Fabrication and Characterization of a SPR Based Fiber Optic Sensor for the Detection of Chlorine Gas Using Silver and Zinc Oxide
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of Probe
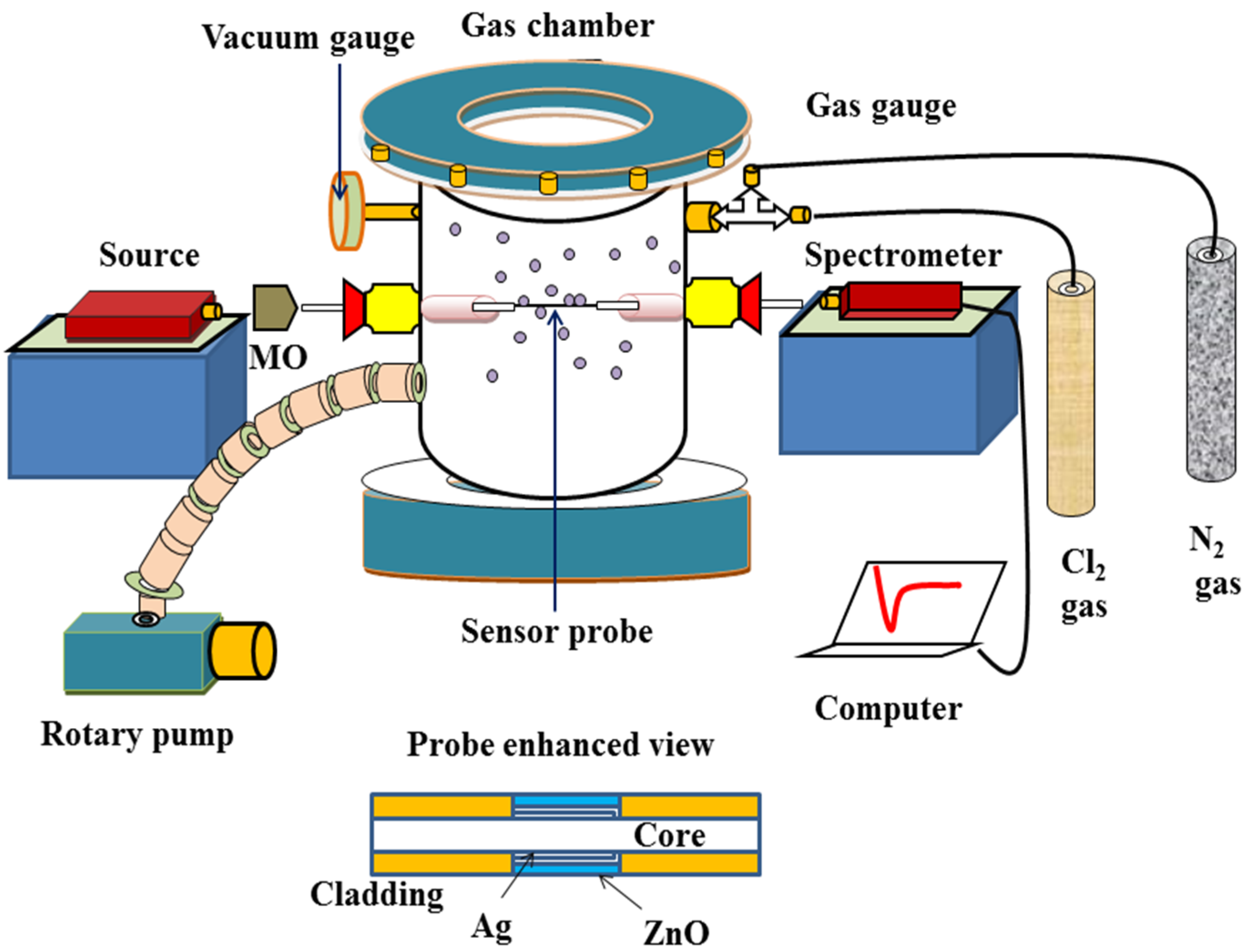
2.2. Experimental Setup
3. Results and Discussion
3.1. Sensing Principle
 2ZnCl2 (s) + O2 (g)
2ZnCl2 (s) + O2 (g)
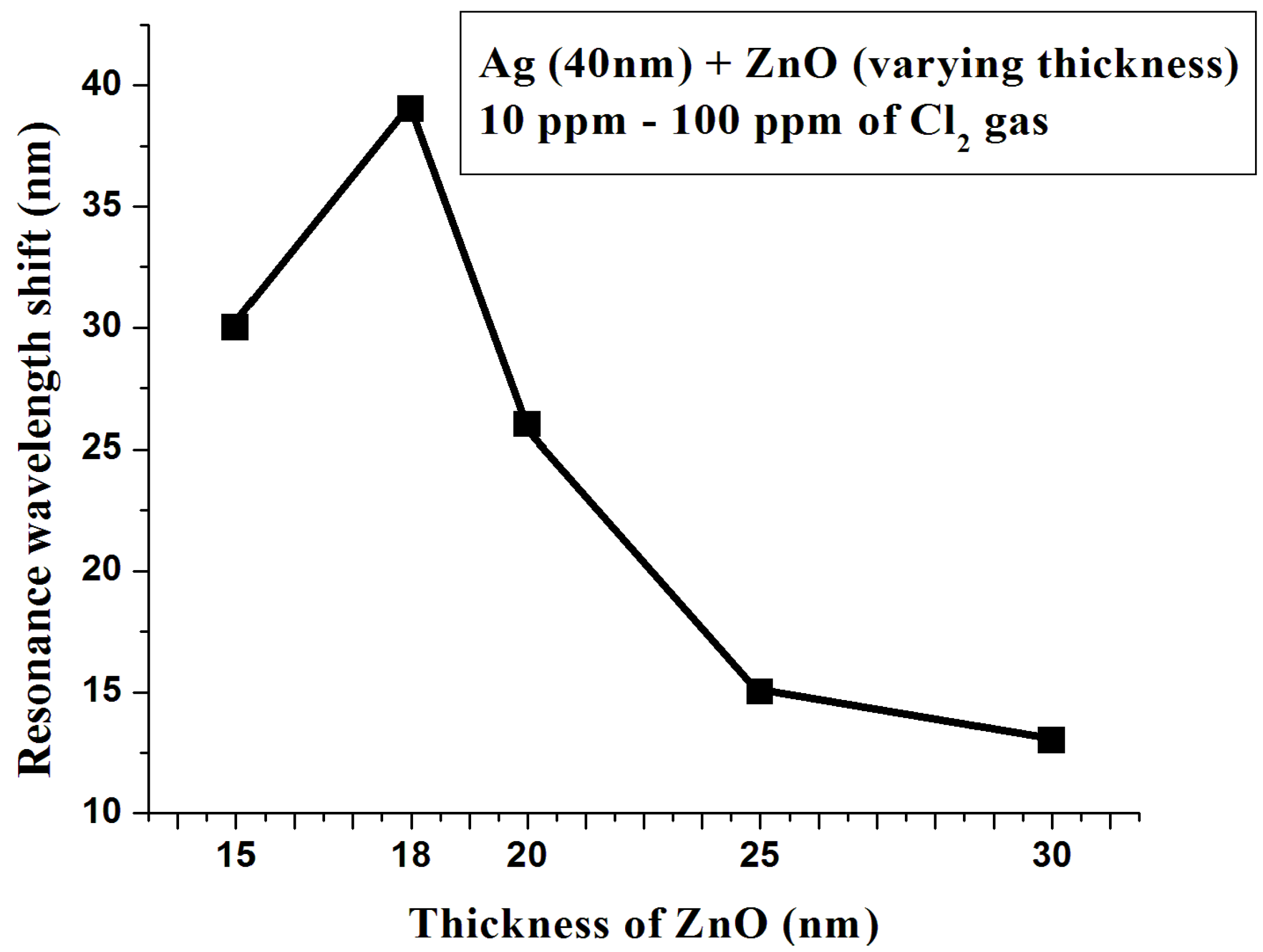
3.2. Thickness Optimization of Zinc Oxide Layer
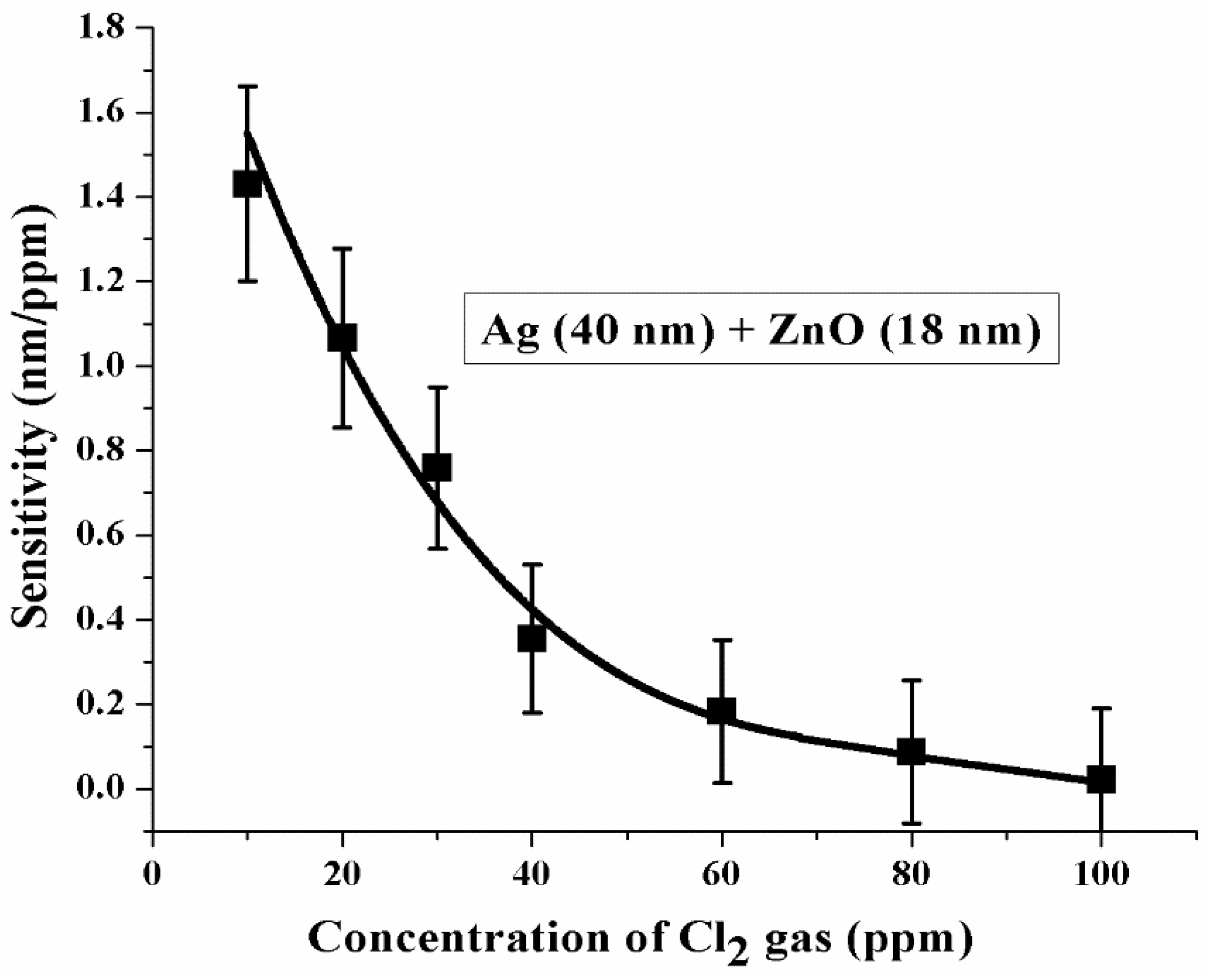
3.3. Characterization of the Sensor Probe


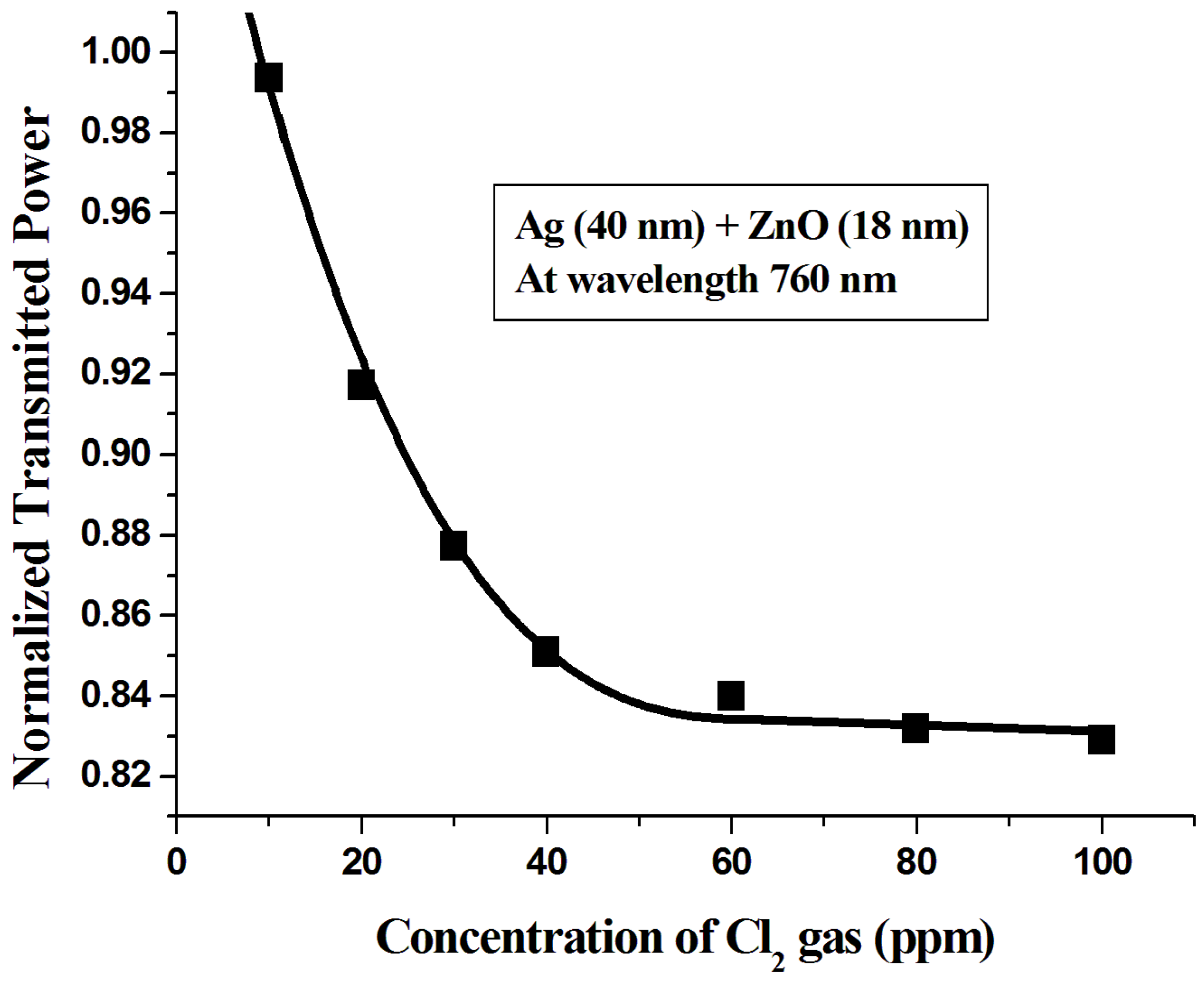

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, C.W.; Martin, J.G. Chlorine gas inhalation: Human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 2010, 7, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, A.; Fabry, P.; Durante, P. Design and testing of a potentiometric chlorine gauge. Sens. Actuators 1985, 7, 245–252. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Mori, Y.; Okajima, Y. Cl2 gas sensor using Na+ conducting solid electrolyte. Chem. Lett. 2000, 109, 34–35. [Google Scholar] [CrossRef]
- Liu, J.; Weppner, W. Limiting-current chlorine gas sensor based on β-alumina solid electrolyte. Sens. Actuators B 1992, 6, 270–273. [Google Scholar] [CrossRef]
