In vitro Model | In vivo Model | Ref. | |
|---|---|---|---|---|---|---|---|
| Chitosan | |||||||
| 1 | Bio-CS-g-PEI | NPs | siRNA | Ovary cancer | HeLa | – | [23] |
| OVCAR-3 | |||||||
| 2 | CS-TAT | NPs | siRNA anti luciferase/survivin | Brest cancer | MCF7 | Mouse | [24,25] |
| CS-nonaArg | |||||||
| 3 | CS-γPGA | NPs | siRNA anti luciferase/GFP | – | HT1080 | – | [26,27] |
| 4 | psi-Pgp-GCt | NPs | siRNA anti Pgp | Brest cancer | MCF7 | Mouse | [28] |
| Hyaluronic Acid | |||||||
| 5 | HA-Chol/2b | NPs | siRNA anti RFP | – | B16F10 | – | [34] |
| 6 | PEG-HA-NP | NPs | siRNA anti Pgp siRNA anti GGCT | Breast cancer | MCF7 | Mouse | [35,36] |
| 7 | CAP/dopa-HA | NPs | siRNA anti luciferase | – | HT29 | Mouse | [37] |
| 8 | CAP-HA-DPA/Zn | NPs | siRNA anti luciferase | – | DU145 | Mouse | [38] |
| 143B HCT116 | |||||||
| Dextran | |||||||
| 9 | Ac-DEX-spermine | NPs | siRNA anti luciferase | – | HeLa | – | [47,48] |
| 10 | DexS/pArg | NPs | siRNA anti GFP | – | HeLa | – | [49] |
| 11 | DexS/pArg/HY | NPs | siRNA anti SPARC | – | Fibroblast | – | [50] |
| Pullulan | |||||||
| 12 | P-PEI-FA | NPs | siRNA anti luciferase | – | HeLa | – | [52] |
| 13 | Ps | NPs | siRNA | – | MDA-MB-231 | – | [53] |
| Other | |||||||
| 14 | CS/alginate | hydrogel | siRNA anti TNFα | Colitis | – | Mouse | [42] |
| 15 | CS/alginate | hydrogel | siRNA anti CD98 | Colitis | Colon-26 RAW 264.7 | Mouse | [43] |
| 16 | PEI/alginate | NPs | siRNA anti VEGFR-3 | – | EPCs | – | [44] |
| 17 | PEG-Tf-CD | NPs | siRNA anti RRM2 | – | – | Mouse | [120,121] |
| Non-human primate Clinical trial | |||||||
3.2.3. Chitosan Based Delivery
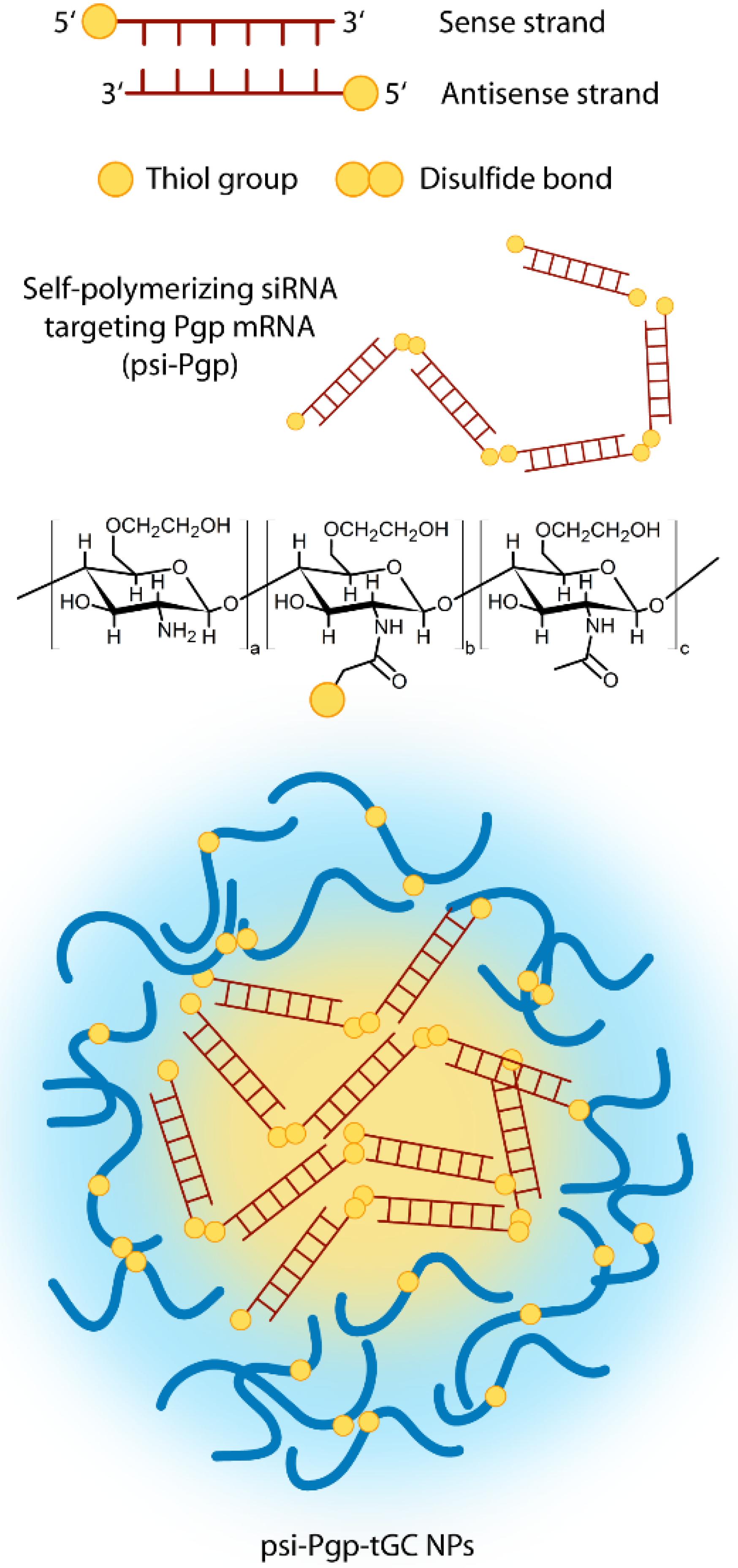
3.2.4. Hyaluronic Acid Based Delivery
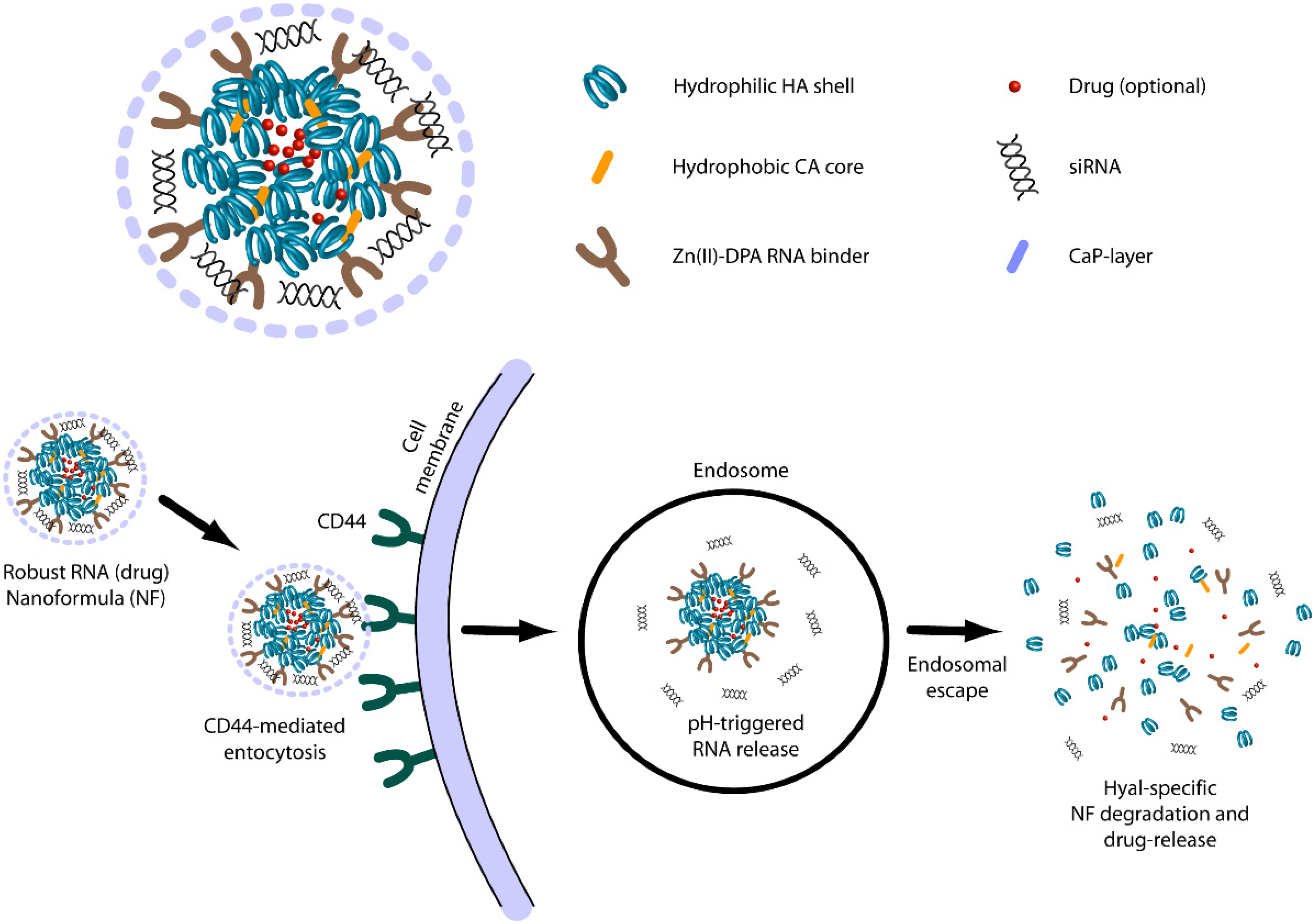
3.2.5. Dextran Based Delivery
3.2.6. Pullulan Based Delivery
