Characterization of Dentine to Assess Bond Strength of Dental Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Raman and SEM of Dentine
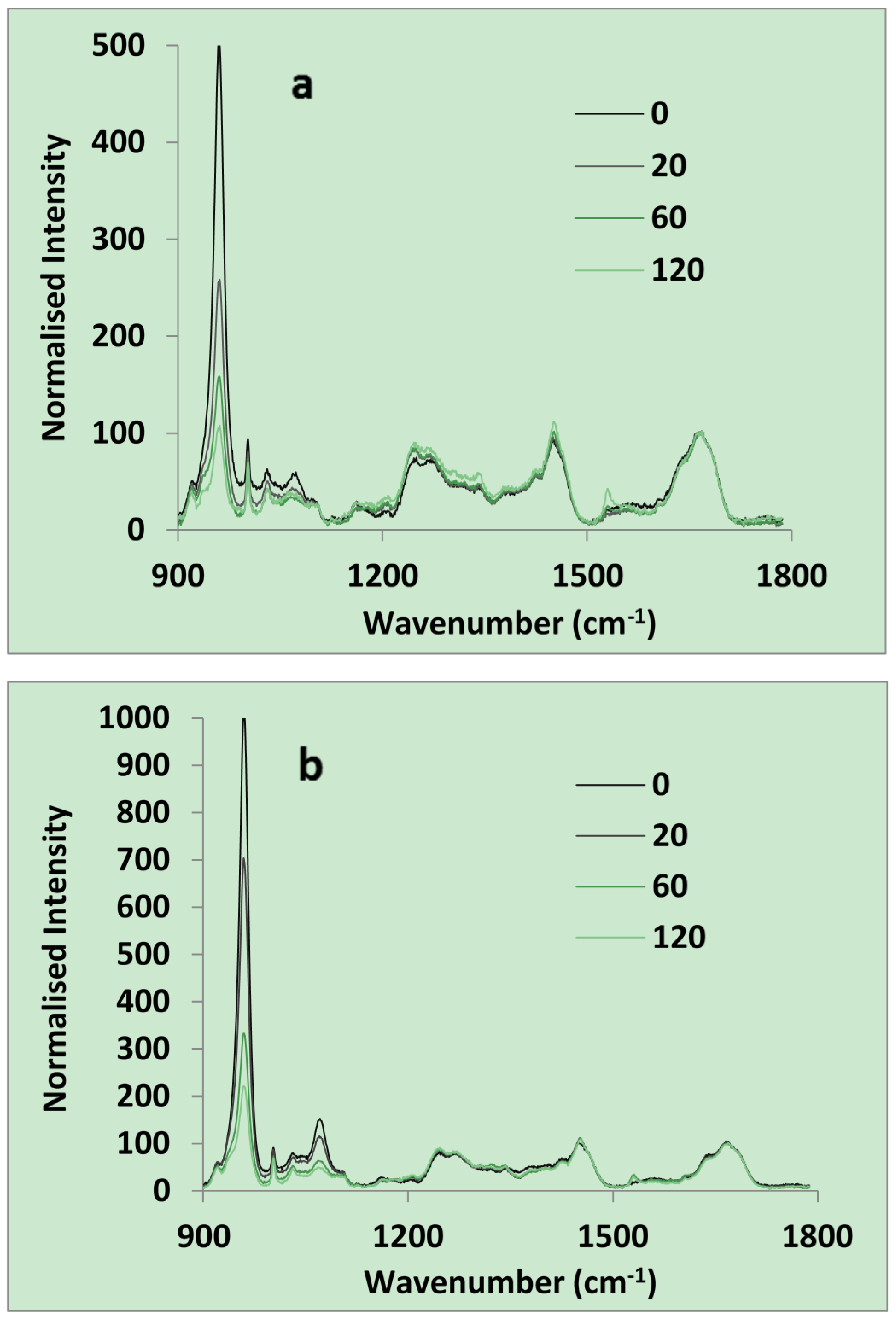
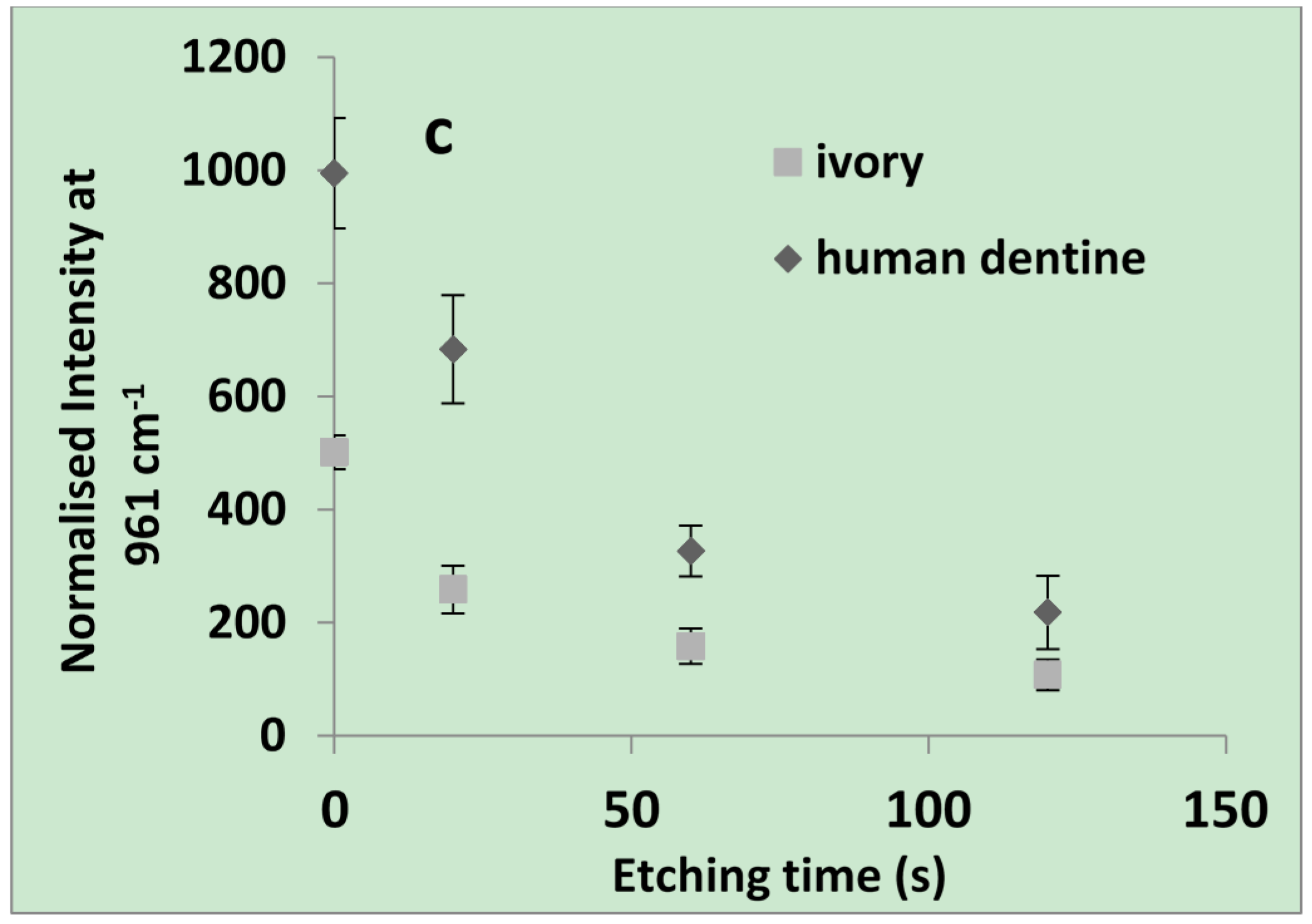

2.1.2. Flexural Properties
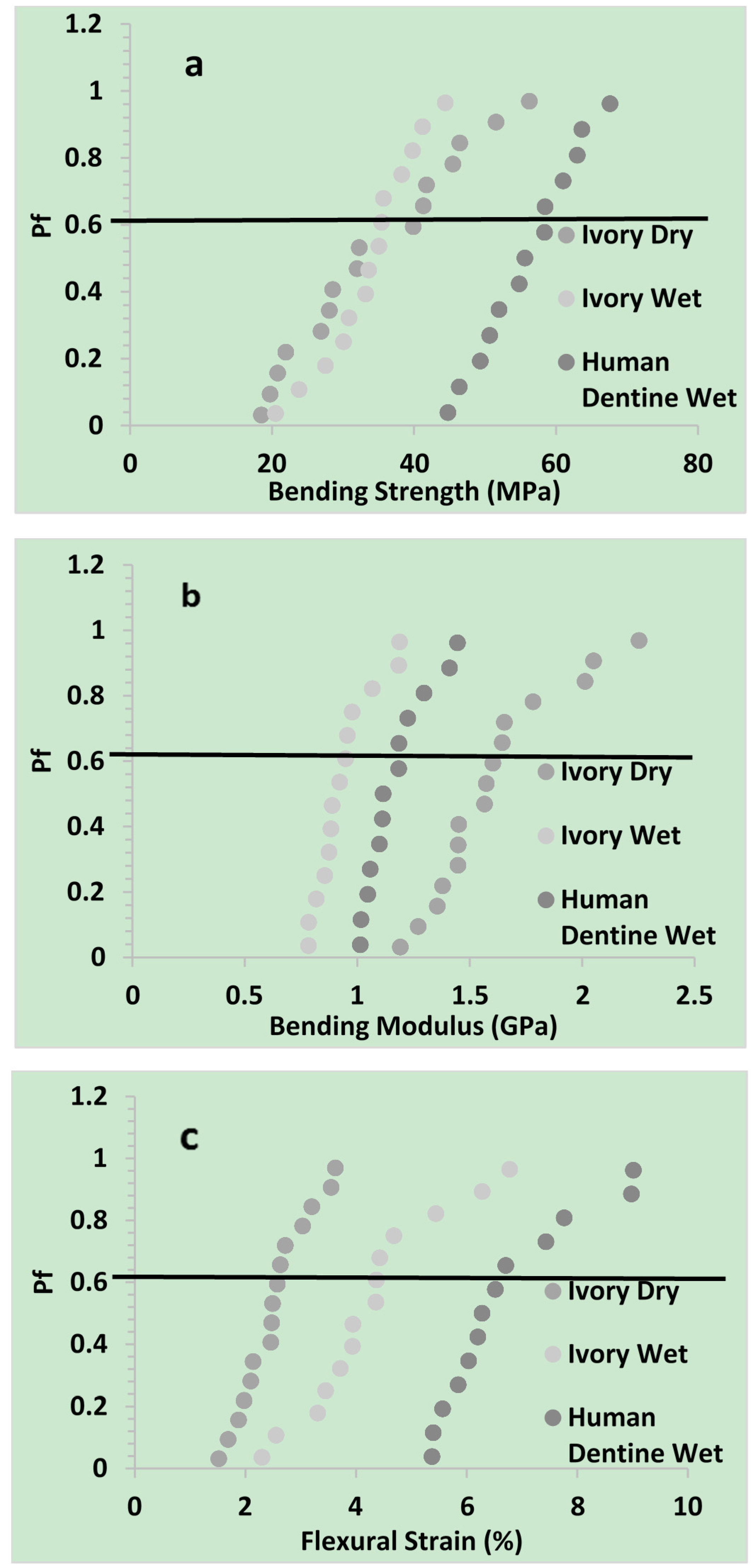
| Three point strength | Weibull parameter | Ivory dry | Ivory wet | Human dentine wet |
|---|---|---|---|---|
| Bending Strength (MPa) | σθ | 38 | 36 | 59 |
| m | 3 | 6 | 8 | |
| R2 | 0.97 | 0.99 | 0.99 | |
| Mean | 34 | 34 | 56 | |
| 95% Cl | 6 | 4 | 4 | |
| Bending Modulus (GPa) | σθ | 1.7 | 1.0 | 1.2 |
| m | 6 | 7 | 8 | |
| R2 | 0.90 | 0.90 | 0.91 | |
| Mean | 1.6 | 0.9 | 1.2 | |
| 95% Cl | 0.1 | 0.1 | 0.1 | |
| Flexural Strain (%) | σθ | 2.7 | 4.7 | 7.2 |
| m | 4 | 4 | 5 | |
| R2 | 0.96 | 0.96 | 0.90 | |
| Mean | 2.5 | 4.3 | 6.7 | |
| 95% Cl | 0.3 | 0.7 | 0.7 |
2.1.3. Shear Bond Strengths of Composites to Dentine

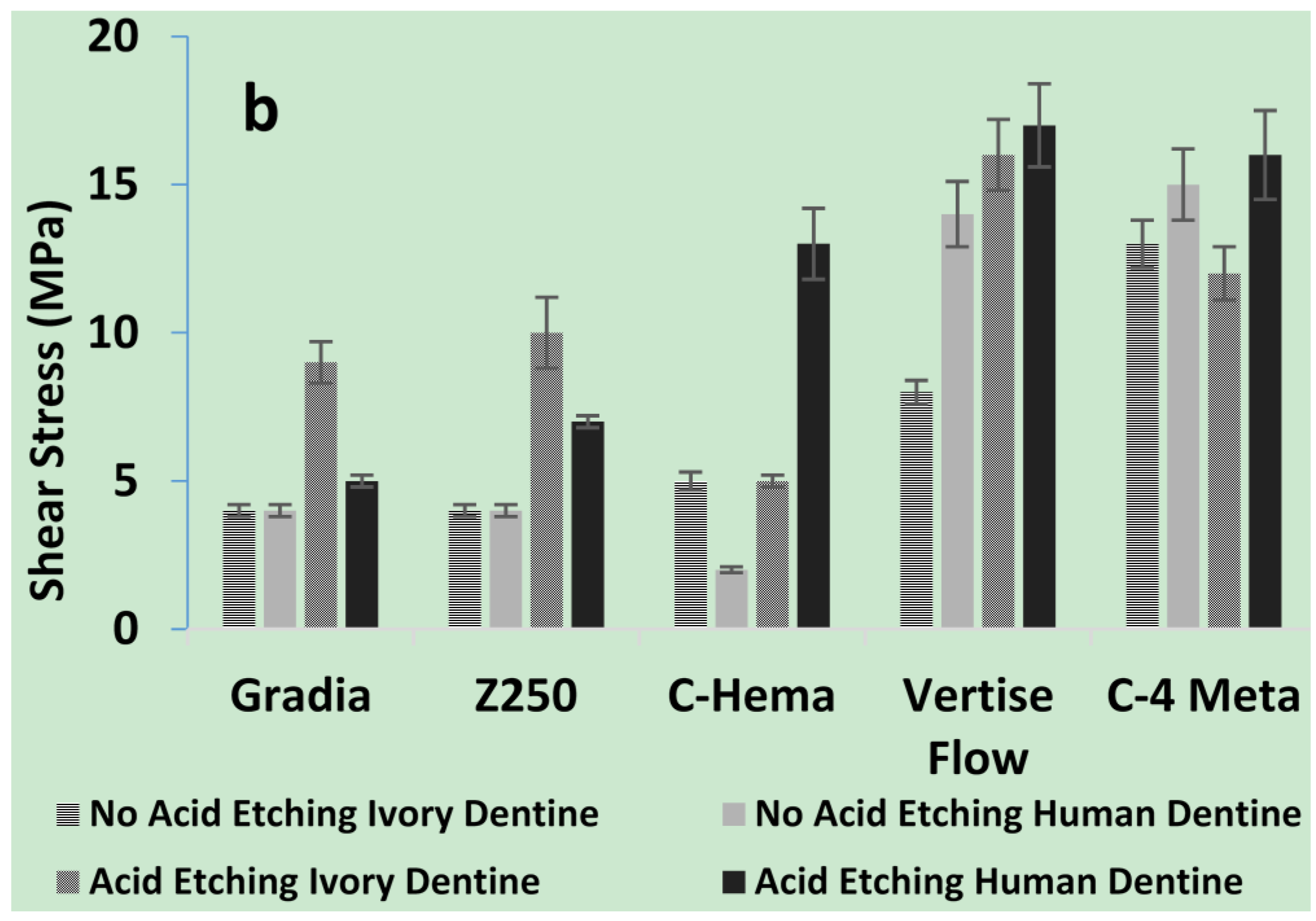
2.1.4. SEM of Composite/Dentine Interfaces

2.2. Discussion
2.2.1. Chemical Properties of Dentine
2.2.2. Microscopic Properties of Dentine
2.2.3. Mechanical Properties
2.2.4. Adhesion Properties
3. Experimental Section
3.1. Dentine Source
3.2. Composite Preparation/Source
3.3. Raman Spectra of Dentine
3.4. Flexural Properties of Dentine
3.5. Shear Bond Strength of Composites to Dentine
- (1)
- ibond application and light cure for 20 s as per manufacturer’s instructions; or
- (2)
- acid etch application for 20 s followed by water rinsing, gentle drying and ibond application and cure; or
- (3)
- acid etch for 20 s, rinse and dry; or
- (4)
- no acid or ibond treatment.