- Mari, C.M.; Terzaghi, G.; Bertolini, M.; Barbi, G.B. A chlorine gas potentiometric sensor. Sens. Actuators B 1992, 8, 41–45. [Google Scholar] [CrossRef]
- Wang, D.; Hu, P.; Xu, J.; Dong, X.; Pan, Q. Fast response chlorine gas sensor based on mesoporous SnO2. Sens. Actuators B 2009, 140, 383–389. [Google Scholar] [CrossRef]
- Patil, D.R.; Patil, L.A. Room temperature chlorine gas sensing using surface modified ZnO thick film resistors. Sens. Actuators B 2007, 123, 546–553. [Google Scholar] [CrossRef]
- Miyata, T.; Seiji, K.; Makoto, I.; Minami, T. High sensitivity chlorine gas sensors using Cu-Phthalocyanine thin films. Appl. Surf. Sci. 2003, 425, 255–259. [Google Scholar]
- Chu, X.; Cheng, Z. High sensitivity chlorine gas sensors using CdSnO3 thick film prepared by co-precipitation method. Sens. Actuators B 2004, 98, 215–217. [Google Scholar] [CrossRef]
- Tabassum, R.; Mishra, S.K.; Gupta, B.D. Surface plasmon resonance based fiber optic hydrogen sulphide gas sensor utilizing Cu/ZnO thin films. Phys. Chem. Chem. Phys. 2013, 15, 11868–11874. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2006, 121, 18–35. [Google Scholar] [CrossRef]
