Hierarchical Composites to Reduce N-Nitrosamines in Cigarette Smoke
Abstract
:1. Introduction
2. Results and Discussion
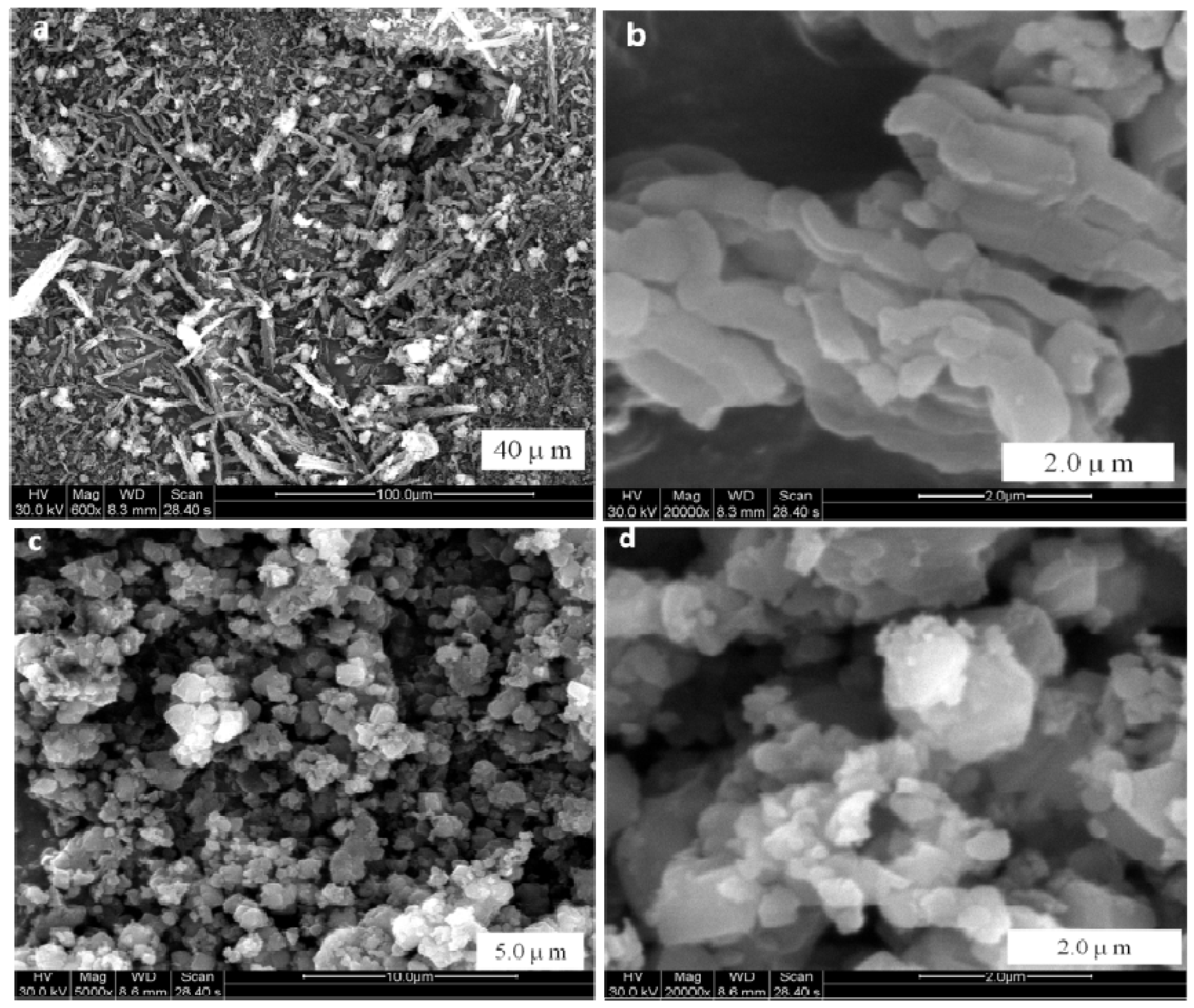
2.1. The Structure Properties of Composite Porous Materials
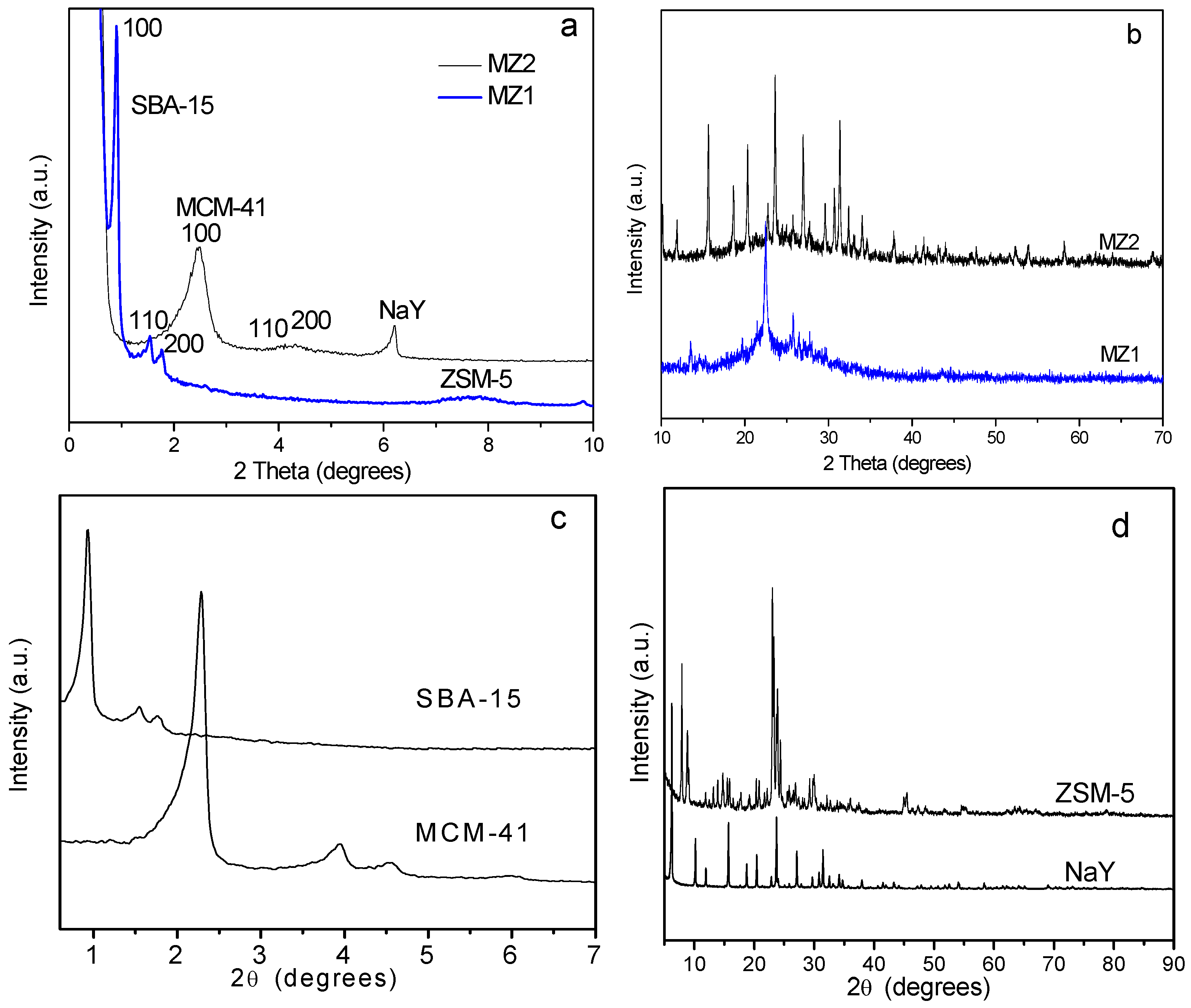
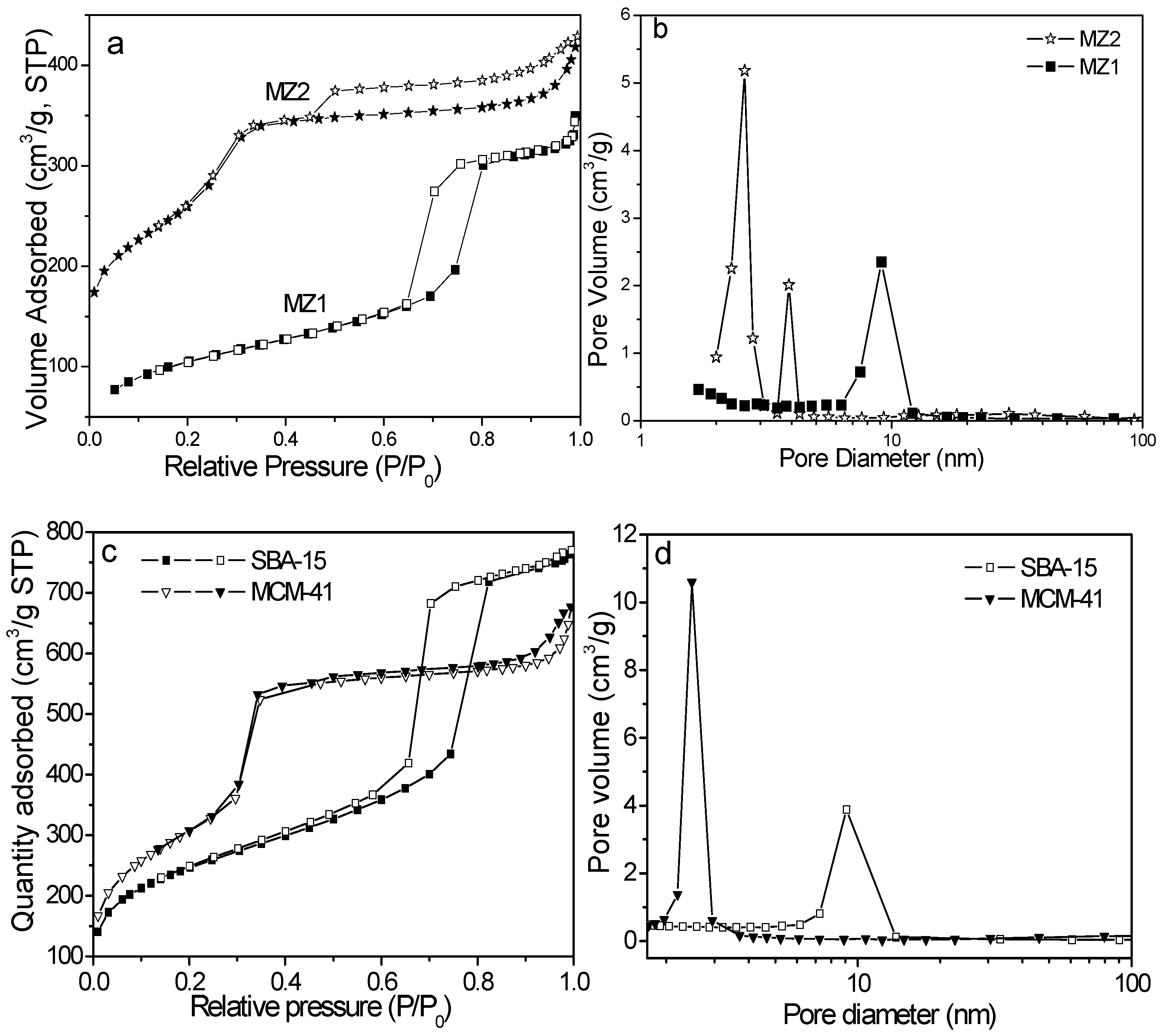
