Applications of Ni3Al Based Intermetallic Alloys—Current Stage and Potential Perceptivities
Abstract
:1. Introduction
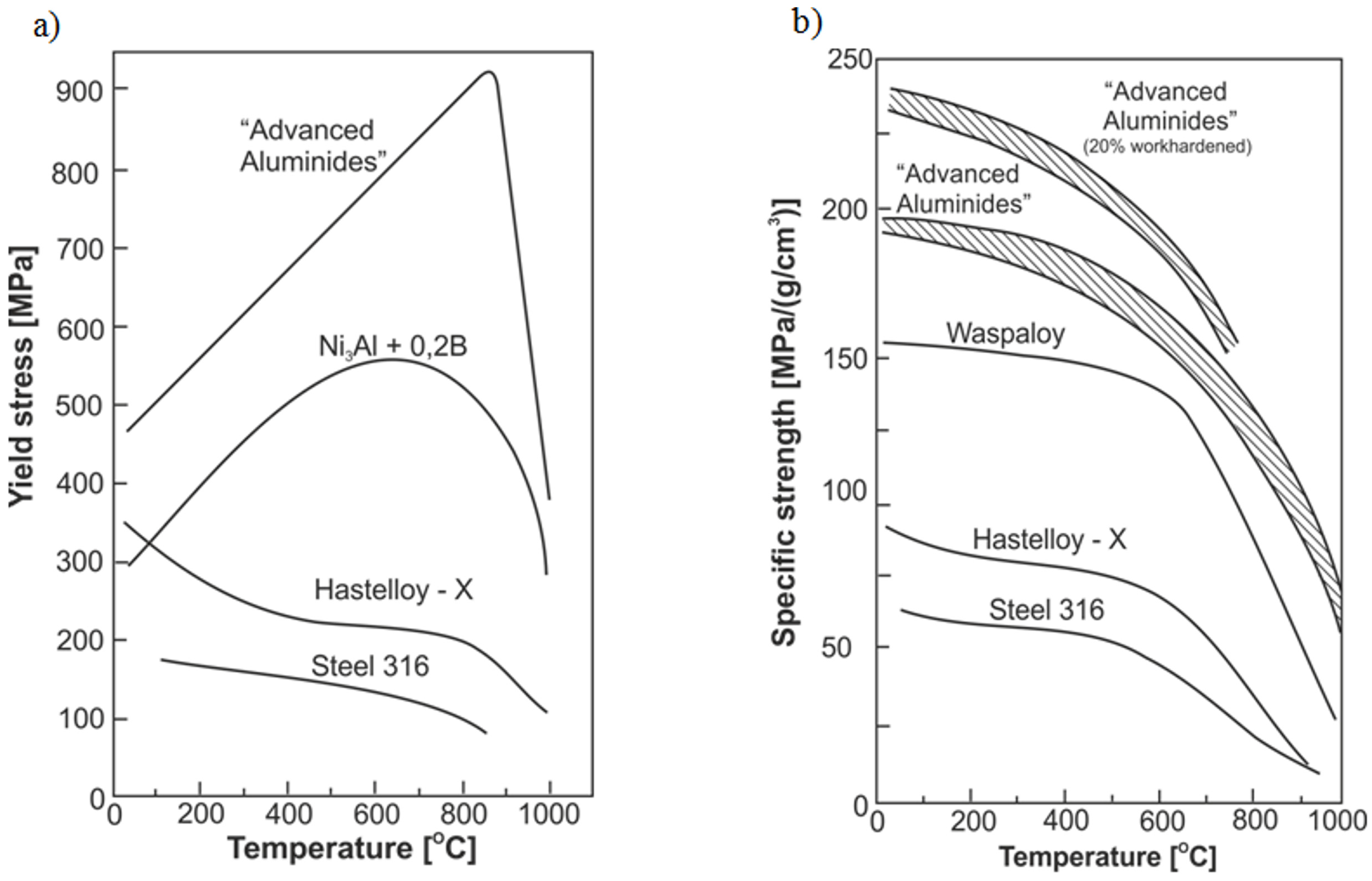
2. Properties of Ni3Al-Based Alloys
| Alloy | Chemical Composition (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cr | Mo | Zr | B | C | Fe | Ti | W | Si | Ni | |
| IC-50 | 11.30 | – | – | 0.60 | 0.02 | – | – | – | – | – | balance |
| IC-221M | 8.0 | 7.70 | 1.43 | 1.70 | 0.008 | – | – | – | – | – | balance |
| IC-218 | 8.65 | 7.87 | – | 0.86 | 0.02 | – | – | – | – | – | balance |
| IC-396 | 7.98 | 7.72 | 3.02 | 0.85 | 0.005 | – | – | – | – | – | balance |
| IC-438 | 8.10 | 5.23 | 7.02 | 0.13 | 0.005 | – | – | – | – | – | balance |
| IC-6 | 7.8 ÷ 8.5 | – | 14.00 | – | 0.03 ÷ 0.15 | – | – | – | – | – | balance ÷ balance |
| VKNA-1V | 8.83 | 5.58 | 3.50 | 0.45 | – | 0.03 | – | 1.54 | 2.82 | – | balance |
| Haynes 214 | 4.50 | 16.00 | – | – | – | 0.03 | 3.00 | – | – | 0.10 | balance |
| FeNiCr (HU) | – | 18.00 | – | – | – | 0.55 | 42.45 | – | – | – | balance |
| Alloy 800 | 0.40 | 21.00 | – | – | – | 0.05 | 45.50 | 0.40 | – | – | balance |
3. Applications of Ni3Al-Based Alloys



3.1. Applications of Bulk Materials
3.1.1. Compressor and Turbine Blades in Aircraft Engines

3.1.2. Turbochargers Rotors in Diesel-Engine Trucks
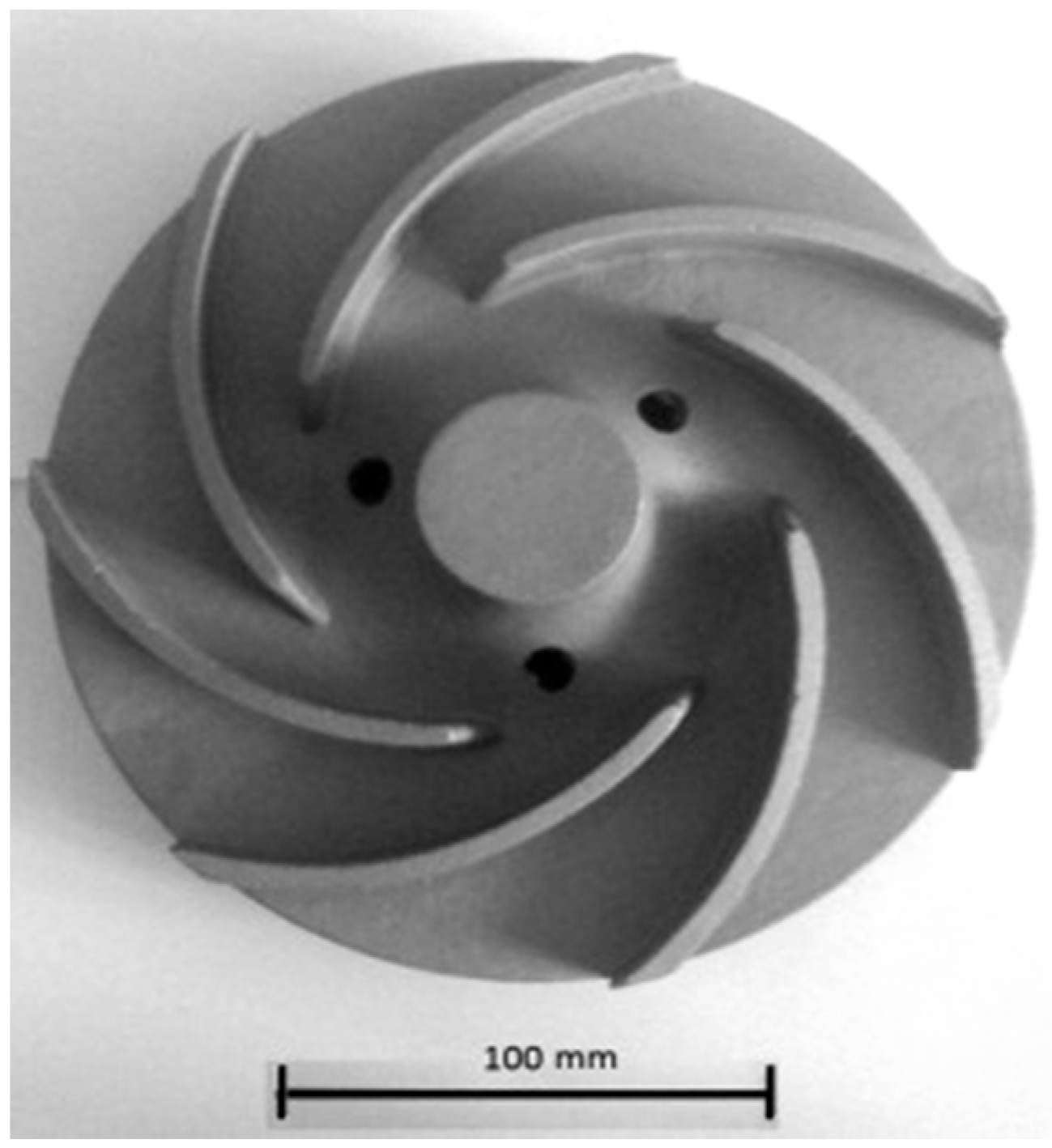
3.1.3. Water Turbine Rotors and Water Pumps
3.1.4. Car Components
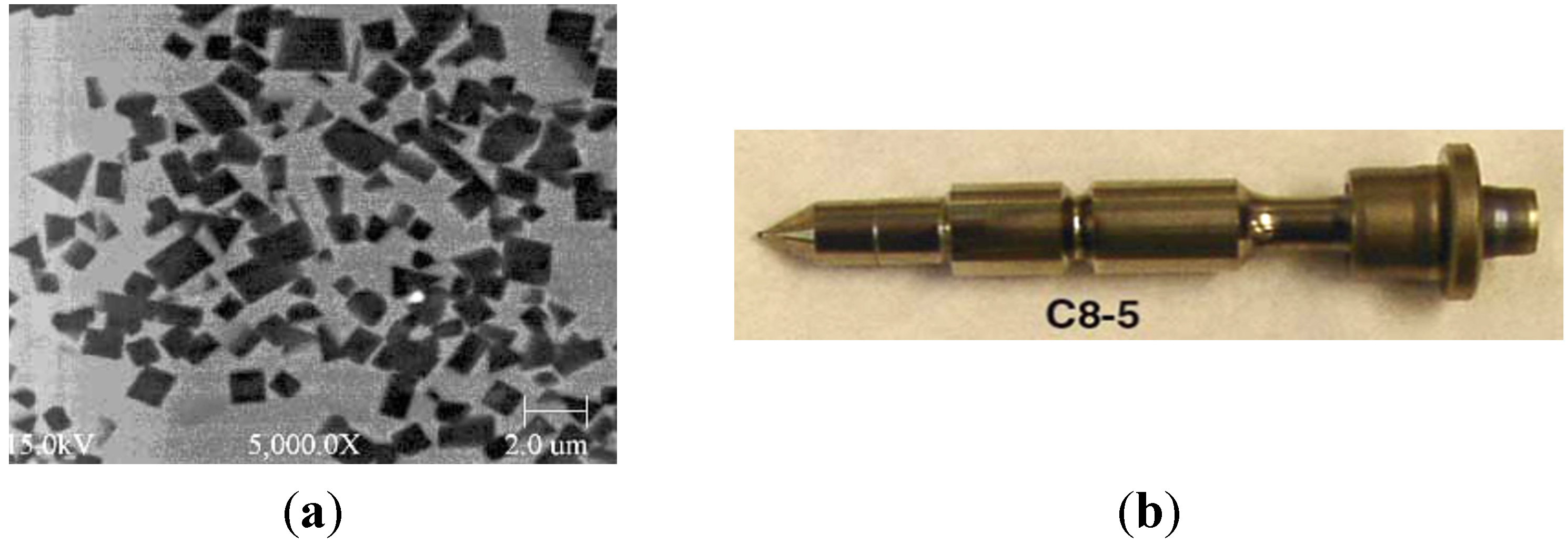
- elements of injection systems (e.g., metering plungers) (Figure 9)—an increased pollution emission of diesel engine and a continuous growth of energy efficiency require higher pressures of fuel injection ensuring more precise control of fuel injection. TiC/Ni3Al composites with a good wear properties against steel elements are needed for applications where components slide and impact against each other. Figure 9b shows element made of TiC-50 vol% Ni3Al which successfully completed the 20-h high pressure (>315 MPa) fuel injection tests [85,86,87].