3.6. Scanning Electron Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. The Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Featherstone, J. The continuum of dental caries—Evidence for a dynamic disease process. J. Dent. Res. 2004, 83, C39–C42. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Mehdawi, I.M.; Pratten, J.; Spratt, D.A.; Knowles, J.C.; Young, A.M. High strength re-mineralizing, antibacterial dental composites with reactive calcium phosphates. Dent. Mater. 2013, 29, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Hiller, K.-A.; Bolay, C.; Petzel, C.; Spagnuolo, G.; Reichl, F.-X.; Schmalz, G.; Schweikl, H. Function of mapk and downstream transcription factors in monomer-induced apoptosis. Biomaterials 2012, 33, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, M.C.G.; Cavalcante, L.M.A.; Pimenta, L.A.F. Influence of phosphoric acid pretreatment on self-etching bond strengths. J. Esthet. Restor. Dent. 2004, 16, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P. Quantifying adhesive penetration in adhesive/dentin interface using confocal raman microspectroscopy. J. Biomed. Mater. Res. 2002, 59, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Lopes, M.; Perdigão, J.; Ambrose, W.W.; Rosa, B.T. The use of flowable composites as filled adhesives. Dent. Mater. 2002, 18, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; Moretto, S.G.; Carvalho, R.C.; Russo, E.M. Influence of intrapulpal pressure simulation on the bond strength of adhesive systems to dentin. Braz. Oral Res. 2008, 22, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, J.; Leary, R.; Perrin, J.; Brydson, R.; Harrington, J.; Markowitz, K.; Milne, S. Characterization of dentine structure in three dimensions using fib-sem. J. Microsc. 