3.2.7. Other Polysaccharide Based Delivery
4. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| ATP binding cassette transporter B1 | ABCB1 |
| Absorption, Distribution, Metabolism and Elimination | ADME |
| Arabinogalactan | AG |
| Biotin | Bio |
| Calcium phosphate | CAP |
| Cluster determinant 44 | CD44 |
| Cisplatin | CDDP |
| Cyclodextrins | CDs |
| Carboxymethylcellulose | CMC |
| Irinotecan | CPT-11 |
| Chitosan | CS |
| Daunorubicin | DNR |
| Doxorubicin | DOX |
| Double stranded RNAs | dsRNAs |
| Docetaxel | DTX |
| Extracellular matrix | ECM |
| Endothelial progenitor cells | EPCs |
| Enhanced permeability and retention effect | EPR |
| Folic acid | FA |
| 5-fluorouracil | 5-FU |
| Flutamide | FLT |
| Folic acid receptor | FR |
| Förster resonance energy transfer | FRET |
| Glycosaminoglycans | GAGs |
| Green fluorescence protein | GFP |
| γ-glutamylcyclotransferase | GGCT |
| Hyaluronic acid | HA |
| HA receptor for endocytosis | HARE |
| Hydroxypropyl methylcellulose | HPC |
| Hydroxyapatite | HY |
| Interpenetrated polymeric networks | IPN |
| Lipofectamine | LF |
| Low molecular weight chitosan | LMWC |
| Lymphatic vessel endothelial hyaluronan receptor-1 | LYVE-1 |
| Multi drug resistance | MDR |
| micro interfering RNAs | miRNAs |
| Mesoporous silica | MPS |
| Microparticles | MPs |
| Mauran | MR |
| Methotrexate | MTX |
| Molecular weight | MW |
| Nucleic acid based drugs | NABDs |
| Nanoparticles | NPs |
| Pullulan | P |
| Pullulan-spermine | Ps |
| p-aminobenzoic acid | PABA |
| Aminohippuric acid-diamine | PAH |
| Pharmacodynamics | PD |
| Photodynamic therapy | PDT |
| Polyethylene glycol | PEG |
| Polyethyleneimine | PEI |
| P-glycoprotein | Pgp |
| Pharmacokinetics | PK |
| Paclitaxel | PTX |
| Red fluorescence protein | RFP |
| Receptor for hyaluronate-mediated motility | RHAMM |