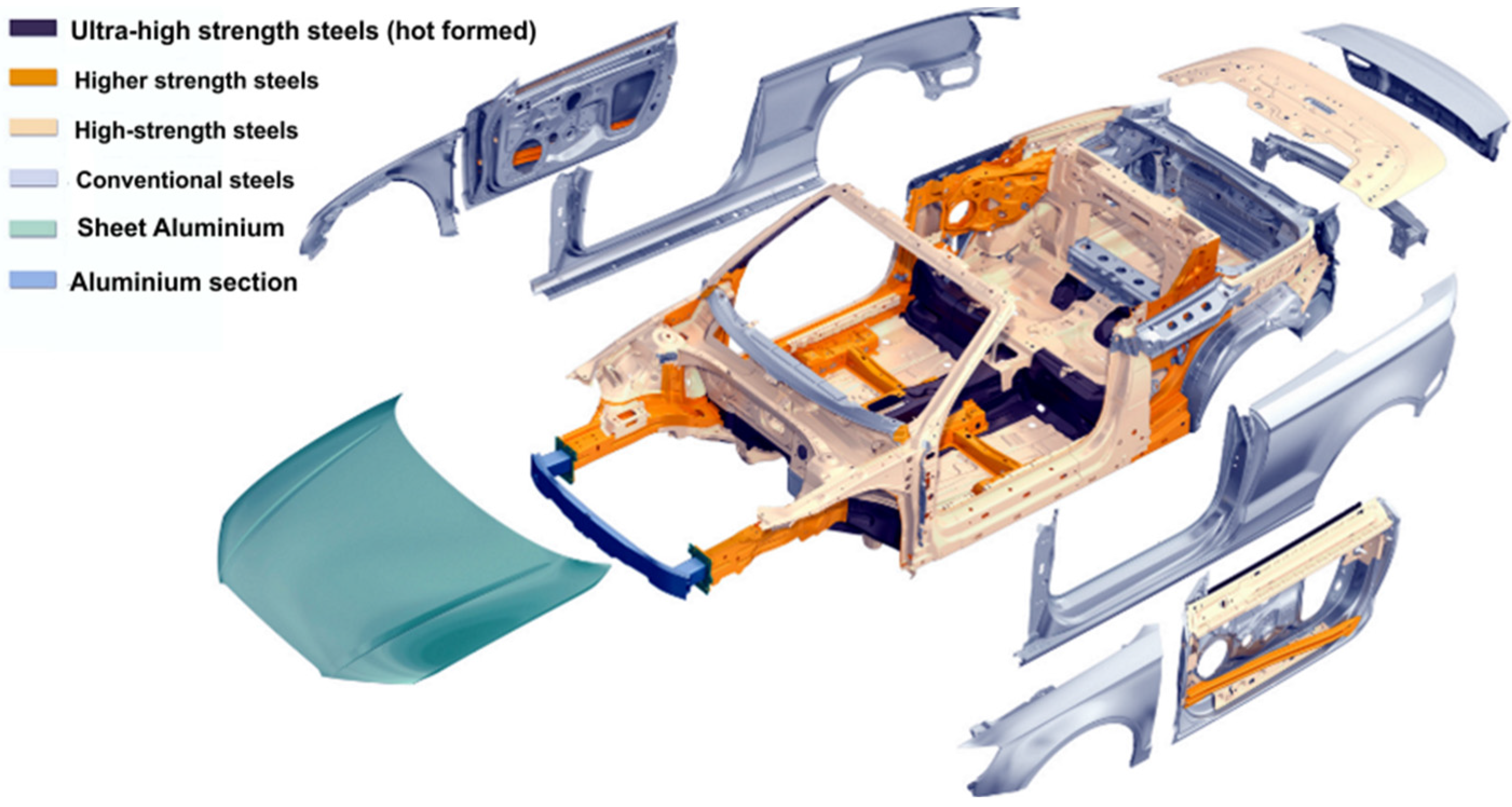
- automotive body material—works concerned on applications of Ni3Al intermetallic alloys to automotive body were recently published [88,89,90,91]. As reported in the papers, this material is lighter and 5 times stronger than stainless steel and exhibits a higher corrosion resistance than currently used automotive materials. Therefore Ni3Al alloys can be used not only as automotive body material and also as elements with superior strength or absorbing energy. However, due to its cost, Ni3Al intermetallic alloys may only be applied to higher end models, e.g.,: Audi, Mercedes and BMW (Figure 10).
3.1.5. A Steel Industry
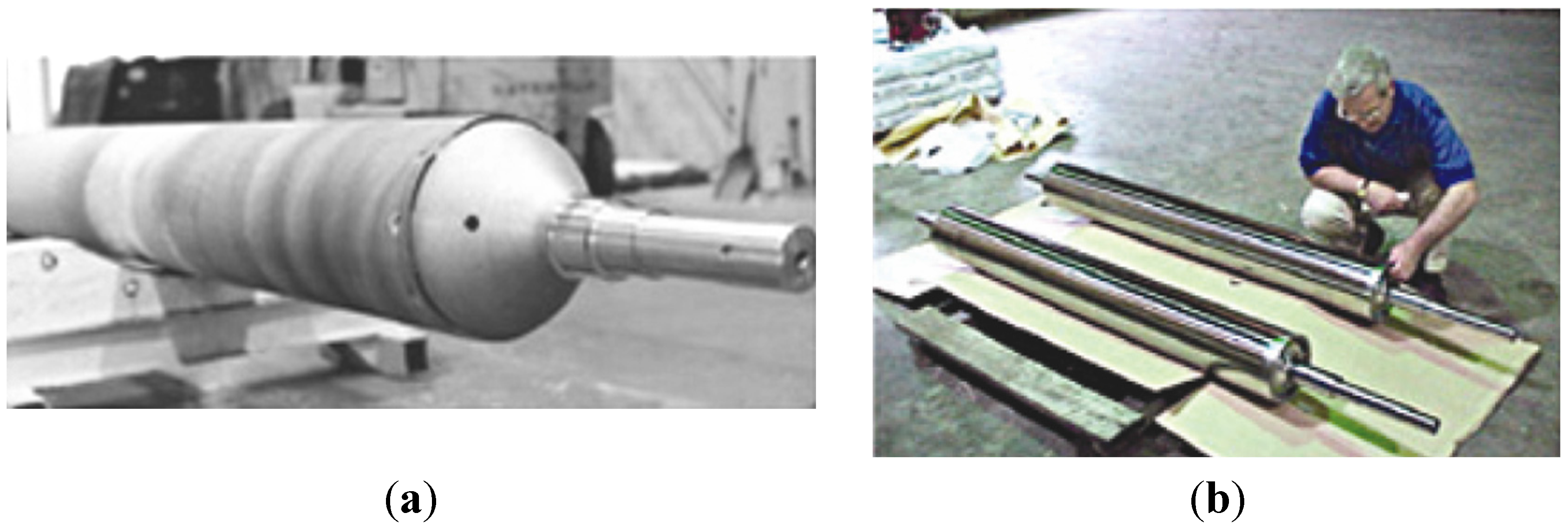


- rails for walking-beam furnace which are used for heating of steels before a hot forging process (Figure 15b) (e.g., firms: Rapid Technologies, BIMAC Corporations, Cast Masters). The rails supports a moving a processed component from the loading end to the exit and after reaching the set temperature in the range of 1100–1200 °C [1,52,54,61].