2010, 240, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-G.; Rosalen, P.; Falsetta, M.; Koo, H. Natural products in caries research: Current (limited) knowledge, challenges and future perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Mine, A.; Poitevin, A.; van Ende, A.; Cardoso, M.V.; van Landuyt, K.L.; Peumans, M.; van Meerbeek, B. Meta-analytical review of parameters involved in dentin bonding. J. Dent. Res. 2012, 91, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Skene, L. Ownership of human tissue and the law. Nat. Rev. Genet. 2002, 3, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Walker, M.P. Chemical profile of adhesive/caries-affected dentin interfaces using raman microspectroscopy. J. Biomed. Mater. Res. A 2007, 81A, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, Z.; Stojšin, I.; Brkanić, T.; Lončar, J. The effect of excessive coca-cola consumption on the development of dental erosions. Stomatol. Glas. Srb. 2012, 59, 148–153. [Google Scholar] [CrossRef]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Gupta, S.K.; Thangaraj, K.; Singh, L. Identification of the source of ivory idol by DNA analysis. J. Forensic Sci. 2011, 56, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Lombardini, M.; Vigorelli, P.; Colombo, M.; Chiesa, M. The role of different toothpastes on preventing dentin erosion: An sem and afm study®. Scanning 2013, 36, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.S.; Shankar, P.; Indira, R. Depth of penetration of four resin sealers into radicular dentinal tubules: A confocal microscopic study. J. Endod. 