| RNA-induced silencing complexes | RISC |
| RNA interference | RNAi |
| Ribonucleotide reductase subunit M2 | RRM2 |
| small interfering RNAs | siRNAs |
| Acidic and rich in cysteine | SPARC |
| Transferrin | Tf |
| Tetrahydrocurcumin | THC |
| Temozolomide | TMZ |
| Vascular endothelial growth factor receptor-3 | VEGFR-3 |
Cell lines
| brain glioblastoma (human) | U87MG |
| breast cancer cell line (human) | MDA-MB-468NL |
| breast cancer cell line (human) | MDA-MB-231 |
| breast cancer cells (human) | MCF7 |
| cervical cancer cell line (human) | HeLa |
| colon adenocarcinoma (human) | HT29 |
| colon cancer (human) | DLD1 |
| colon carcinoma cells (human) | HCT116 |
| colon cell line (human) | CRL1790 |
| colon cells (human) | Colon-26 |
| colorectal adenocarcinoma cells (human) | Caco-2 |
| connective tissue fibroblast cells (mouse) | L929 |
| fibroblast (murine) | NIH3T3 |
| fibrosarcoma cell line (human) | HT1080 |
| head and neck squamous cell carcinoma (human) | SCC7 |
| hepatic cancer cell line (human) | JHH6 |
| hepatocellular carcinoma cell (human) | HepG2 |
| kidney cell (African green monkey) | COS-7 |
| lung adenocarcinoma epithelial cell line (human) | A549 |
| lung carcinoma cell line (human) | H1299 |
| macrophage cells (murine) | RAW264.7 |
| melanoma cell line (mouse) | B16F10 |
| non-small-cell lung carcinoma (human) | NSCLC-NCIH358 |
| ovary cancer cells (human) | OVCAR-3 |
| ovary cell line (chinese hamster) | AA8 |
| prostate tumor cells (human) | DU145 |
| prostate tumor cells (human) | 143B |
| umbilical vein endothelial (human) | HUVEC |
Conflict of Interest
References
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef] [PubMed]
- Polysaccharides: Structural Diversity and Functiojnal Versatility; Marcel Dekker: New York, NY, USA, 2005.