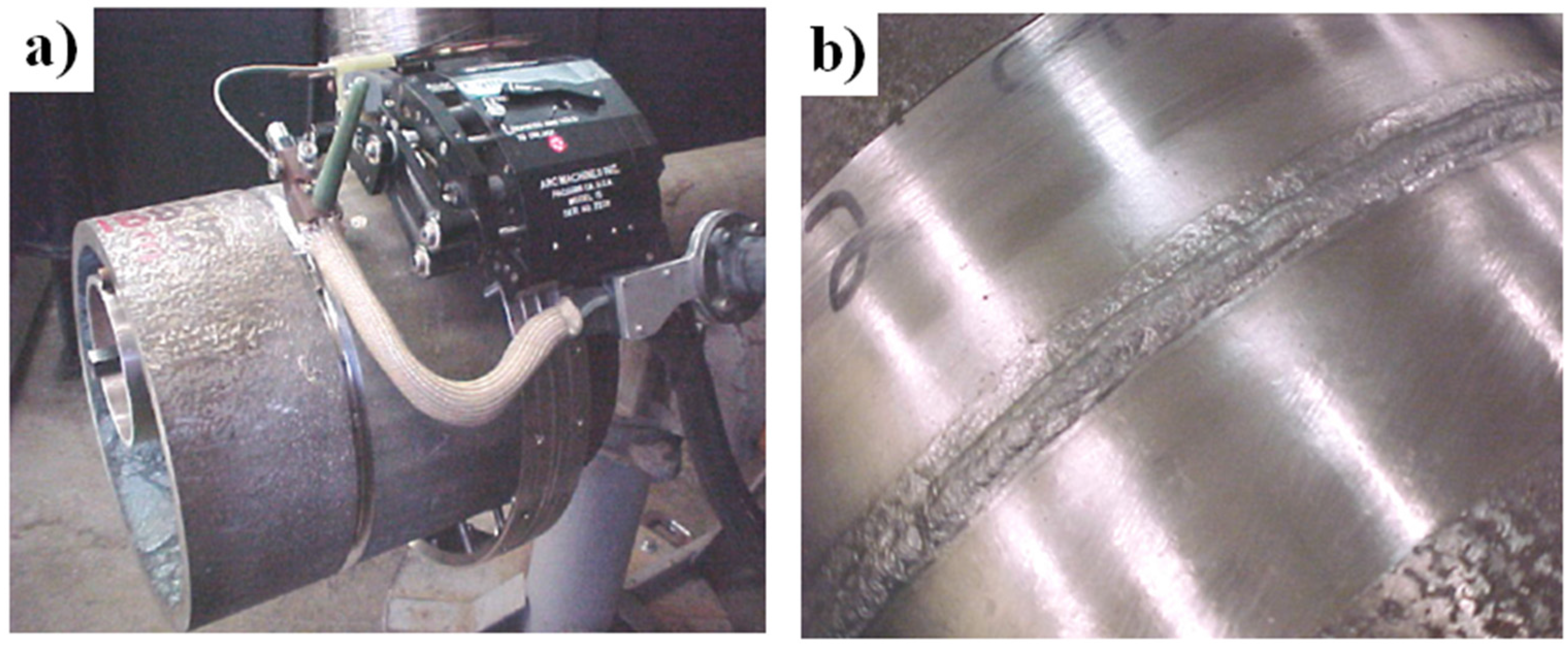
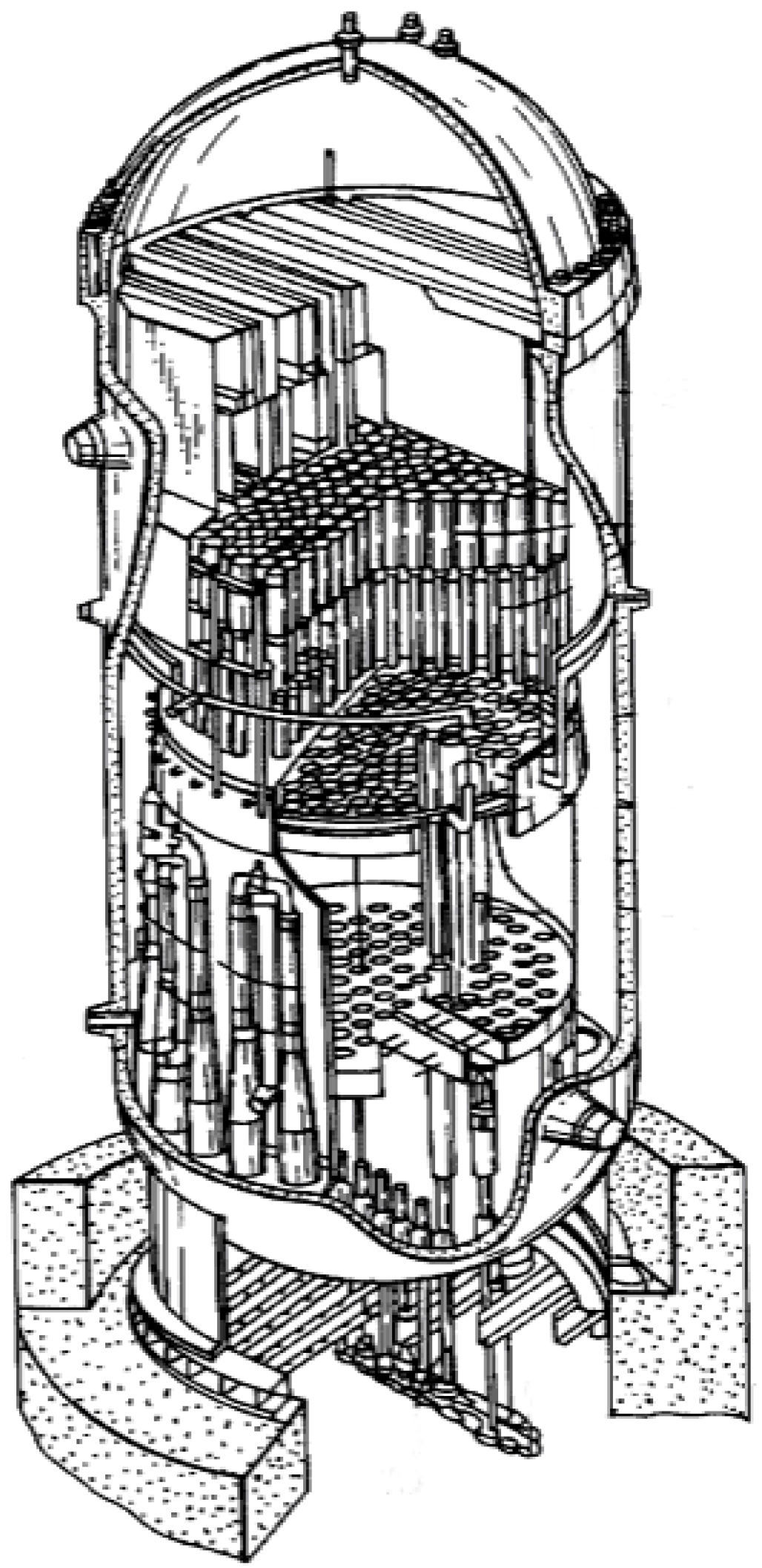
- die blocks for closed-die hot forming process (United Defense LP/Steel Products Division, Metallamics) [6,9,10,12,97,98,99,100,101,102]. A higher wear resistance, a higher strength and a resistance to thermal fatigue are the main advantages of Ni3Al components, which are taken into consideration in this application. It was reported that dies made of IC-221M alloy exhibit almost 10-times higher durability than that made of HU steel (Figure 15c). Ni3Al alloy forging dies was used to successfully forge 100,000 pieces of a part known as a “brake spider” [6,97].

3.2. Applications of Ni3Al Thin Foils
- -
- based on a directional solidification and a cold rolling—proposed by Hirano et al. from National Institute for Materials Science NIMS (Japan) with a collaboration of Ni3Al thin foils group established in 2000, e.g., Oak Ridge National Laboratory and Oregon State University (USA), Max-Planck Institute fur Eisenforschung (Germany), Oregon State University [106,107,108,109,110,111,112,113,114];
- -
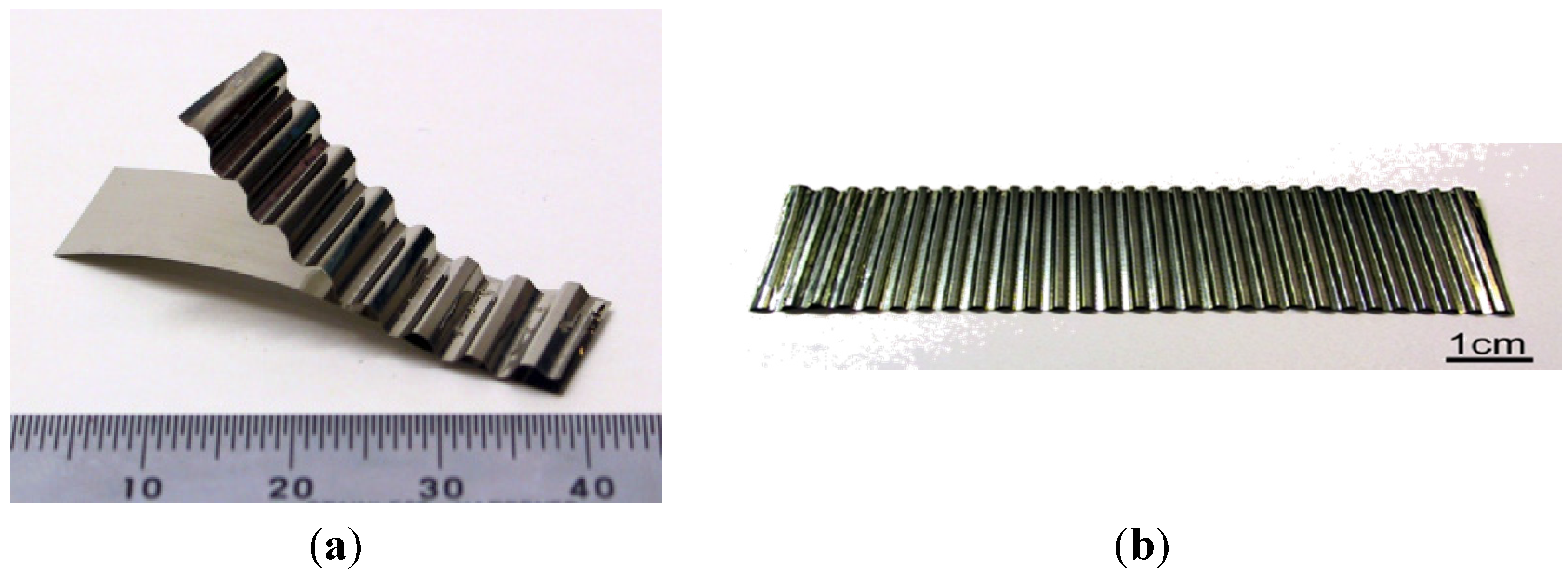
- without a costly and time-consuming directional crystallization; based on a controlled deformation of the conventionally casted alloys—proposed by Bojar et al. from Military University of Technology (MUT) (Poland). Moreover, this technology was found to give a final product with a higher ductility and a better strength properties (also with nanostructure) than those of Ni3Al foils produced by Hirano group (Figure 17 and Figure 18) [115,116,117,118,119,120].