2012, 38, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Chng, H.K.; Palamara, J.E.; Messer, H.H. Effect of hydrogen peroxide and sodium perborate on biomechanical properties of human dentin. J. Endod. 2002, 28, 62–67. [Google Scholar] [CrossRef] [PubMed]
- McKittrick, J.; Chen, P.Y.; Tombolato, L.; Novitskaya, E.E.; Trim, M.W.; Hirata, G.A.; Olevsky, E.A.; Horstemeyer, M.F.; Meyers, M.A. Energy absorbent natural materials and bioinspired design strategies: A review. Mater. Sci. Eng. C 2010, 30, 331–342. [Google Scholar] [CrossRef]
- Hayashi, M.; Koychev, E.V.; Okamura, K.; Sugeta, A.; Hongo, C.; Okuyama, K.; Ebisu, S. Heat treatment strengthens human dentin. J. Dent. Res. 2008, 87, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Bedini, R.; Pameijer, C.H.; Somma, F. Flexural properties of endodontic posts and human root dentin. Dent. Mater. 2007, 23, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Imbeni, V.; Nalla, R.K.; Bosi, C.; Kinney, J.H.; Ritchie, R.O. In vitro fracture toughness of human dentin. J. Biomed. Mater. Res. A 2003, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Okamura, K.; Koychev, E.V.; Furuya, Y.; Sugeta, A.; Ota, T.; Ebisu, S. Effects of rehydration on dentin strengthened by heating or uv irradiation. J. Dent. Res. 2010, 89, 154–158. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, M.J.; Mitraki, A. Natural fibrous proteins: Structural analysis, assembly, and applications. In Proteins in Solution and at Interfaces: Methods and Applications in Biotechnology and Materials Science; Wiley: Hoboken, NJ, USA, 2013; pp. 219–232. [Google Scholar]