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.L. Colon targeted delivery systems: Review of polysaccharides for encapsulation and delivery. Crit. Rev. Food Sci. Nutr. 2005, 45, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccharides: Theory and Applications; Blackie Academic & Professional, Chapman & Hall: London, UK, 1995. [Google Scholar]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Wang, P.G. Polysaccharide-based systems in drug and gene delivery. Adv. Drug Deliv. Rev. 2013, 65, 1121–1122. [Google Scholar] [CrossRef]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Chaturvedi, K.; More, U.A.; Nadagouda, M.N.; Aminabhavi, T.M. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Control Release 2014, 193, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.; Di, M.C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.; Lee, I.H.; Yu, M.; Kim, H.; Chae, S.Y.; Jon, S. Conjugated chitosan as a novel platform for oral delivery of paclitaxel. J. Med. Chem. 2008, 51, 6442–6449. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, H.; Lee, I.H.; Jon, S. In vivo antitumor effects of chitosan-conjugated docetaxel after oral administration. J. Control Release 2009, 140, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.W.; Kim, I.S.; et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Chiu, Y.L.; Chen, Y.M.; Ho, Y.C.; Sung, H.W. Intracellularly monitoring/imaging the release of doxorubicin from pH-responsive nanoparticles using F+Ârster resonance energy transfer. Biomaterials 2011, 32, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Koo, H.; Jeong, H.; Huh, M.S.; Choi, Y.; Jeong, S.Y.; Byun, Y.; Choi, K.; Kim, K.; Kwon, I.C. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J. Control Release 2011, 152, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Elgindy, N.; Elkhodairy, K.; Molokhia, A.; Elzoghby, A. Biopolymeric microparticles combined with lyophilized monophase dispersions for controlled flutamide release. Int. J. Pharm. 2011, 411, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.J.; Gil, M.H.; Figueiredo, M.M. Delivery of cisplatin from thermosensitive co-cross-linked chitosan hydrogels. Eur. Polymer J. 2013, 49, 2504–2510. [Google Scholar] [CrossRef]
- Darvishi, M.H.; Nomani, A.; Amini, M.; Shokrgozar, M.A.; Dinarvand, R. Novel biotinylated chitosan-graft-polyethyleneimine copolymer as a targeted non-viral vector for anti-EGF receptor siRNA delivery in cancer cells. Int. J. Pharm. 2013, 456, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, W.; Li, Y.; Liu, S.; Jin, M.; Wang, Y.; Jia, L.; Gao, Z. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials 2013, 34, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, E.J.; Lee, J.; Rhim, T.; Lee, S.K.; Lee, K.Y. Preparation and characterization of nonaarginine-modified chitosan nanoparticles for siRNA delivery. Carbohydr. Polym. 2013, 92, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Hsiao, C.W.; Ho, Y.C.; Chen, H.L.; Sung, H.W. Disulfide bond-conjugated dual PEGylated siRNAs for prolonged multiple gene silencing. Biomaterials 2013, 34, 6930–6937. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Peng, S.F.; Chiu, Y.L.; Hsiao, C.W.; Liu, H.Y.; Lim, W.H.; Lu, H.M.; Sung, H.W. Enhancement of efficiency of chitosan-based complexes for gene transfection with poly(gamma-glutamic acid) by augmenting their cellular uptake and intracellular unpackage. J. Control Release 2014, 193, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Song, S.; Lee, S.J.; Park, S.; Kim, K.; Kim, M.G.; Son, S.; Koo, H.; Kwon, I.C.; Jeong, J.H.; et al. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J. Control Release 2014, 198C, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chem. Eur. J. 2013, 19, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Thati, S.; Bagby, T.R.; Diab, H.M.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J. Control. Release 2010, 146, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Aillon, K.L.; Cai, S.; Christian, J.M.; Davies, N.M.; Berkland, C.J.; Forrest, M.L. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int. J. Pharm. 2010, 392, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.G.; Moon, M.; Lee, S.; Jeong, Y.Y. Paclitaxel loaded hyaluronic acid nanoparticles for targeted cancer therapy: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2015, 72, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, Y.; Zhang, Y.M.; Yang, Y.; Su, Y.; Chen, J.T.; Liu, Y. Polysaccharide-gold nanocluster supramolecular conjugates as a versatile platform for the targeted delivery of anticancer drugs. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Jang, M.; Kim, J.H.; Ahn, H.J. Tumor-specific delivery of siRNA using supramolecular assembly of hyaluronic acid nanoparticles and 2b RNA-binding protein/siRNA complexes. Biomaterials 2014, 35, 7121–7132. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Liu, Y.; Gao, H.; Kuang, Q.; Zhang, Q.; Tang, J.; Huang, K.; Chen, X.; Zhang, Z.; He, Q. Enhanced gene delivery efficiency of cationic liposomes coated with PEGylated hyaluronic acid for anti P-glycoprotein siRNA: A potential candidate for overcoming multi-drug resistance. Int. J. Pharm. 2014, 477, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Liu, Y.; Gao, H.; Kuang, Q.; Zhang, Q.; Tang, J.; Fu, H.; Zhang, Z.; He, Q. PEGylated hyaluronic acid-modified liposomal delivery system with anti-gamma-glutamylcyclotransferase siRNA for drug-resistant MCF-7 breast cancer therapy. J. Pharm. Sci. 2014, 104, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, J.E.; Byun, E.; Kim, N.W.; Lee, K.; Lee, H.; Sim, S.J.; Lee, D.S.; Jeong, J.H. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Control Release 2014, 192, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Silvestre, O.F.; Huang, X.; Min, K.H.; Howard, G.P.; Hida, N.; Jin, A.J.; Carvajal, N.; Lee, S.W.; Hong, J.I.; et al. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano 2014, 8, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Metaxa, A.F.; Efthimiadou, E.K.; Boukos, N.; Fragogeorgi, E.A.; Loudos, G.; Kordas, G. Hollow microspheres based on—Folic acid modified—Hydroxypropyl Cellulose and synthetic multi-responsive bio-copolymer for targeted cancer therapy: Controlled release of daunorubicin, in vitro and in vivo studies. J. Colloid Interface Sci. 2014, 435, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Thipapun Plyduang, L.L. Carboxymethylcellulose-tetrahydrocurcumin conjugates for colon-specific delivery of a novel anti-cancer agent, 4-amino tetrahydrocurcumin. Eur. J. Pharm. Biopharm. 2014, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Aiello, M.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Viennois, E.; Xiao, B.; Canup, B.S.; Geem, D.; Denning, T.L.; Merlin, D. Fab'-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control. Release 2014, 186, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.; Zhang, Z.; Baker, M.T.; Zhang, B.; Gewirtz, A.T.; et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 2014, 146, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, G.D.; Tan, Y.Z.; Wang, H.J. Inhibition of lymphangiogenesis of endothelial progenitor cells with VEGFR-3 siRNA delivered with PEI-alginate nanoparticles. Int. J. Biol. Sci. 2014, 10, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.F.; Xu, X.D.; Zhang, J.; Yang, J.; Gong, Y.H.; Lei, Q.; Jia, H.Z.; Li, C.; Zhuo, R.X.; Zhang, X.Z. Encapsulation of an adamantane-doxorubicin prodrug in pH-responsive polysaccharide capsules for controlled release. ACS Appl. Mater. Interfaces 2012, 4, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Pramod, P.S.; Shah, R.; Chaphekar, S.; Balasubramanian, N.; Jayakannan, M. Polysaccharide nano-vesicular multidrug carriers for synergistic killing of cancer cells. Nanoscale 2014, 6, 11841–11855. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Schubert, S.; Wich, P.R.; Cui, L.; Cohen, J.A.; Mynar, J.L.; Frechet, J.M. Acid-degradable cationic dextran particles for the delivery of siRNA therapeutics. Bioconjug. Chem. 2011, 22, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Ganas, C.; Weiss, A.; Nazarenus, M.; Rosler, S.; Kissel, T.; Rivera, G.P.; Parak, W.J. Biodegradable capsules as non-viral vectors for in vitro delivery of PEI/siRNA polyplexes for efficient gene silencing. J. Control Release 2014, 196, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.F.; Mundargi, R.C.; Chen, M.H.; Lessig, J.; Neu, B.; Venkatraman, S.S.; Wong, T.T. Layer-by-layer nanoparticles as an efficient siRNA delivery vehicle for SPARC silencing. Small 2014, 10, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hong, G.Y.; Jeong, Y.I.; Kang, M.S.; Oh, J.S.; Song, C.E.; Lee, H.C. Paclitaxel-incorporated nanoparticles of hydrophobized polysaccharide and their antitumor activity. Int. J. Pharm. 2012, 433, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, B.; Bao, Y. Efficient targeted pDNA/siRNA delivery with folate-low-molecular-weight polyethyleneimine-modified pullulan as non-viral carrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Tabata, Y.; Meng, J.; Xu, H. The effect of serum in culture on RNAi efficacy through modulation of polyplexes size. Biomaterials 2014, 35, 567–577. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, E.; Borselli, C.; Netti, P. Transport of large molecules in hyaluronic acid-based membranes and solution. J. Membr. Sci. 2006, 273, 84–88. [Google Scholar] [CrossRef]