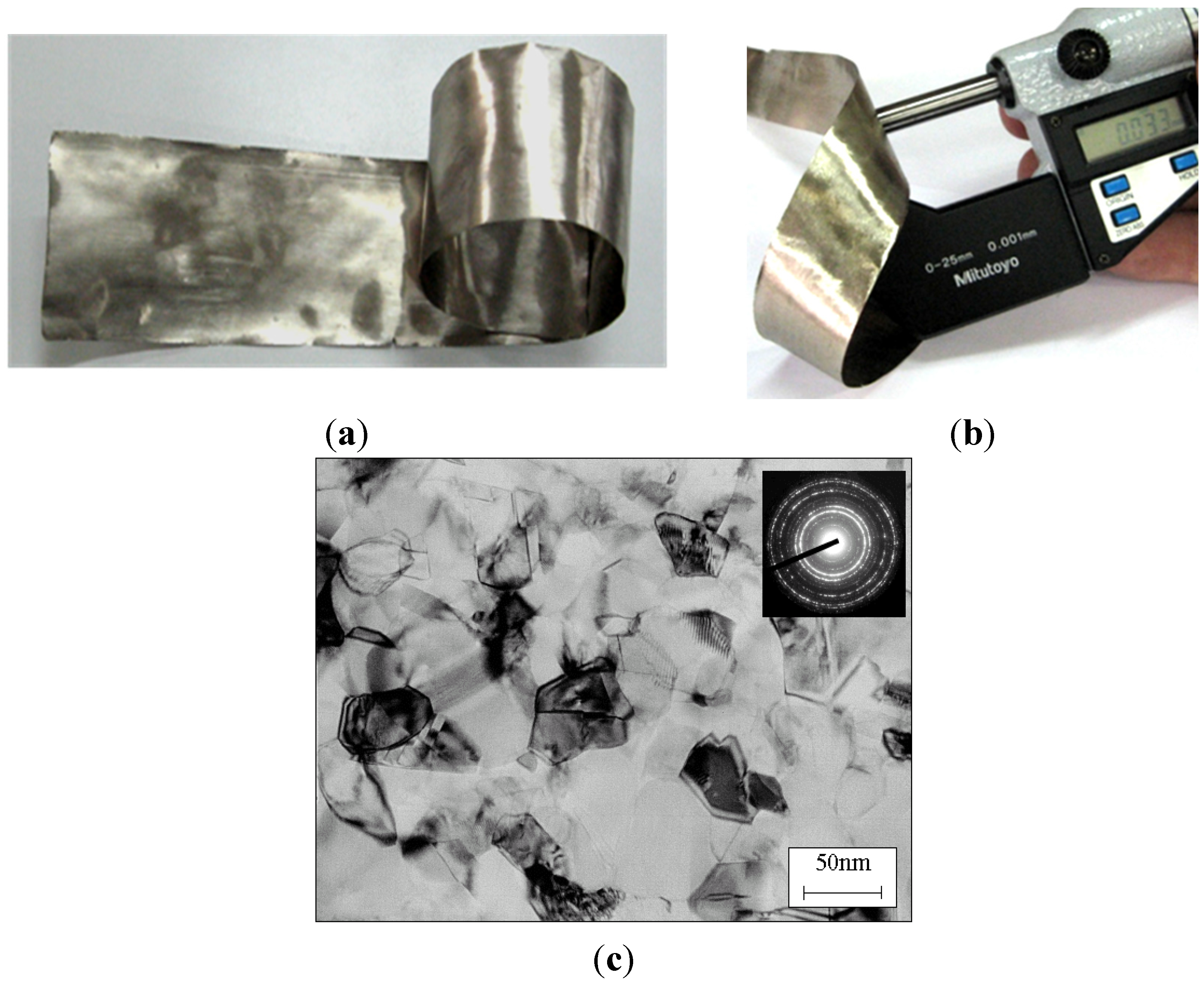
- fabrication of microsensors of, e.g., acceleration, pressure, flow (e.g., to remote respiratory monitoring system, monitoring the levels of chemical contaminants) and gyroscopes,
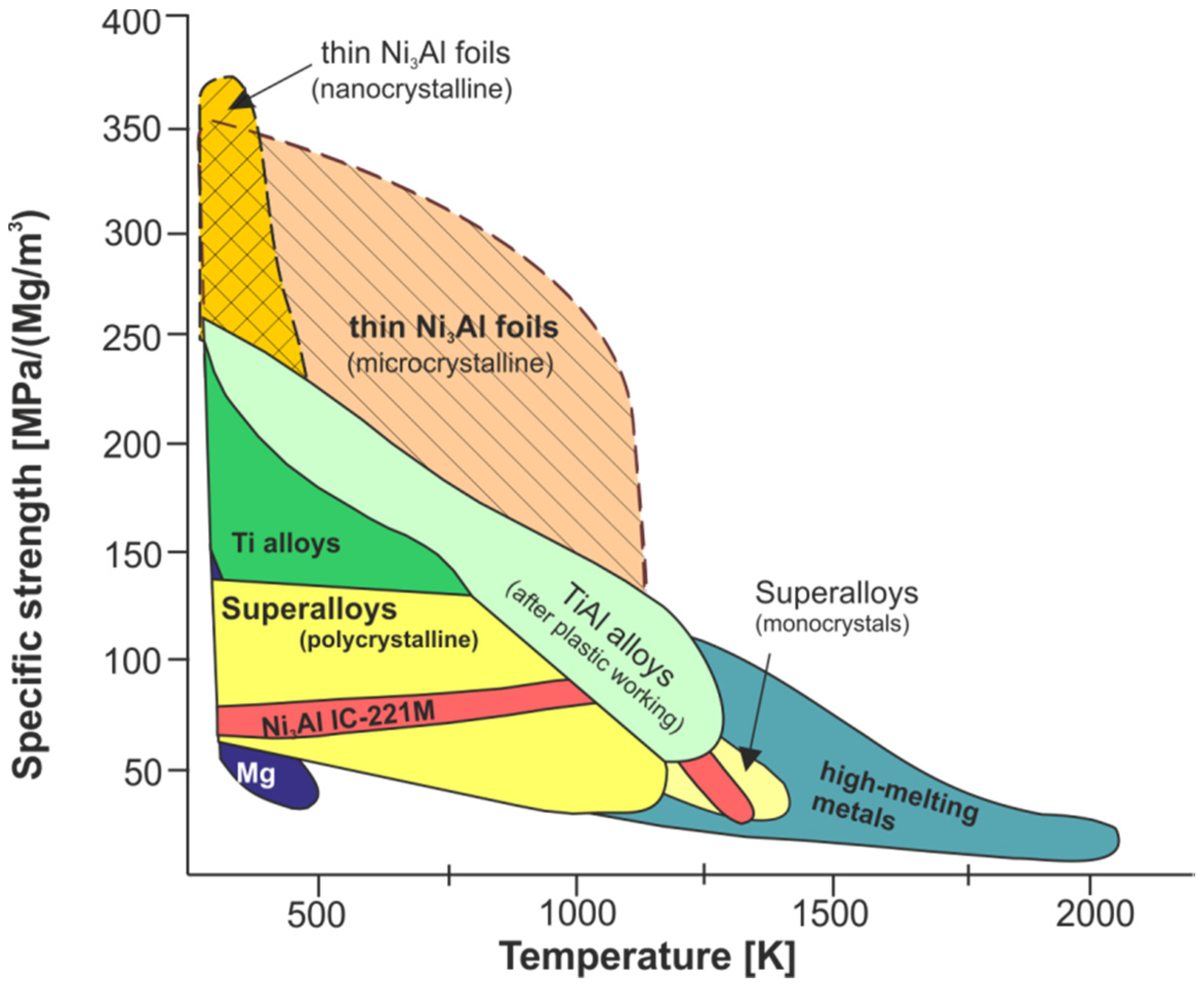
| Material | Density (g/cm3) | Young Modulus (GPa) | Tensile Strength (MPa) | Specific Stiffness (GN/kg·m) | Specific Strength (MN/kg·m) |
|---|---|---|---|---|---|
| Silicon 1 | 2.33 | 129–187 | 4000 | 55–80 | 1.7 |
| Silica 1 | 2.20 | 73 | 1000 | 33 | 0.45 |
| Nickel 1 | 8.90 | 207 | 500 | 23 | 0.06 |
| Aluminum 1 | 2.71 | 69 | 300 | 25 | 0.11 |
| Alumina 1 | 3.97 | 393 | 2000 | 99 | 0.50 |
| Silicon carbide 1 | 3.30 | 430 | 2000 | 130 | 0.30 |
| Diamond 1 | 3.51 | 1035 | 1000 | 295 | 0.28 |
| Ni3Al (micro) 2 | 7.5 | 200 | 2300 | 26 | 0.31 |
| Ni3Al (nano) 2 | 7.5 | 200 | 2900 | 26 | 0.39 |
3.2.1. Structure with a Highly Developed Active Surface, e.g., “Honeycombs”, Heat Exchangers, Catalysts and Also Filters

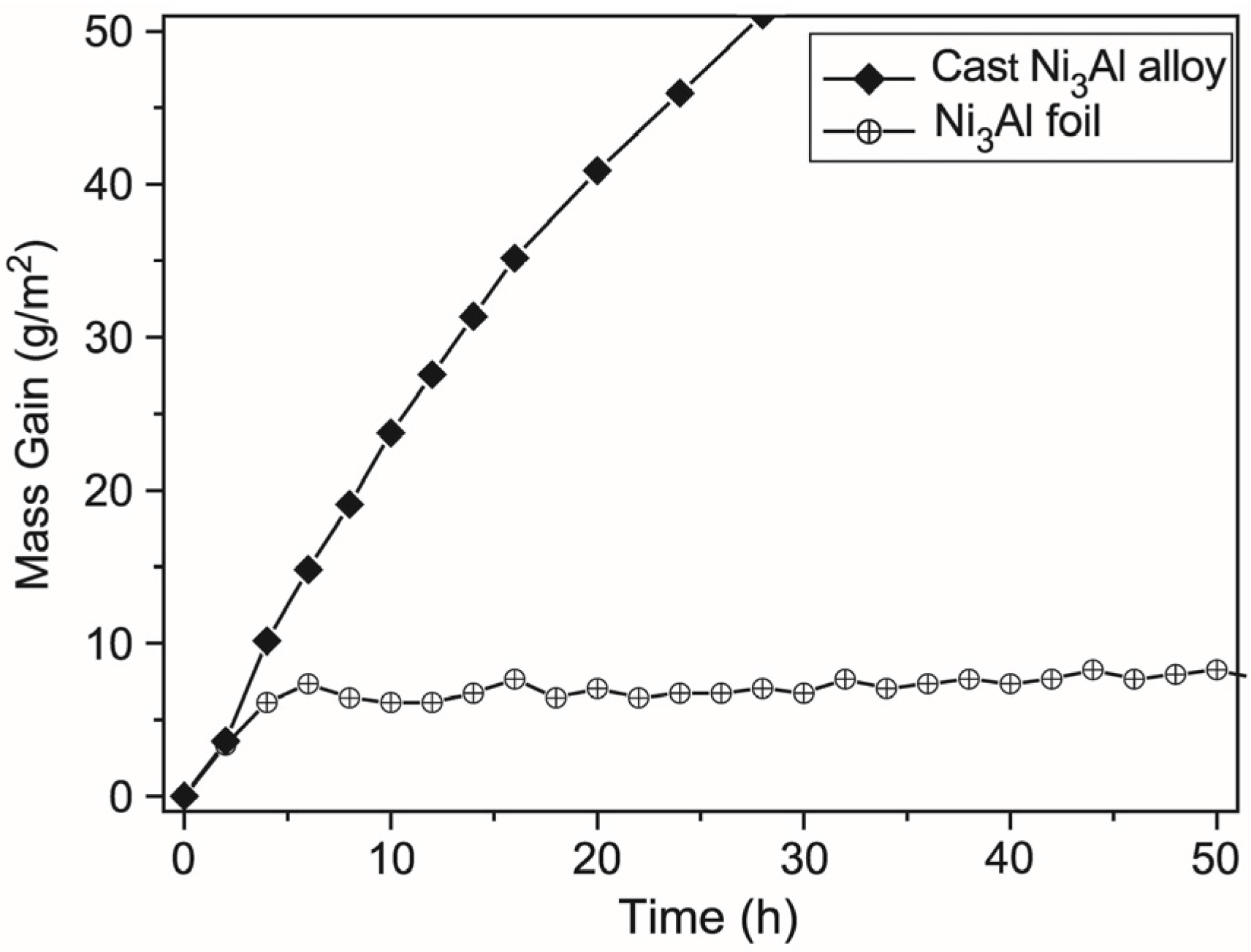
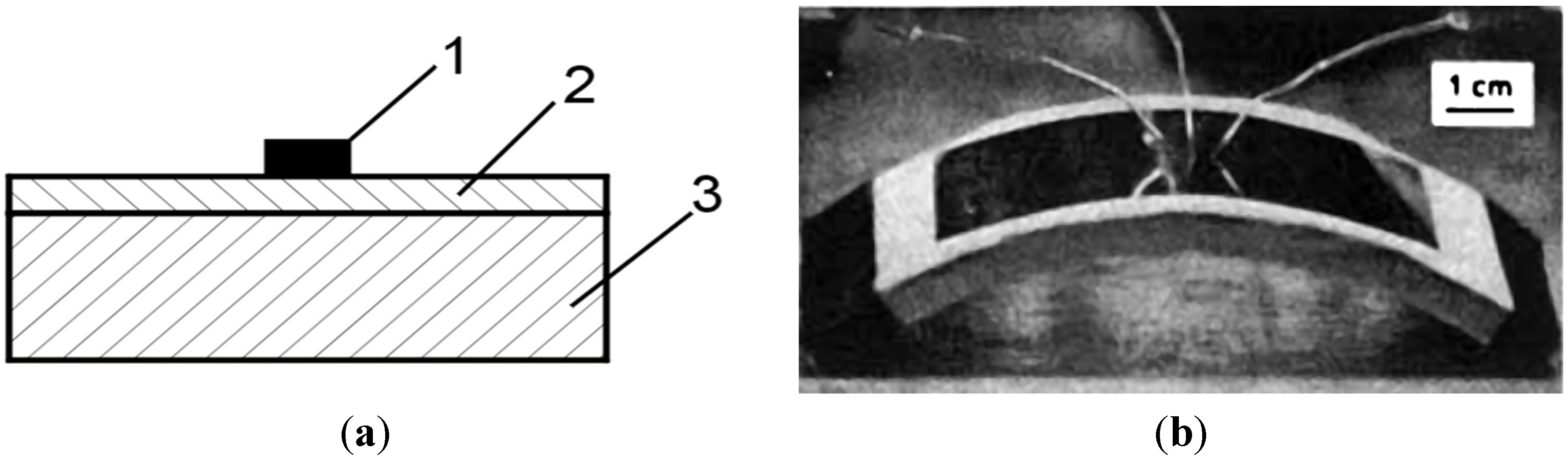
- thermocatalytic air purification systems—the main function of this type of devices is to remove all kinds of toxic chemicals including warfare agents (e.g.,: sarin, mustard gas—Figure 23) and dangerous biological agents from the air. In contrast to conventional filtering devices (where after the hazardous substances are retained in the filters causing the need for their frequent replacement) designed device using thermocatalytic processes, remove them completely. Therefore, the “hot filter” works more efficiently and much faster as compared to conventional filtration systems [32,131].