| Sample | SBET a (m2 g−1) | Vt b (cm3 g−1) | Vmic c (cm3 g−1) | Vmeso d (cm3 g−1) | DBJH e (nm) |
|---|---|---|---|---|---|
| MZ1 | 545 | 0.62 | 0.09 | 0.53 | 9.10 |
| MZ2 | 916 | 0.61 | 0.05 | 0.56 | 2.68 |
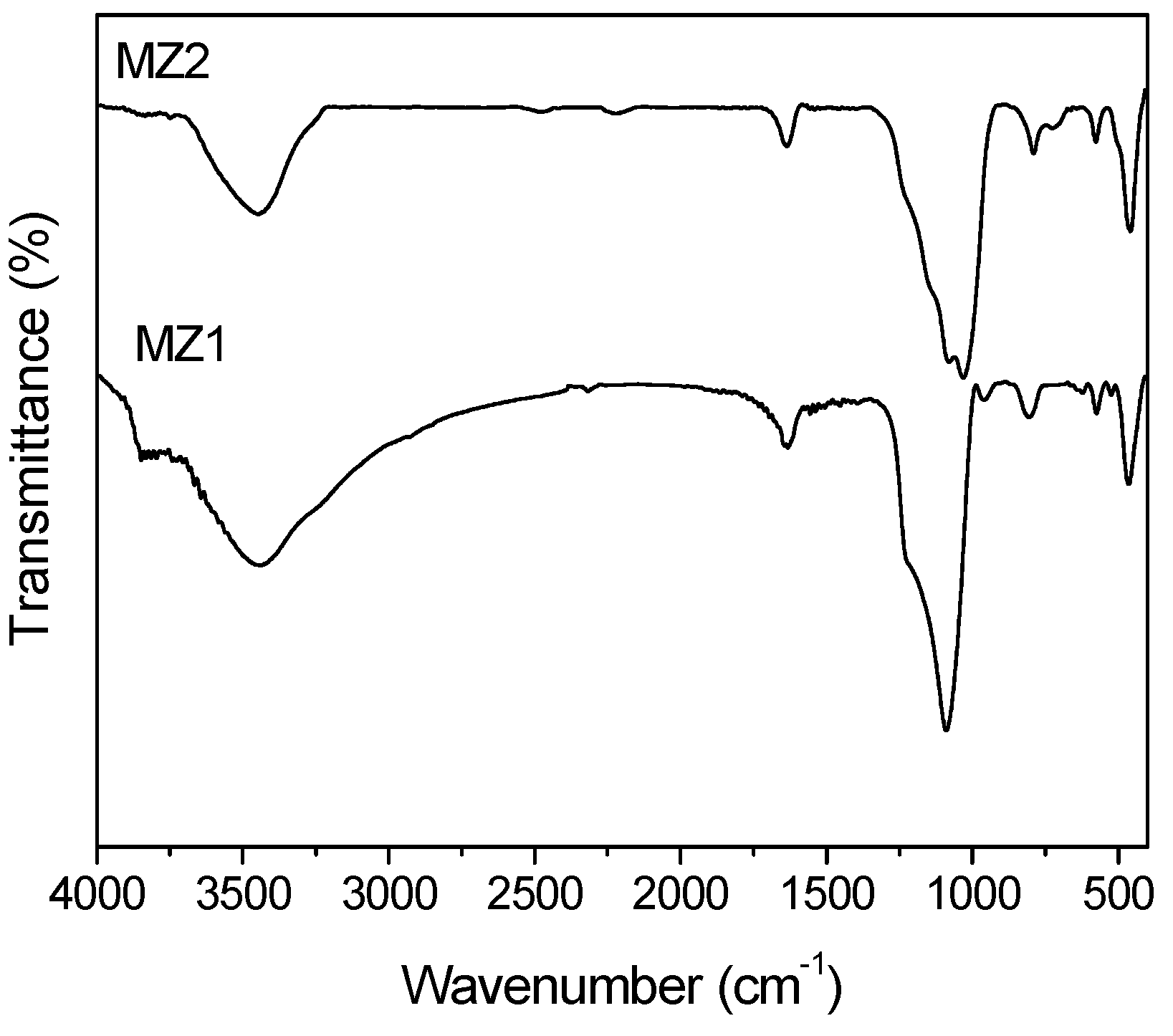
2.2. Adsorption of Volatile N-Nitrosamines from the Gaseous Phase