- Wang, Q.; Wang, X.M.; Tian, L.L.; Cheng, Z.J.; Cui, F.Z. In situ remineralizaiton of partially demineralized human dentine mediated by a biomimetic non-collagen peptide. Soft Matter 2011, 7, 9673–9680. [Google Scholar] [CrossRef]
- Dibdin, G.; Poole, D. Surface area and pore size analysis for human enamel and dentine by water vapour sorption. Arch. Oral Biol. 1982, 27, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Roop Kumar, R.; Wang, M. Modulus and hardness evaluations of sintered bioceramic powders and functionally graded bioactive composites by nano-indentation technique. Mater. Sci. Eng. A 2002, 338, 230–236. [Google Scholar] [CrossRef]
- Profeta, A.C. Dentine bonding agents comprising calcium-silicates to support proactive dental care: Origins, development and future. Dent. Mater. J. 2014, 33, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Burke, F.; Hussain, A.; Nolan, L.; Fleming, G. Methods used in dentine bonding tests: An analysis of 102 investigations on bond strength. Eur. J. Prosthodont. Restor. Dent. 2008, 16, 158–165. [Google Scholar] [PubMed]
- Krifka, S.; Börzsönyi, A.; Koch, A.; Hiller, K.-A.; Schmalz, G.; Friedl, K.-H. Bond strength of adhesive systems to dentin and enamel—human vs. Bovine primary teeth in vitro. Dent. Mater. 2008, 24, 888–894. [Google Scholar]
- Nicolae, L.C.; Shelton, R.M.; Cooper, P.R.; Martin, R.A.; Palin, W.M. The effect of udma/tegdma mixtures and bioglass incorporation on the mechanical and physical properties of resin and resin-based composite materials. Conf. Pap. Sci. 2014, 2014, 6143–6147. [Google Scholar]