- Turco, G.; Donati, I.; Grassi, M.; Marchioli, G.; Lapasin, R.; Paoletti, S. Mechanical spectroscopy and relaxometry on alginate hydrogels: A comparative analysis for structural characterization and network mesh size determination. Biomacromolecules 2011, 12, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.; Morris, E.; Rees, D.; Smith, P.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Smidsrod, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G.; Lapasin, R.; Colombo, I. Understanding Drug Release and Absorption Mechanisms: A Physical and Mathematical Aproach; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Abrami, M.; D'Agostino, I.; Milcovich, G.; Fiorentino, S.; Farra, R.; Asaro, F.; Lapasin, R.; Grassi, G.; Grassi, M. Physical characterization of alginate-Pluronic F127 gel for endoluminal NABDs delivery. Soft Matter 2014, 10, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Grassi, G. Application of mathematical modeling in sustained release delivery systems. Expert Opin. Drug Deliv. 2014, 11, 1299–1321. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Bioinspired drug delivery systems. Curr. Opin. Biotechnol. 2013, 24, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Song, W.; Blanco-Fernandez, B.; Alvarez-Lorenzo, C.; Mano, J.F. Synthesis of temperature-responsive dextran-MA/PNIPAAm particles for controlled drug delivery using superhydrophobic surfaces. Pharm. Res . 2011, 28, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, M. Pharmakokinetic und Arzneimittelentwicklung. Deutske Apotheker 1994, Z.4, 23–32. (In German) [Google Scholar]
- Muller, K.; Fedosov, D.A.; Gompper, G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Tschudi, K.; Nam, J.; Lu, X.; Ma, A.W. Particle margination and its implications on intravenous anticancer drug delivery. AAPS PharmSciTech 2014, 15, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Sikes, R.A. Chemistry and pharmacology of anticancer drugs. Br. J. Cancer 2007, 97, 1713. [Google Scholar] [CrossRef]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Biopolymers In Drug Delivery: Recent Advances and Challenges; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012.
- Hennenfent, K.L.; Govindan, R. Novel formulations of taxanes: A review. Old wine in a new bottle? Ann. Oncol. 2006, 17, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Weiszhár, Z.; Czúcz, J.; Révész, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS applied to over 900 drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Poulose, A.C.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Bacterial exopolysaccharide based nanoparticles for sustained drug delivery, cancer chemotherapy and bioimaging. Carbohydr. Polym. 2013, 91, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pinhassi, R.I.; Assaraf, Y.G.; Farber, S.; Stark, M.; Ickowicz, D.; Drori, S.; Domb, A.J.; Livney, Y.D. Arabinogalactan-folic acid-drug conjugate for targeted delivery and target-activated release of anticancer drugs to folate receptor-overexpressing cells. Biomacromolecules 2010, 11, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Stella, B.; Arpicco, S.; Cattel, L. Macromolecules as taxane delivery systems. Expert Opin. Drug Deliv. 2011, 8, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Neri, R. Pharmacology and pharmacokinetics of flutamide. Urology 1989, 34, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Martínez-Máñez, R.; Sancenon, F.; Hoffmann, K.; Rurack, K. The supramolecular chemistry of organic-inorganic hybrid materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 5924–5948. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Jothy, S. CD44 and its partners in metastasis. Clin. Exp. Metastasis 2003, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control Release 2014, 174, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Tzircotis, G.; Thorne, R.F.; Isacke, C.M. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J. Cell Sci. 2005, 118, 5119–5128. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Andy, M.L.; Bystrom, K.; Perlmutter, H.D.; Kristol, D.S. Cyclodextrin inclusion complexes: Studies of the variation in the size of alicyclic guests. J. Am. Chem. Soc. 1989, 111, 6765–6772. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathi, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.M.; Khoury, J.T.; Schaaff, T.G.; Shafigullin, M.N.; Vezmar, I.; Whetten, R.L. Optical absorption spectra of nanocrystal gold molecules. J. Phys. Chem. B 1997, 101, 3706–3712. [Google Scholar] [CrossRef]