| Sample | NDMA | NPYR | NHMI | |||
|---|---|---|---|---|---|---|
| Passed (mmol g−1) | Adsorbed (mmol g−1) | Passed (mmol g−1) | Adsorbed (mmol g−1) | Passed (mmol g−1) | Adsorbed (mmol g−1) | |
| MZ1 | 1.67 | 1.64 | 1.13 | 1.13 | 0.74 | 0.74 |
| MZ2 | 1.63 | 1.61 | 1.13 | 1.13 | 0.74 | 0.74 |
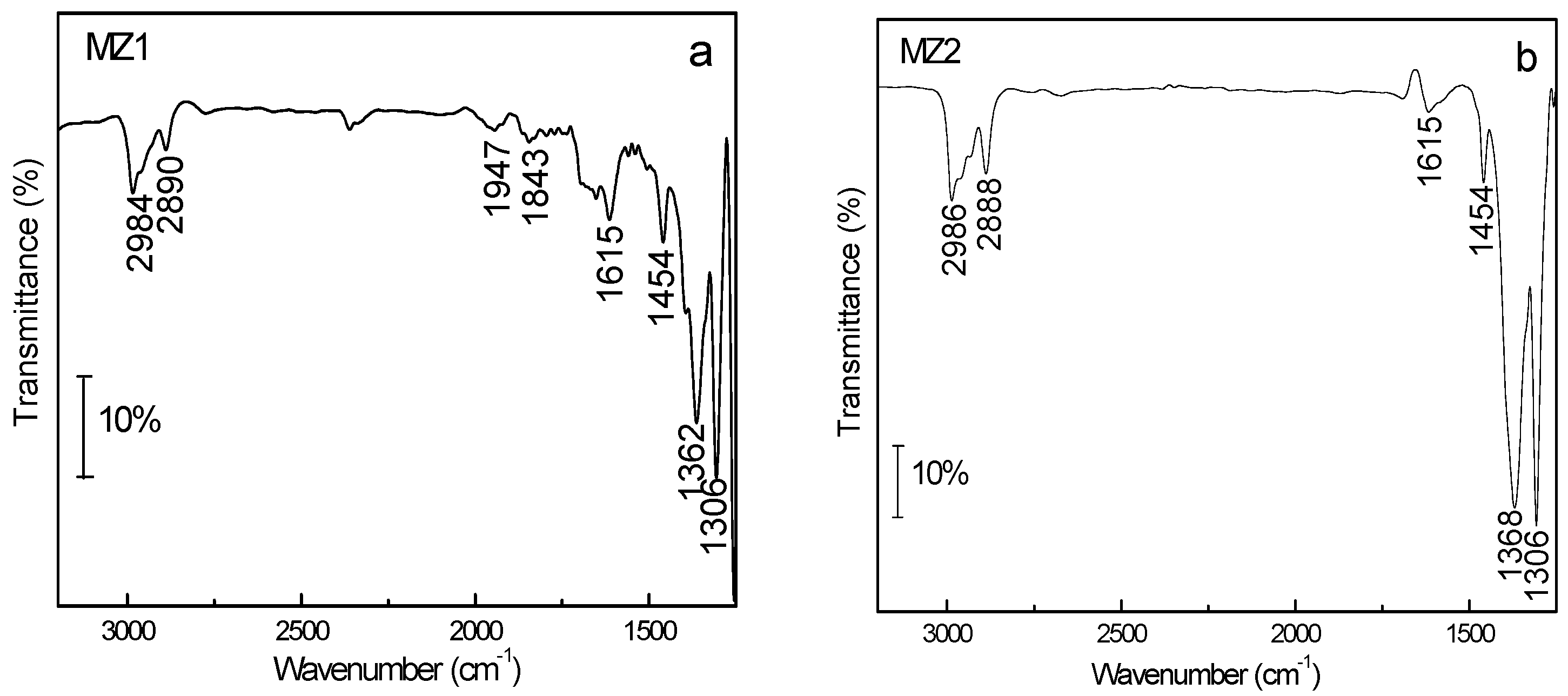
2.3. Application of Hierarchical Composites as the Filter Additive
| Sample | NFDPM (mg cig−1) | Nicotine (mg cig−1) | TPM (mg cig−1) | Water (mg cig−1) | Puff No. | CO (mg cig−1) |
|---|---|---|---|---|---|---|
| Control | 10.6 | 0.95 | 13.0 | 1.44 | 7.3 | 9.9 |