- Watanabe, T.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of prior acid etching on bonding durability of single-step adhesives. Oper. Dent. 2008, 33, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.A.; Pugach, M.K.; Hilton, J.F.; Watanabe, L.G.; Marshall, S.J.; Marshall, G.W., Jr. The influence of the dentin smear layer on adhesion: A self-etching primer vs. A total-etch system. Dent. Mater. 2003, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Ozel Bektas, O.; Eren, D.; Akin, E.G.; Akin, H. Evaluation of a self-adhering flowable composite in terms of micro-shear bond strength and microleakage. Acta Odontol. Scand. 2013, 71, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Margvelashvili, M.; Goracci, C.; Papacchini, F.; Ferrari, M. Bonding and sealing ability of a new self-adhering flowable composite resin in class i restorations. Clin. Oral Investig. 2013, 17, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; Peumans, M.; De Munck, J.; Lambrechts, P.; van Meerbeek, B. The role of hema in one-step self-etch adhesives. Dent. Mater. 2008, 24, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, W.; Thalacker, C.; Guggenberger, R. Siloranes in dental composites. Dent. Mater. 2005, 21, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Kleverlaan, C.J.; Feilzer, A.J. Polymerization shrinkage and contraction stress of dental resin composites. Dent. Mater. 2005, 21, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ferracane, J.L. Alternatives in polymerization contraction stress management. Crit. Rev. Oral Biol. Med. 2004, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Silikas, N.; Zhang, Z.-T.; Watts, D.C. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Silikas, N.; Zhang, Z.