- Varnavski, O.; Ramakrishna, G.; Kim, J.; Lee, D.; Goodson, T. Critical size for the observation of quantum confinement in optically excited gold clusters. J. Am. Chem. Soc. 2010, 132, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Citrin, P.; Wertheim, G. Photoemission from surface-atom core levels, surface densities of states, and metal-atom clusters: A unified picture. Phys. Rev. B 1983, 27, 3176–3200. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Marini, J.C. Ribozymes: Structure, function, and potential therapy for dominant genetic disorders. Ann. Med. 1996, 28, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Dawson, P.; Guarnieri, G.; Kandolf, R.; Grassi, M. Therapeutic potential of hammerhead ribozymes in the treatment of hyper-proliferative diseases. Curr. Pharm. Biotechnol. 2004, 5, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Schneider, A.; Engel, S.; Racchi, G.; Kandolf, R.; Kuhn, A. Hammerhead ribozymes targeted against cyclin E and E2F1 cooperate to down-regulate coronary smooth muscle cell proliferation. J. Gene Med. 2005, 7, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Farra, R.; Grassi, M.; Pozzato, G.; Giansante, C.; Fiotti, N.; Grassi, G. Improving siRNA bio-distribution and minimizing side effects. Curr. Drug Metab. 2011, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Farra, R.; Tonon, F.; Abrami, M.; Grassi, M.; Musiani, F.; Zanconati, F.; Pozzato, G.; Grassi, G. Translation elongation. In Translation and its Regulation in Cancer Biology and Medicine; Parsyan, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 241–265. [Google Scholar]
- Dapas, B.; Farra, R.; Grassi, M.; Giansante, C.; Fiotti, N.; Uxa, L.; Rainaldi, G.; Mercatanti, A.; Colombatti, A.; Spessotto, P.; et al. Role of E2F1-cyclin E1-cyclin E2 circuit in human coronary smooth muscle cell proliferation and therapeutic potential of its downregulation by siRNAs. Mol. Med. 2009, 15, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Dapas, B.; Pozzato, G.; Scaggiante, B.; Agostini, F.; Zennaro, C.; Grassi, M.; Rosso, N.; Giansante, C.; Fiotti, N.; et al. Effects of E2F1-cyclin E1-E2 circuit down regulation in hepatocellular carcinoma cells. Dig. Liver Dis. 2011, 43, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Werth, D.; Grassi, G.; Konjer, N.; Dapas, B.; Farra, R.; Giansante, C.; Kandolf, R.; Guarnieri, G.; Nordheim, A.; Heidenreich, O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur. J. Cell Biol. 2010, 89, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Dapas, B.; Pozzato, G.; Giansante, C.; Heidenreich, O.; Uxa, L.; Zennaro, C.; Guarnieri, G.; Grassi, G. Serum response factor depletion affects the proliferation of the hepatocellular carcinoma cells HepG2 and JHH6. Biochimie 2010, 92, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Cavallaro, G.; Scirè, S.; Scaggiante, B.; Daps, B.; Farra, R.; Baiz, D.; Giansante, C.; Guarnieri, G.; Perin, D.; et al. Current strategies to improve the efficacy and the delivery of nucleic acid based drugs. Curr. Signal Transduct. Ther. 2010, 5, 92–120. [Google Scholar] [CrossRef]
- Lang, C.; Sauter, M.; Szalay, G.; Racchi, G.; Grassi, G.; Rainaldi, G.; Mercatanti, A.; Lang, F.; Kandolf, R.; Klingel, K. Connective tissue growth factor: A crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J. Mol. Med. 2008, 86, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Dapas, B.; Farra, R.; Grassi, M.; Racchi, G.; Klingel, K.; Kandolf, R.; Heidenreich, O.; Mercatahnti, A.; rainaldi, G.; et al. Potential applications of small interfering RNAs in the cardiovascular field. Drug Future 2006, 31, 513–525. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, J.; Zheng, S.; Ding, Y.; Guo, S.; Zhang, H.; Zhang, X.; Du, Q.; Liang, Z. Elimination pathways of systemically delivered siRNA. Mol. Ther. 2011, 19, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Layzer, J.M.; McCaffrey, A.P.; Tanner, A.K.; Huang, Z.; Kay, M.A.; Sullenger, B.A. In vivo activity of nuclease-resistant siRNAs. RNA 2004, 10, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Wisse, E.; Jacobs, F.; Topal, B.; Frederik, P.; De, G.B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008, 15, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y. Gene therapy: A battle against biological barriers. Curr. Mol. Med. 2001, 1, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Schatzlein, A.G.; Uchegbu, I.F. Gene delivery with synthetic (non viral) carriers. Int. J. Pharm. 