3.2.2. Electronic Equipment
- electronic devices on a substrate made of IC-50 alloy (see Table 1)
- liquid crystal displays
3.2.3. Mechanical Systems and Other
- micro gears and micromotors
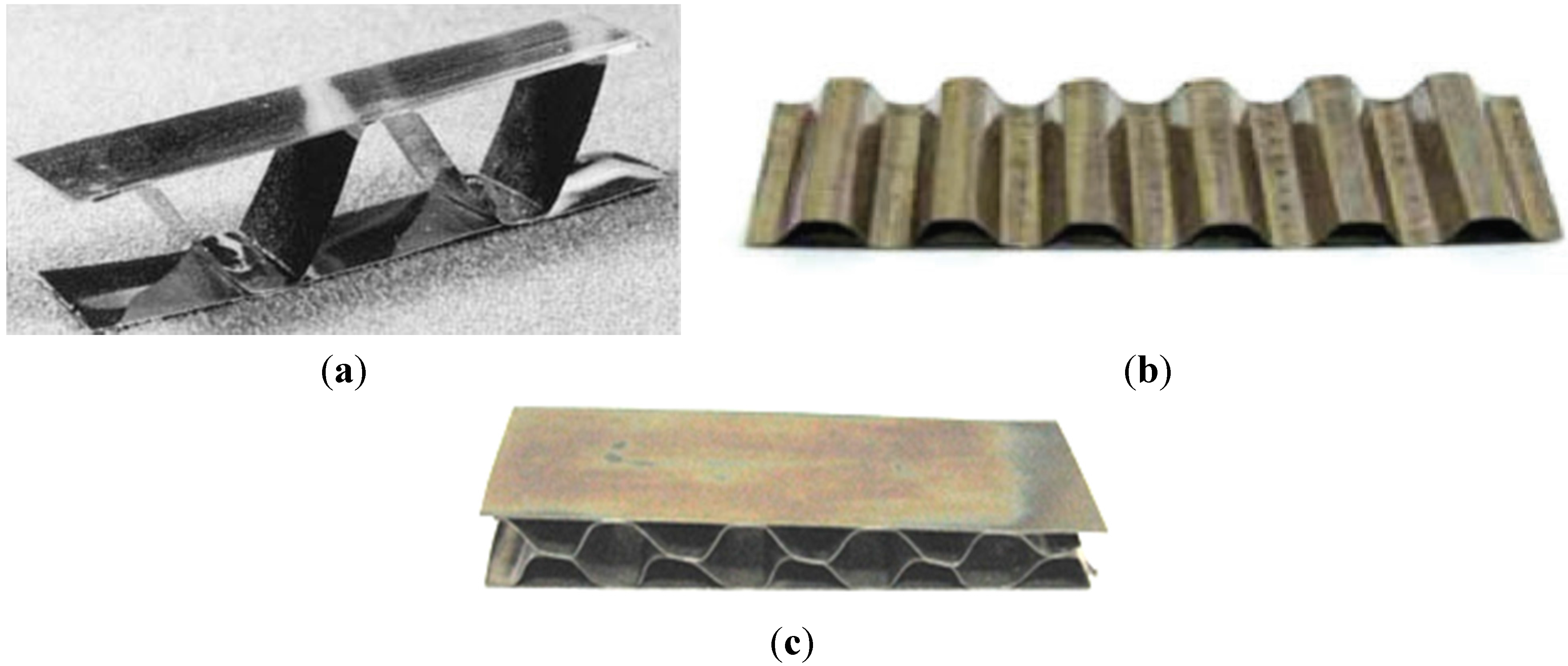
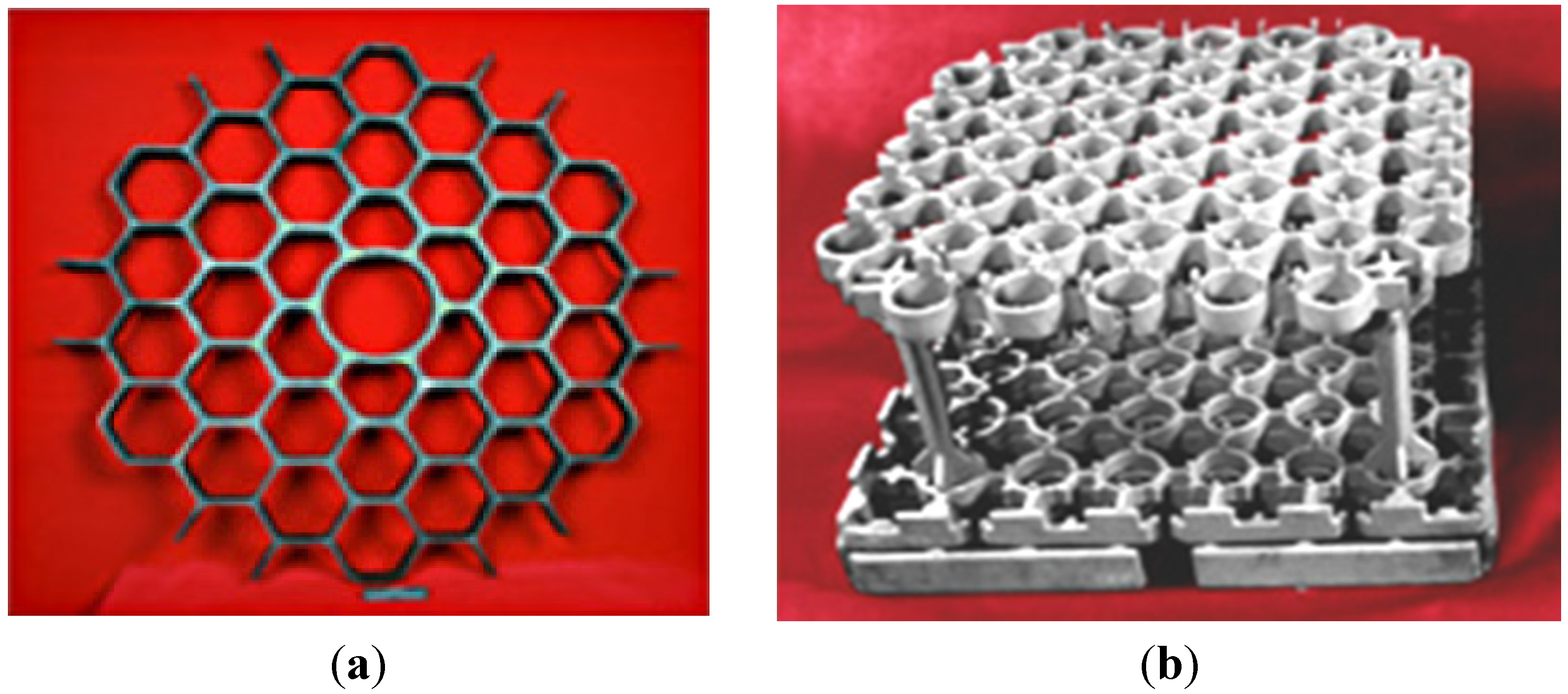
- honeycomb structures—a LFB (lighter, faster, better) is one of the most important criteria in modern designing (Figure 28). An extremely high specific strength (Figure 19) combined with a high operating temperature (900–1000 °C) and corrosion resistance makes the Ni3Al thin foils a great structural materials.



4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intermetallic Compound. Encyclopedia Britannica. Available online: http://www.britannica.com/EBchecked/topic/290430/intermetallic-compound (accessed on 2 January 2015).
- Varin, R.A. Intermetallics: Crystal structures. In Concise Encyclopedia of Structure of Materials; Martin, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 235–238. [Google Scholar]
- Liu, C.T.; Pope, D.P. Ni3Al and its alloys. In Structural Applications of Intermetallic Compounds; Westbrook, J.H., Fleischer, R.L., Eds.; John Wiley & Son, Ltd.: Hoboken, NJ, USA, 2000; pp. 15–32. [Google Scholar]
- Li, Z.; Gao, W. High temperature corrosion of intermetallics. In Intermetallics Research Progress; Berdovsky, Y.N., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 1–64. [Google Scholar]
- National Materials Advisory Board. Intermetallic Alloy Development; NMAB-487-1; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Advanced Materials. Intermetallics for Manufacturing, Industrial Technologies Program. U.S. Departament of Energy. Available online: http://www1.eere.energy.gov/manufacturing/industries_technologies/imf/pdfs/intermetallics.pdf (accessed on 2 January 2015).