-T.; Watts, D.C. Diffusion and concurrent solubility of self-adhering and new resin–matrix composites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Obici, A.C.; Sinhoreti, M.A.C.; Frollini, E.; Correr Sobrinho, L.; Consani, S. Degree of conversion of z250 composite determined by fourier transform infrared spectroscopy: Comparison of techniques, storage periods and photo-activation methods. Mater. Res. 2004, 7, 605–610. [Google Scholar] [CrossRef]
- Xia, W.; Razi, M.M.; Ashley, P.; Neel, E.A.; Hofmann, M.; Young, A. Quantifying effects of interactions between polyacrylic acid and chlorhexidine in dicalcium phosphate–forming cements. J. Mater. Chem. B 2014, 2, 1673–1680. [Google Scholar] [CrossRef]
- International Organization for Standards. Dentistry—Adhesion-Notched-Edge Shear Bond Strength Test; International Organization for Standards: Geneva, Switzerland, 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaqat, S.; Aljabo, A.; Khan, M.A.; Nuba, H.B.; Bozec, L.; Ashley, P.; Young, A. Characterization of Dentine to Assess Bond Strength of Dental Composites. Materials 2015, 8, 2110-2126. https://doi.org/10.3390/ma8052110
Liaqat S, Aljabo A, Khan MA, Nuba HB, Bozec L, Ashley P, Young A. Characterization of Dentine to Assess Bond Strength of Dental Composites. Materials. 2015; 8(5):2110-2126. https://doi.org/10.3390/ma8052110
Chicago/Turabian StyleLiaqat, Saad, Anas Aljabo, Muhammad Adnan Khan, Hesham Ben Nuba, Laurent Bozec, Paul Ashley, and Anne Young. 2015. "Characterization of Dentine to Assess Bond Strength of Dental Composites" Materials 8, no. 5: 2110-2126. https://doi.org/10.3390/ma8052110