2001, 229, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Amin, A.R.; Wang, X.; Zuckerman, J.E.; Choi, C.H.; Zhou, B.; Wang, D.; Nannapaneni, S.; Koenig, L.; Chen, Z.; et al. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J. Control. Release 2012, 159, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Koping-Hoggard, M.; Tubulekas, I.; Guan, H.; Edwards, K.; Nilsson, M.; Varum, K.M.; Artursson, P. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001, 8, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Methot, S.; Tran-Khanh, N.; Buschmann, M.D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 2006, 27, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Thibault, M.; Nimesh, S.; Lavertu, M.; Buschmann, M.D. Intracellular trafficking and decondensation kinetics of chitosan-pDNA polyplexes. Mol. Ther. 2010, 18, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Malmo, J.; Sorgard, H.; Varum, K.M.; Strand, S.P. siRNA delivery with chitosan nanoparticles: Molecular properties favoring efficient gene silencing. J. Control Release 2012, 158, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.X.; Ho, Y.C.; Chen, H.L.; Peng, S.F.; Hsiao, C.W.; Sung, H.W. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/siRNA complexes via the inclusion of a negatively charged poly(gamma-glutamic acid). Biomaterials 2010, 31, 8780–8788. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, B.; Lu, Y.; Ouahab, A.; Li, Q.; Tu, J. A novel tumor-targeted delivery system with hydrophobized hyaluronic acid-spermine conjugates (HHSCs) for efficient receptor-mediated siRNA delivery. Int. J. Pharm. 2011, 414, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Maruyama, A.; Kawano, S.; Nishimura, Y.; Asayama, S.; Nogawa, M.; Ikejima, K.; Hori, M.; Akaike, T.; Lemasters, J.J.; et al. Targeted gene transfer to sinusoidal endothelial cells and expression in vivo. Transplant Proc. 1999, 31, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Silvestre, O.F.; Huang, X.; Hida, N.; Liu, G.; Ho, D.N.; Lee, S.; Lee, S.W.; Hong, J.I.; Chen, X. A nanoparticle formula for delivering siRNA or miRNAs to tumor cells in cell culture and in vivo. Nat. Protoc. 2014, 9, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Naeye, B.; Raemdonck, K.; Remaut, K.; Sproat, B.; Demeester, J.; De Smedt, S.C. PEGylation of biodegradable dextran nanogels for siRNA delivery. Eur. J. Pharm. Sci. 2010, 40, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeye, B.; Deschout, H.; Roding, M.; Rudemo, M.; Delanghe, J.; Devreese, K.; Demeester, J.; Braeckmans, K.; De Smedt, S.C.; Raemdonck, K. Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials 2011, 32, 9120–9127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patnaik, S.; Arif, M.; Pathak, A.; Singh, N.; Gupta, K.C. PEI-alginate nanocomposites: Efficient non-viral vectors for nucleic acids. Int. J. Pharm. 2010, 385, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lieskovan, S.; Heidel, J.D.; Bartlett, D.W.; Davis, M.E.; Triche, T.J. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res. 2005, 65, 8984–8992. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Davis, M.E. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug. Chem. 2007, 18, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549–15554. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Deng, W.; Hyun, S.H.; Thompson, D.H. Development of a low toxicity, effective pDNA vector based on noncovalent assembly of bioresponsive amino-beta-cyclodextrin:adamantane-poly(vinyl alcohol)-poly(ethylene glycol) transfection complexes. Bioconjug Chem. 2012, 23, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; DeFrees, K.; Hyun, S.H.; Thompson, D.H. Pendant polymer: amino-beta-cyclodextrin: siRNA guest: host nanoparticles as efficient vectors for gene silencing. J. Am. Chem. Soc. 2012, 134, 7596–7599. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posocco, B.; Dreussi, E.; De Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G.; et al. Polysaccharides for the Delivery of Antitumor Drugs. Materials 2015, 8, 2569-2615. https://doi.org/10.3390/ma8052569
Posocco B, Dreussi E, De Santa J, Toffoli G, Abrami M, Musiani F, Grassi M, Farra R, Tonon F, Grassi G, et al. Polysaccharides for the Delivery of Antitumor Drugs. Materials. 2015; 8(5):2569-2615. https://doi.org/10.3390/ma8052569
Chicago/Turabian StylePosocco, Bianca, Eva Dreussi, Jacopo De Santa, Giuseppe Toffoli, Michela Abrami, Francesco Musiani, Mario Grassi, Rossella Farra, Federica Tonon, Gabriele Grassi, and et al. 2015. "Polysaccharides for the Delivery of Antitumor Drugs" Materials 8, no. 5: 2569-2615. https://doi.org/10.3390/ma8052569