- Schulson, E.M. Brittle fracture and toughening. In Physical Metallurgy and Processing of Intermetallic Compounds; Stoloff, N.S., Sikka, V.K., Eds.; Chapman & Hall: London, UK, 1996; pp. 56–94. [Google Scholar]
- Yamaguchi, M.; Shirai, Y. Defect structures. In Physical Metallurgy and Processing of Intermetallic Compounds; Stoloff, N.S., Sikka, V.K., Eds.; Chapman & Hall: London, UK, 1996; pp. 3–27. [Google Scholar]
- Deevi, S.C.; Sikka, V.K.; Liu, C.T. Processing, properties, and applications of nickel and iron aluminides. Prog. Mater. Sci. 1997, 42, 177–192. [Google Scholar] [CrossRef]
- Stoloff, N.S.; Liu, C.T.; Deevi, S.C. Emerging applications of intermetallics. Intermetallics 2000, 8, 1313–1320. [Google Scholar] [CrossRef]
- Aoki, K.; Izumi, O. Improvement in room temperature ductility of the intermetallic compound Ni3Al by boron addition. J. Jpn. Inst. Met. 1979, 43, 358–359. [Google Scholar]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Senderowski, C.; Zasada, D.; Durejko, T.; Bojar, Z. Characterization of as-synthesized and mechanically milled Fe–Al powders produced by the self-disintegration method. Powder Technol. 2014, 263, 96–103. [Google Scholar] [CrossRef]
- Senderowski, C. Nanocomposite Fe–Al intermetallic coating obtained by gas detonation spraying of milled self-decomposing powder. J. Therm. Spray Technol. 2014, 23, 1124–1134. [Google Scholar] [CrossRef]
- Pawłowski, A.; Czeppe, T.; Major, Ł.; Senderowski, C. Structure morphology of Fe–Al coating detonation sprayed onto carbon steel substrate. Arch. Metall. Mater. 2009, 54, 783–788. [Google Scholar]
- Łyszkowski, R.; Bystrzycki, J. Influence of temperature and strain rate on the microstructure and flow stress of iron aluminides. Arch. Metall. Mater. 2007, 52, 347–350. [Google Scholar]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Karczewski, K.; Jóźwiak, S.; Bojar, Z. Mechanisms of strength properties anomaly of Fe–Al sinters by compression tests at elevated temperature. Arch. Metall. Mater. 2007, 52, 361–366. [Google Scholar]
- Sikka, V.K.; Deevi, S.C.; Viswanathan, S.; Swindeman, R.W.; Santella, M.L. Advances in processing of Ni3Al-based intermetallics and applications. Intermetallics 2000, 8, 1329–1337. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Liu, C.T.; Stringer, J.; Mundy, J.N.; Horton, L.L.; Angelini, P. Ordered intermetallic alloys: An assessment. Intermetallics 1997, 5, 579–596. [Google Scholar] [CrossRef]
- Jang, J.H.; Xu, Y.; Demura, M.; Wee, D.M.; Hirano, T. Catalytic activity improvement of Ni3Al foils for methanol decomposition by oxidation-reduction pretreatment. Appl. Catal. A Gen. 2011, 398, 161–167. [Google Scholar] [CrossRef]
- Jang, J.H.; Xu, Y.; Chun, D.H.; Demura, M.; Wee, D.M.; Hirano, T. Effects of steam addition on the spontaneous activation in Ni3Al foil catalysts during methanol decomposition. J. Mol. Catal. A Chem. 2009, 307, 21–28. [Google Scholar] [CrossRef]
- Hirano, T.; Xu, Y.; Demura, M. Catalytic properties of Ni3Al foils for hydrogen production. Adv. Mater. Res. 2011, 306, 130–133. [Google Scholar] [CrossRef]
- Chun, D.H.; Xu, Y.; Demura, M.; Kishida, K.; Oh, M.H.; Hirano, T.; Wee, D.M. Spontaneous catalytic activation of Ni3Al thin foils in methanol decomposition. J. Catal. 2006, 243, 99–107. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Sakurai, J.; Teraoka, Y.; Yoshigoe, A.; Demura, M.; Hirano, T. Effect of water vapor and hydrogen treatments on the surface structure of Ni3Al foil. Appl. Surf. Sci. 2014, 315, 475–480. [Google Scholar] [CrossRef]
- Jozwik, P.; Salerno, M.; Stępniowski, W.J.; Bojar, Z.; Krawczyk, K. Decomposition of cyclohexane on Ni3Al thin foil intermetallic catalyst. Materials 2014, 7, 7039–7047. [Google Scholar] [CrossRef]
- Jozwik, P.; Bojar, Z.; Winiarek, P. Catalytic activity of Ni3Al foils in decomposition of selected chemical compounds. Mater. Eng. 2010, 3, 654–657. [Google Scholar]
- Jozwik, P.; Bojar, Z.; Grabowski, R. Catalytic activity of Ni3Al foils in methanol reforming. Mater. Sci. Forum 2010, 636, 895–900. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Jóźwik, P.; Jankiewicz, B.; Stępniowski, W.J.; Bojar, Z. Catalytic stability and surface analysis of microcrystalline Ni3Al thin foils in methanol decomposition. Appl. Surf. Sci. 2014, 293, 169–176. [Google Scholar] [CrossRef]
- Jozwik, P.; Bojar, Z.; Grabowski, R. Catalytic properties of thin Ni3Al nano and microcrystalline foils in methanol decomposition. In Proceedings of the Annual International Conference on Materials Science, Metal & Manufacturing, Singapore, 12–13 December 2011; pp. 83–87.
- Jozwik, P. Military Application of Micro, Ultra and Nanocrystalline Alloys Ni3Al-Technology Demonstrator of Thermoactive Elements for Contaminated Air Treatment Systems; Final Report of Research Project OR00004905; MUT: Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Arkatova, L.A. The deposition of coke during carbon dioxide reforming of methane over intermetallides. Catal. Today 2010, 157, 170–176. [Google Scholar] [CrossRef]
- Arkatova, L.A.; Pakhnutov, O.V.; Shmakov, A.N.; Naiborodenko, Y.S.; Kasatsky, N.G. Pt-implanted intermetallides as the catalysts for CH4–CO2 reforming. Catal. Today 2011, 171, 156–167. [Google Scholar] [CrossRef]
- Arkatova, L.A. Influence of nickel content on catalytic activity and stability of the systems, based on intermetallic Ni3Al in the conversion of natural gas using carbon dioxide. Russ. J. Phys. Chem. A 2010, 84, 566–572. [Google Scholar] [CrossRef]
- Zhai, W.; Shi, X.; Yao, J.; Ibrahim, A.M.M.; Xu, Z.; Zhu, Q.; Xiao, Y.; Chen, L.; Zhang, Q. Investigation of mechanical and tribological behaviors of multilayer graphene reinforced Ni3Al matrix composites. Compos. Part B Eng. 2015, 70, 149–155. [Google Scholar] [CrossRef]
- Zhu, S.; Li, F.; Ma, J.; Chenga, J.; Yina, B.; Yanga, J.; Qiao, Z.; Liu, W. Tribological properties of Ni3Al matrix composites with addition of silver and barium salt. Tribol. Int. 2015, 84, 118–123. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Z.; Lu, X.; Li, K.; Du, Y.; Long, J.; Xu, T.; Zhang, H.; Chen, L.; Kong, Y.; et al. Microstructure and composition of the grain/binder interface in WC–Ni3Al composites. Int. J. Refract. Met. Hard Mater. 2014, 44, 88–93. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Yang, J.; Liu, W.; Xu, Q. Effect of particle size on tribological behavior of Ni3Al matrix high temperature self-lubricating composites. Tribol. Int. 2011, 44, 1800–1809. [Google Scholar] [CrossRef]
- Shi, X.; Zhai, W.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Din, A.Q.; Zhang, Q. Tribological performance of Ni3Al–15 wt% Ti3SiC2 composites against Al2O3, Si3N4 and WC–6Co from 25 to 800 °C. Wear 2013, 303, 244–254. [Google Scholar] [CrossRef]
- Ye, Q.H. Recent developments in high temperature intermetallics research in China. Intermetallics 2000, 8, 503–509. [Google Scholar] [CrossRef]
- Liu, C.T.; George, E.P.; McKamey, C.G. Current Status of Research and Development on Nickel and Iron Aluminides. Available online: http://www.osti.gov/scitech/servlets/purl/10108395 (accessed on 2 January 2015).
- Liu, C.T.; Jemian, W.; Inouye, H.; Cathcart, J.V.; David, S.A.; Horton, J.A. Initial Development of Nickel and Nickel-Iron Aluminides for Structural Uses. Raport ORNL-6067. Available online: http://web.ornl.gov/info/reports/1984/3445600339417.pdf (accessed on 16 January 2015).
- Nathal, V.M.; Gayda, J.; Noebe, R.D.; Gleeson, B.M.; Sordelet, D.J. NiAl-Based Approach for Rocket Combustion Chambers. U.S. Patent US6,886,327 B1, 3 May 2005. [Google Scholar]
- Karin, G.; Luo, H.; Feng, D.; Li, C. Ni3Al-based intermetallic alloys as a new type of high-temperature and wear-resistant materials. J. Iron Steel Res. 2007, 14, 21–25. [Google Scholar] [CrossRef] also in Proceedings of the Sino-Swedish Structural Materials Symposium. 2007, pp. 21–25. Available online: http://publications.lib.chalmers.se/records/fulltext/61053/local_61053.pdf (accessed on 2 January 2015).
- Zhu, S.; Bi, Q.; Yang, J.; Qiao, Z.; Ma, J.; Li, F.; Yin, B.; Liu, W. Tribological behavior of Ni3Al alloy at dry friction and under sea water environment. Tribol. Int. 2014, 75, 24–30. [Google Scholar] [CrossRef]
- Wu, L.; Yao, J.; Dong, H.; He, Y.; Xu, N.; Zou, J.; Huang, B.; Liu, C.T. The corrosion behavior of porous Ni3Al intermetallic materials in strong alkali solution. Intermetallics 2011, 19, 1759–1765. [Google Scholar] [CrossRef]
- Sulka, G.; Jóźwik, P. Electrochemical behavior of Ni3Al-based intermetallic alloys in NaOH. Intermetallics 2011, 19, 974–981. [Google Scholar] [CrossRef]
- Podrez-Radziszewska, M.; Jóźwik, P. Influence of heat treatment on resistance to electrochemical corrosion of the strain-hardened strips made of the Ni3Al phase based alloys. Arch. Civil Mech. Eng. 2011, 11, 1011–1021. [Google Scholar] [CrossRef]
- Gleeson, B.M.; Sordelet, D.J. Pt Metal Modified γ-Ni + γ'-Ni3Al Alloy Compositions for High Temperature Degradation Resistant Structural Alloys. U.S. Patent US8,821,654 B2, 2 February 2014. [Google Scholar]
- Nazmy, M.Y. Creep. In Physical Metallurgy and Processing of Intermetallic Compounds; Stoloff, N.S., Sikka, V.K., Eds.; Chapman & Hall: London, UK, 1996; pp. 95–125. [Google Scholar]
- Santella, M.; Sikka, V.K.; Sorrell, Ch.; Angelini, A.P. Intermetallic Alloy Development for the Steel Industry. Available online: https://www1.eere.energy.gov/manufacturing/industries_technologies/imf/pdfs/intmetalloydevsteel.pdf (accessed on 2 January 2015).
- Liu, C.T.; Sikka, V.K. Nickel aluminides for structural use. J. Met. 1986, 38, 19–21. [Google Scholar]
- Mengel, J.; Martocci, A.; Fabina, L.; Petrusha, R.; Chango, R.; Angelini, P.; Sikka, V.K.; Santella, M. Large-Scale Evaluation of Nickel Aluminide Rolls in a Heat-Treat Furnace at Bethlehem Steel’s (Now ISG) Burns Harbor Plate Mill, Project Report. Available online: http://web.ornl.gov/~webworks/cppr/y2001/rpt/119155.pdf (accessed on 6 January 2015).
- Bazyleva, O.A.; Bondarenko, A.; Morozova, G.I.; Timofeeva, O.B. Structure, chemical compostion, and phase composition of intermetallic alloy VKNA-1V after high-temperature heat treatment and process heating. Mater. Sci. Heat Treat. 2014, 56, 229–234. [Google Scholar] [CrossRef]
- Sikka, V.K.; Sanatella, M.L.; Orth, J.E. Processing and Operating Experience of Ni3Al-Based Intermetallic Alloy IC-221M. Available online: http://webapp1.dlib.indiana.edu/virtual_disk_library/index.cgi/4297581/FID1887/m97006000.pdf (accessed on 15 January 2015).
- Sikka, V.K.; Santella, M.L.; Orth, J.E. Processing and operating experience of Ni3Al-based intermetallic alloy IC-221M. Mater. Sci. Eng. A 1997, 239–240, 564–569. [Google Scholar] [CrossRef]
- Lipsitt, H.A.; Blackburn, M.J.; Dimiduk, D.M. Commercializing intermetallic alloys: Seeking a complete technology. In Proceedings of the 3rd International Symposium on Structural Intermetallic, Snow King Resort, Jackson Hole, WY, USA, 23–27 September 2001; pp. 73–82.
- Li, S.; Song, J.; Zhou, C.; Gong, S.; Han, Y. Microstructure evolution of NiCoCrAlY overlay coating for Ni3Al based alloy IC6 turbine vane during long term engine test. Intermetallics 2005, 13, 309–314. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Exo-Melt process for melting and casting intermetallics. Intermetallics 1997, 5, 17–27. [Google Scholar] [CrossRef]
- Krause, C. Nickel Aluminides: Breaking into the Marketplace. Available online: http://web.ornl.gov/info/ornlreview/rev28-4/text/nickel.htm (accessed on 6 January 2015).