- Batzil, M.; Diebold, U. Surface studies of gas sensing metal oxides. Phys. Chem. Chem. Phys. 2007, 9, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Basu, S. Improved zinc oxide film for gas sensor applications. Bull. Mater. Sci. 2002, 25, 513–515. [Google Scholar] [CrossRef]
- Reather, H. Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Springer: New York, NY, USA, 1988. [Google Scholar]
- Jorgenson, R.C.; Yee, S.S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Lal, S.; Link, S.; Halas, N.J. Nano-optics from sensing to wave guiding. Nat. Photonics 2007, 1, 641–648. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, B.D. Simulation of a surface plasmon resonance-based fiber-optic sensor for gas sensing in visible range using films of nanocomposites. Meas. Sci. Technol. 2010, 21. [Google Scholar] [CrossRef]
- Bhatia, P.; Gupta, B.D. Surface Plasmon resonance based fiber optic refractive index sensor: Sensitivity enhancement. Appl. Opt. 2011, 50, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Gupta, B.D. Surface plasmon resonance based fiber optic sensor for the detection of CrO42− using Ag/ITO/hydrogel layers. Anal. Methods 2014, 6, 5191–5197. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Sundstrom, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators B 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Gupta, B.D.; Verma, R.K. Surface plasmon resonance-based fiber optic sensors: Principle, probe designs, and some applications. J. Sens. 2009, 171. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, B.D. Fabrication and characterization of a surface plasmon resonance based fiber optic sensor using gel entrapment technique for the detection of low glucose concentration. Sens. Actuators B 2013, 177, 589–595. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR based fiber optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced grapheme oxide prepared by in situ polymerization. Sens. Actuators B 2014, 199, 190–200. [Google Scholar] [CrossRef]
- Mishra, S.K.; Rani, S.; Gupta, B.D. Surface plasmon resonance based fiber optic hydrogen sulphide gas sensor utilizing nickel oxide doped ITO thin film. Sens. Actuators B 2014, 195, 215–222. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Fiber optic SPR sensor for the detection of melamine using molecular imprinting. Sens. Actuators B 2015, 212, 404–410. [Google Scholar] [CrossRef]
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Surface plasmon resonance based fiber optic chlorine gas sensor utilizing Ag/ZnO thin film. In Proceedings of the 12th International Conference on Fiber Optics and Photonics, Kharagpur, India, 13–16 December 2014; The Optical Society (OSA): Washington, DC, USA.
- Fontana, E. Thickness optimization of metal films for the development of surface-plasmon-based sensors for non-absorbing media. Appl. Opt. 2008, 45, 7632–7642. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Shalabney, A.; Abdulhalim, I. Electromagnetic fields distribution in multilayer thin film structures and the origin of sensitivity enhancement in surface plasmon resonance sensors. Sens. Actuators A 2010, 159, 24–32. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usha, S.P.; Mishra, S.K.; Gupta, B.D. Fabrication and Characterization of a SPR Based Fiber Optic Sensor for the Detection of Chlorine Gas Using Silver and Zinc Oxide. Materials 2015, 8, 2204-2216. https://doi.org/10.3390/ma8052204
Usha SP, Mishra SK, Gupta BD. Fabrication and Characterization of a SPR Based Fiber Optic Sensor for the Detection of Chlorine Gas Using Silver and Zinc Oxide. Materials. 2015; 8(5):2204-2216. https://doi.org/10.3390/ma8052204
Chicago/Turabian StyleUsha, Sruthi P., Satyendra K. Mishra, and Banshi D. Gupta. 2015. "Fabrication and Characterization of a SPR Based Fiber Optic Sensor for the Detection of Chlorine Gas Using Silver and Zinc Oxide" Materials 8, no. 5: 2204-2216. https://doi.org/10.3390/ma8052204