- Sorell, C.A. Advanced Industrial Materials (AIM) Program activities Impact Metalcasting. OIT EXPO; 1999. Available online: http://science.energy.gov/~/media/bes/mse/pdf/reports-and-activities (accessed on 5 June 2008). [Google Scholar]
- Santella, M.L.; Sikka, V.K. Certain aspects of the melting, casting and welding of Ni3Al alloys. Available online: http://www.osti.gov/scitech/biblio/10158764 (accessed on 6 January 2015).
- Han, Y.F.; Xing, Z.P.; Chaturvedi, M.C. Structural intermetallics. In Proceedings of the Second International Symposium on Structural Intermetallics, Seven Springs Mountain Resort, Champion, PA, USA, 21–25 September 1997; p. 731.
- Pietrzak, K.; Kaliński, D.; Chmielewski, M.; Chmielewski, T.; Włosiński, W.; Choręgiewicz, K. Processing of intermetallics with Al2O3 or steel joints obtained by friction welding technique. In Proceedings of the 12th Conference of the European Ceramic Society—ECerS XII, Stockholm, Sweden, 19–23 June 2011; Available online: http://www.ippt.pan.pl/Repository/o510.pdf (accessed on 6 January 2015).
- Han, Y.F.; Chen, R.Z. R&D of cast superalloys and processing for gas turbine blades in BIAM. Acta Metall. Sin. 1996, 9, 457–463. [Google Scholar]
- Huo, X.; Zhang, J.S.; Wang, B.L.; Wu, F.J.; Han, Y.F. Evaluation of NiCrAlYSi overlay coating on Ni3Al based alloy IC-6 after an engine test. Surf. Coat. Technol. 1999, 114, 174–180. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Han, Y. Effects of boron and carbon contents on long-term aging of Ni3Al-base single crystal alloy IC6SX. Procedia Eng. 2012, 27, 1054–1060. [Google Scholar] [CrossRef]
- Mathur, H.; Bhowmik, A. Making Metals Take the Heat: Candidates Line up to Steal the Superalloy Crown...; Science Articles; University of Cambridge. Available online: http://www.thenakedscientists.com/HTML/articles/article/making-metals-take-the-heat (accessed on 25 December 2014).
- Zhao, X.; Huang, Z.; Tan, Y.; Zhang, Q.; Yu, Q.; Xu, H. New Ni3Al-based directionally-solidified superalloy IC10. J. Aeronaut. Mater. 2006, 26, 20–24. [Google Scholar]
- Zhang, H.; Wen, W.; Cui, H. An experimental study on constitutive equations of alloy IC10 over a wide range of temperatures and strain rates. Mater. Des. 2012, 36, 130–135. [Google Scholar] [CrossRef]
- Gorbovets, M.A.; Bazyleva, O.A.; Belyaev, M.S.; Khodinev, I.A. Low-cycle fatigue of VKNA type single-crystal intermetallic alloy under “hard” loading conditions. Metallurgist 2014, 58, 724–728. [Google Scholar] [CrossRef]
- Povarova, K.B.; Kazanskaja, N.K.; Buntushkin, V.P.; Kostogryz, V.G.; Baharev, V.G.; Mironov, V.I.; Bazyleva, O.A.; Drozdov, A.A.; Bannyh, I.O. Thermal stability of alloy structure on Ni3Al basis and its application in rotor blades of small-size turbine engines. Metals. 2003, 3. Available online: http://www.omkb.ru/english/pages/news/termostabilnost.htm (accessed on 25 December 2014).
- Kablov, E.N.; Lomberg, B.S.; Buntushkin, V.P.; Golubovskii, E.P.; Muboyadzhyan, S.A. Intermetalic Ni3Al alloy: Promising material for turbine blades. Met. Sci. Treat. 2002, 44, 284–287. [Google Scholar] [CrossRef]
- United Engine Corporation. A Worthy Pavel Solovyev Prize. Available online: http://www.uk-odk.ru/eng/presscenter/odk_news/?ELEMENT_ID=2102 (accessed on 10 January 2015).
- Kalashinkov Group. Certification of the PD-14 Engine Begins This Year. Available online: http://rostec.ru/en/news/2610 (accessed on 12 January 2015).
- Rostechnologiesblog. JSC “Aviadvigatel” Preparing Flight Test of Engine PD-14. Available online: https://rostechnologiesblog.wordpress.com/2014/12/09/jsc-aviadvigatel-preparing-flight-test-of-engine-pd-14 (accessed on 12 January 2015).
- Sikka, V.K.; Mavity, J.T.; Anderson, K. Report: Processing of Nickel Aluminides and Their Industrial Applications. Available online: http://www.osti.gov/scitech/biblio/5061830 (accessed on 12 January 2015).
- Patten, J.W. Nickel aluminides for diesel engines. In High Temperature Aluminides and Intermetallics; TMS: Warrendale, PA, USA, 1990; pp. 493–503. [Google Scholar]
- Chang, J.T.; Yeh, C.H.; He, J.L.; Chen, K.C. Cavitation erosion and corrosion behavior of Ni–Al intermetallic coating. Wear 2003, 255, 162–169. [Google Scholar] [CrossRef]
- Zasada, D.; Zarański, Z.; Jasionowski, R. The influence of grain size of Ni3Al alloy on cavitation wear of Ni3Al intermetallic after cold rolling and recovery during incubation period. Mater. Sci. 2010, 3, 650–653. [Google Scholar]
- Zasada, D. Final Report of Research Project Analysis of Wear Mechanisms of Intermetallic Based Alloy; MUT: Warsaw, Poland, 2010. [Google Scholar]
- Mehdi, A. Nickel—Aluminide Based Wear Resistant Material for Piston Rings. Patent No. WO02,088,407, 7 November 2002. [Google Scholar]
- Kraemer, J.; Brill, U. (Exhaust) Valve of Internal Combustion Engine—Made at Least Completion of Intermetallic Phases of Nickel and Aluminum. Patent DE 3935496 C1, 26 November 1990. [Google Scholar]
- Heavy Vehicle Propulsion Materials. Annual Progress Report 2004. U.S. Department of Energy. Available online: http://web.ornl.gov/sci/propulsionmaterials/pdfs/HV_04_AN.pdf (accessed on 16 January 2015).
- Heavy Vehicle Propulsion Materials. U.S. Department of Energy. Office of Freedom CAR and Vehicle Technology Program. Available online: http://engine-materials.ornl.gov/Highlights.html (accessed on 4 December 2005).
- Becher, P.F.; Waters, S.B. Intermetallic-bonded cermets. In Heavy Vehicle Propulsion Material Program; Quartly Progress Report 2003; Johnson, D.R., Ed.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, April–June 2003. [Google Scholar]
- Kwan, W.L. Mechanical and Thermal Properties of Intermetallic Ni3Al for Automotive Body Applications; Project Report; UTeM: Melaka, Malaysia, 2010. [Google Scholar]
- Kumar, H.G.; Sivaro, T.; Anand, J.S. A novel intermetallic nickel aluminide (Ni3Al) as an alternative body material. Int. J. Eng. Technol. 2011, 11, 208–215. [Google Scholar]
- Anand, J.S. Intermetallic Nickel Aluminides Being an Alternative for Automotive Body Applications; Lambert Academic Publisher: Saarbrücken, Germany, 2011. [Google Scholar]
- Kumar, G.K.; Subramonian, S.; Anand, J.S. An overview of intermetallic nickel aluminides (Ni3Al) as an alternative automotive body material. In Proceedings of the International Conference on Design and Concurrent Engineering, Melaka, Malaysia, 20–21 September 2010; pp. 132–135.
- Caricos. Available online: http://www.caricos.com/cars/a/audi/2015_audi_a3_cabrio/1920x1080/95.html (accessed on 2 January 2015).
- Sikka, V.K.; Dailey, R.E. Use of Ni3Al-Based Alloys for Walking-Beam Furnaces. CRADA Final Report. Available online: http://www.osti.gov/scitech/servlets/purl/392810 (accessed on 2 January 2014).
- National Materials Advisory Board. Materials Technologies for the Process of the Future; NMAB-496; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Sikka, V.K. Commercialization of Nickel and Iron Aluminides. Available online: http://www.osti.gov/scitech/servlets/purl/443989 (accessed on 8 January 2010).
- Sikka, V.K.; Sanatella, L.; Viswanathan, S.; Swindeman, R.W. In-Service Testing of Ni3Al Coupons and Trays in Carburizing Furnaces at Delphi Saginaw. CRADA Final Report. Available online: http://www.osti.gov/scitech/biblio/310024 (accessed on 15 January 2015).
- Sikka, V.K. Newly Developed Ni3Al Heat Treating Furnace Assemblies are Being Commercialized at Delphi. Available online: https://www1.eere.energy.gov/manufacturing/industries_technologies/imf/pdfs/ni3alheatfurnace.pdf (accessed on 12 January 2015).
- Sikka, V.K.; Santella, M.L.; Swindeman, R.W.; Aramayo, G. Intermetallic Alloy Development and Technology Transfer, Advanced Intermetallics/Metals and Composites. pp. 89–107. Available online: http://www.ms.ornl.gov/programs/energyeff/aim/annual (accessed on 6 January 2010).
- Sikka, V.K.; Santella, M.L. Intermetallic Alloy Development and Technology Transfer. In Report of Advanced Industrial Materials (AIM) Program. Available online: http://web.ornl.gov/~webworks/cppr/y2001/rpt/106779.pdf (accessed on 6 January 2010).
- Angelini, P.; Sims, G. Advanced Industrial Materials (AIM). Program Compilation of Project Summaries and Significant Accomplishments, 2000; Metals and Ceramics Division Oak Ridge National Laboratory: Oak Ridge, TN, USA, May 2000. [Google Scholar]
- Sikka, V.K. Ni3Al Enables Improvement in Forging Dies. Available online: https://www1.eere.energy.gov/manufacturing/industries_technologies/imf/pdfs/ni3alenablesimp.pdf (accessed on 12 January 2015).
- Liu, C.T.; Bloom, E.E. Ni3Al-Based Alloys for Die and Tool Application. U.S. Patent US6,238,620 B1, 29 May 2001. [Google Scholar]
- Abrameit, C.; Müller, S.; Wanderka, N. Stability of γ' phase in the stoichiometric Ni3Al alloy under ion irradiation. Scr. Metall. Mater. 1995, 32, 1519–1523. [Google Scholar] [CrossRef]
- Katsumaro, F.; Akimichi, H. Irradiated Intermetallic Compound Containing Part of Light-Water Reactor. U.S. Patent US5,735,974, 7 April 1998. [Google Scholar]
- Corwin, W.R.; Burchell, T.D.; Hayner, G.O.; Katoh, Y.; McGreevy, T.E.; Nansad, R.K.; Ren, W.; Snead, L.L.; Stoller, R.E.; Wilson, D.F.; et al. Nuclear Energy Systems; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 31 December 2005. [Google Scholar]
- Ni3Al Foils Group. Available online: http://www.nims.go.jp/imc/Ni3Alfoil (accessed on 12 January 2006).
- Hirano, T.; Demura, M.; Kishida, K. Method for Manufacturing Ni3Al Alloy Foil. Patent No. JP2,003,034,832, 7 February 2003. [Google Scholar]
- Demura, M.; Suga, Y.; Umezawa, O.; Kishida, K.; George, E.P.; Hirano, T. Fabrication of Ni3Al thin foil by cold-rolling. Intermetallics 2001, 9, 157–167. [Google Scholar] [CrossRef]
- Demura, M.; Kishida, K.; Suga, Y.; Takanashi, M.; Hirano, T. Fabrication of thin Ni3Al foils by cold rolling. Scr. Mater. 2002, 47, 267–272. [Google Scholar] [CrossRef]
- Borodianska, W.; Demura, M.; Kishida, K.; Hirano, T. Fabrication of thin foils of binary Ni–Al two phase alloys by cold rolling. Intermetallics 2002, 10, 255–262. [Google Scholar] [CrossRef]
- Frontiers of Materials Research at NIMS, Research Staff and Current Work. Tsukuba, Japan, September 2012 Edition ed. pp. 65–66. Available online: http://www.nims.go.jp/eng/publicity/publication/nims-research-guide_list.html (accessed on 15 January 2015).
- Kishida, K.; Demura, M.; Hirano, T. Development of Intermetallic Foils for Automotive Exhaust Gas Catalyst Supports. Report 2002. Available online: http://www.nims.go.jp/eng/research/database/index.html (accessed on 10 January 2008).
- Kim, S.H.; Oh, M.H.; Kishida, K.; Hirano, T.; Wee, D.M. Cyclic oxidation behavior and recrystalization of cold-rolled Ni3Al foils. Mater. Lett. 2004, 58, 2867–2871. [Google Scholar] [CrossRef]
- Hirano, T.; Demura, M.; Kishida, K. Process for Producing Heat-Resistant Intermetallic Compound Ni3Al Foil Having Room-Temperature Ductility and Heat-Resistant Intermetallic Compound Ni3Al Foil Having Room-Temperature Ductility. U.S. Patent No. US2,003,136,480, 24 July 2003. [Google Scholar]
- Bojar, Z.; Józwik, P.; Bystrzycki, J. Tensile properties and fracture behavior of nanocrystalline Ni3Al intermetallic foil. Scr. Mater. 2006, 55, 399–402. [Google Scholar] [CrossRef]
- Jozwik, P.; Bojar, Z. Analysis of grain size effect on tensile properties of Ni3Al—Based intermetallic strips. Arch. Metall. Mater. 2007, 52, 321–327. [Google Scholar]
- Jozwik, P.; Bojar, Z. Influence of heat treatment on structure and mechanical properties of Ni3Al-based alloys. Arch. Metall. Mater. 2010, 55, 237–245. [Google Scholar]
- Jozwik, P. Mechanical Properties and Fracture of Ni3Al-based Intermetallic Alloys. Ph.D. Thesis, MUT, Warsaw, Poland, 2004. [Google Scholar]
- Polkowski, W.; Jozwik, P.; Bojar, Z. Differential speed rolling of Ni3Al based intermetallic alloy—Analysis of the deformation process. Mater. Lett. 2015, 139, 46–49. [Google Scholar] [CrossRef]
- Polkowski, W.; Jozwik, P.; Bojar, Z. EBSD and X-ray diffraction study on the recrystallization of cold rolled Ni3Al based intermetallic alloy. J. Alloy. Compds. 2014, 614, 226–233. [Google Scholar] [CrossRef]
- Spearing, S.M. Materials issues in microelectromechanical systems (MEMS). Acta Mater. 2000, 48, 179–196. [Google Scholar] [CrossRef]
- Burns, D.E.; Zang, Y.; Teutsch, M.; Bade, K.; Akta, J.; Hemkera, K.J. Development of Ni-based superalloys for microelectromechanical systems. Scr. Mater. 2012, 67, 459–462. [Google Scholar] [CrossRef]
- Microtechnology-Based Energy. Chemical and Biological Systems. Available online: http://mecs.oregonstate.edu (accessed on 15 January 2015).
- Advanced Microgravity Acceleration Measurement Systems (AMAMS) Being Developed. Available online: http://microgravity.grc.nasa.gov (accessed on 15 January 2015).
- An Introduction to MEMS (Micro-electromechanical Systems). Available online: http://www.lboro.ac.uk/microsites/mechman/research/ipm-ktn/pdf/Technology_review/an-introduction-to-mems.pdf (accessed on 18 January 2015).
- What is MEMS Technology? Material Provided Courtesy of Dr. Michael Huff of MEMS and Nanotechnology Exchange at the Corporation for National Research Initiatives. Available online: https://www.mems-exchange.org/MEMS/what-is.html (accessed on 18 January 2015).
- Romig, A.D., Jr.; Dugger, M.T.; McWhorter, P.J. Materials issues in microelectromechanical devices: Science, engineering, manufacturability and reliability. Acta Mater. 2003, 51, 5837–5866. [Google Scholar] [CrossRef]
- Nanonet, Nanotechnology Research Network Center of Japan. Available online: http://www.nanonet.go.jp (accessed on 10 January 2015).
- National Science Foundation (NSF). MEMS-Micro Electro Mechanical System. Available online: http://www.nsf.gov/od/lpa/nsf50/nsfoutreach/htm/n50_z2/pages_z3/32_pg.htm (accessed on 15 January 2015).
- Micro Electro Mechanical Systems (MEMS). Available online: http://www.sandia.gov/mstc/mems/ (accessed on 5 January 2015).
- Jóźwik, P.; Karcz, M.; Badur, J. Numerical modeling of a microreactor for thermocatalytic decomposition of toxic compounds. Chem. Process Eng. 2011, 32, 215–227. [Google Scholar] [CrossRef]
- Hirano, T.; Demura, M.; Kishida, K.; Minamida, K.; Xu, Y. Laser spot welding of cold-rolled boron-free Ni3Al foils. Metall. Mater. Trans. A 2007, 38A, 1041–1047. [Google Scholar] [CrossRef]
- Oikawa, M.; Minamida, K.; Demura, M.; Kishida, K.; Hirano, T. Development of laser welding lethods for cold-rolled thin foils of Ni3Al alloys. J. Jpn. Inst. Met. 2003, 67, 185–188. [Google Scholar]
- Józwik, P.; Bojar, Z.; Kołodziejczak, P. Microjoining of Ni3Al based intermetallic thin foils. Mater. Sci. Technol. 2010, 26, 473–477. [Google Scholar] [CrossRef]
- Durejko, T.; Jóźwik, P.; Bojar, Z. Joining of Ni3Al microcrystalline foils by SHS reaction. Arch. Metall. Mater. 2009, 4, 717–723. [Google Scholar]
- Jozwik, P.; Bojar, Z.; Zasada, D.; Paszula, J. Experimental investigation of explosive welding of Ni3Al—Based alloy. In Proceedings of the 11th International Conference on advanced Materials, Rio de Janeiro, Brazil, 20–25 September 2009.
- Sikka, S.V.; Deevi, S. Electronic Circuits Having NiAl and Ni3Al Substrates. U.S. Patent US5,965,274, 12 October 1999. [Google Scholar]
- Suo, Z.; Ma, E.Y.; Gleskova, H.; Wagner, S. Mechanics of rollable and foldable film-on-foil electronics. Appl. Phys. Lett. 1999, 74, 1177–1179. [Google Scholar] [CrossRef]
- Theiss, S.D.; Wu, C.C.; Lu, M.; Sturm, J.C.; Wagner, S. Flexible, lightweight steel-foil substrates for a Si:H thin-film transistor. MRS Proc. 1997, 471, 21–26. [Google Scholar] [CrossRef]
- Hirano, T.; Demura, M.; Kishida, K.; Umezawa, O.; George, E.P.; Hirano, T. Ductile thin foils of Ni3Al. In Mechanical Properties of Structural Films; Christopher, L., Muhlstein, Ch.L., Brown, S.B., Eds.; ASTM International: Bridgeport, CT, USA, 2001; pp. 248–261. [Google Scholar]
- Bikonda, O.; Castro, G.R.; Torrelles, X.; Wendler, F.; Moritz, W. Surface-induced disorder on the clean Ni3Al(111) surface. Phys. Rev. B 2005, 72, 195430. [Google Scholar] [CrossRef]
- Krupski, A. Growth morphology of thin films on metallic and oxide surfaces. J. Phys. Condens. Matter 2014, 26, 053001. [Google Scholar] [CrossRef] [PubMed]
- Miśków, K.; Krupski, A.; Wandelt, K. Growth morphology of Pb films on Ni3Al(111). Vacuum 2014, 101, 71–78. [Google Scholar] [CrossRef]
- Min, B.I.; Freeman, A.J.; Jansen, H.J.F. Magnetism, electronic structure, and Fermi surface of Ni3Al. Phys. Rev. B 1988, 37, 6757. [Google Scholar] [CrossRef]
- Han, Y.; Unal, B.; Evans, J.W. Nonadiabatic forces in ion-solid interactions: The initial stages of radiation damage. Phys. Rev. Lett. 2012, 108, 216101. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Srinivasan, S.G. First-principles study of self- and solute diffusion mechanisms in γ'-Ni3Al. Phys. Rev. B 2012, 86, 014112. [Google Scholar] [CrossRef]
- Degen, S.; Krupski, A.; Kralj, M.; Langner, A.; Becker, C.; Sokolowski, M.; Wandelt, K. Determination of the coincidence lattice of an ultra thin Al2O3 film on Ni3Al(111). Surf. Sci. 2005, 576, L57–L64. [Google Scholar] [CrossRef]
- Kresse, G.; Schmid, M.; Napetschnig, E.; Shishkin, M.; Koehler, L.; Varga, P. Structure of the ultrathin aluminum oxide film on NiAl(110). Science 2005, 308, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Moors, M.; Krupski, A.; Degen, S.; Kralj, M.; Becker, C.; Wandelt, K. Scanning tunneling microscopy and spectroscopy investigations of copper phthalocyanine adsorbed on Al2O3/Ni3Al(111). Appl. Surf. Sci. 2008, 254, 4251–4257. [Google Scholar] [CrossRef]
- Lehnert, A.; Krupski, A.; Degen, S.; Franke, K.; Decker, S.; Rusponi, S.; Kralj, M.; Becker, C.; Brune, H.; Wandelt, K.; et al. Nucleation of ordered Fe islands on Al2O3/Ni3Al(111). Surf. Sci. 2006, 600, 1804–1808. [Google Scholar] [CrossRef]
- Synthetic Multifunctional Materials for Structural and Ballistic and Blast Protection. Available online: http://www.darpa.mil/dso/thrust/madev/smfm/project.html (accessed on 10 May 2008).
- Hirano, T.; Demura, M.; Kishida, K.; Hong, H.U.; Suga, Y. Mechanical properties of cold-rolled thin foils of Ni3Al. In Proceedings of the 3rd International symposium on Structural Intermetallics, Snow King Resort, Jackson Hole, WY, USA, 23–27 September 2001; pp. 765–774.
- Schafrik, R.E. A perspective on intermetallic commercialization for aero-turbine applications. In Proceedings of the 3rd International symposium on Structural Intermetallics, Snow King Resort, Jackson Hole, WY, USA, 23–27 September 2001; pp. 13–17.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozwik, P.; Polkowski, W.; Bojar, Z. Applications of Ni3Al Based Intermetallic Alloys—Current Stage and Potential Perceptivities. Materials 2015, 8, 2537-2568. https://doi.org/10.3390/ma8052537
Jozwik P, Polkowski W, Bojar Z. Applications of Ni3Al Based Intermetallic Alloys—Current Stage and Potential Perceptivities. Materials. 2015; 8(5):2537-2568. https://doi.org/10.3390/ma8052537
Chicago/Turabian StyleJozwik, Pawel, Wojciech Polkowski, and Zbigniew Bojar. 2015. "Applications of Ni3Al Based Intermetallic Alloys—Current Stage and Potential Perceptivities" Materials 8, no. 5: 2537-2568. https://doi.org/10.3390/ma8052537