| Carbon | 9.6 | 0.92 | 12.0 | 1.45 | 7.3 | 9.6 |
| MZ1 | 9.4 | 0.89 | 11.9 | 1.56 | 7.3 | 9.9 |
| MZ2 | 8.8 | 0.79 | 10.8 | 1.24 | 7.1 | 9.9 |
| Sample | NAB | NAT | NNK | NNN | ||||
|---|---|---|---|---|---|---|---|---|
| Amount (ng cig−1) | Removed (%) | Amount (ng cig−1) | Removed (%) | Amount (ng cig−1) | Removed (%) | Amount (ng cig−1) | Removed (%) | |
| Control | 3.18 | - | 26.8 | - | 11.1 | - | 14.4 | - |
| Carbon | 3.05 | 4.1 | 28.3 | - | 10.9 | 1.8 | 15.5 | - |
| MZ1 | 2.76 | 13.2 | 24.4 | 9 | 11.6 | - | 12.2 | 15.3 |
| MZ2 | 2.19 | 31.1 | 19.3 | 28 | 7.97 | 28.2 | 9.98 | 30.7 |
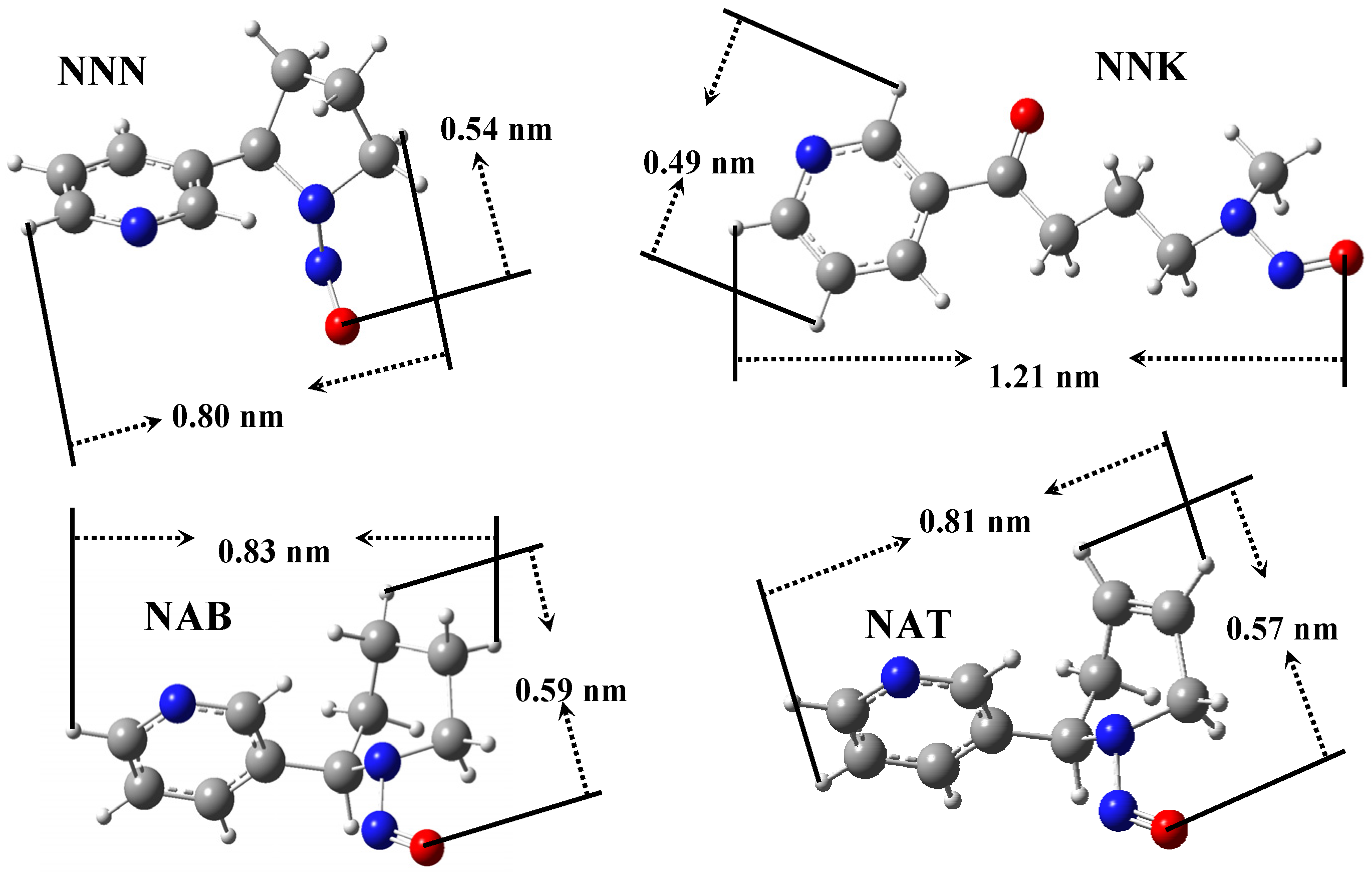
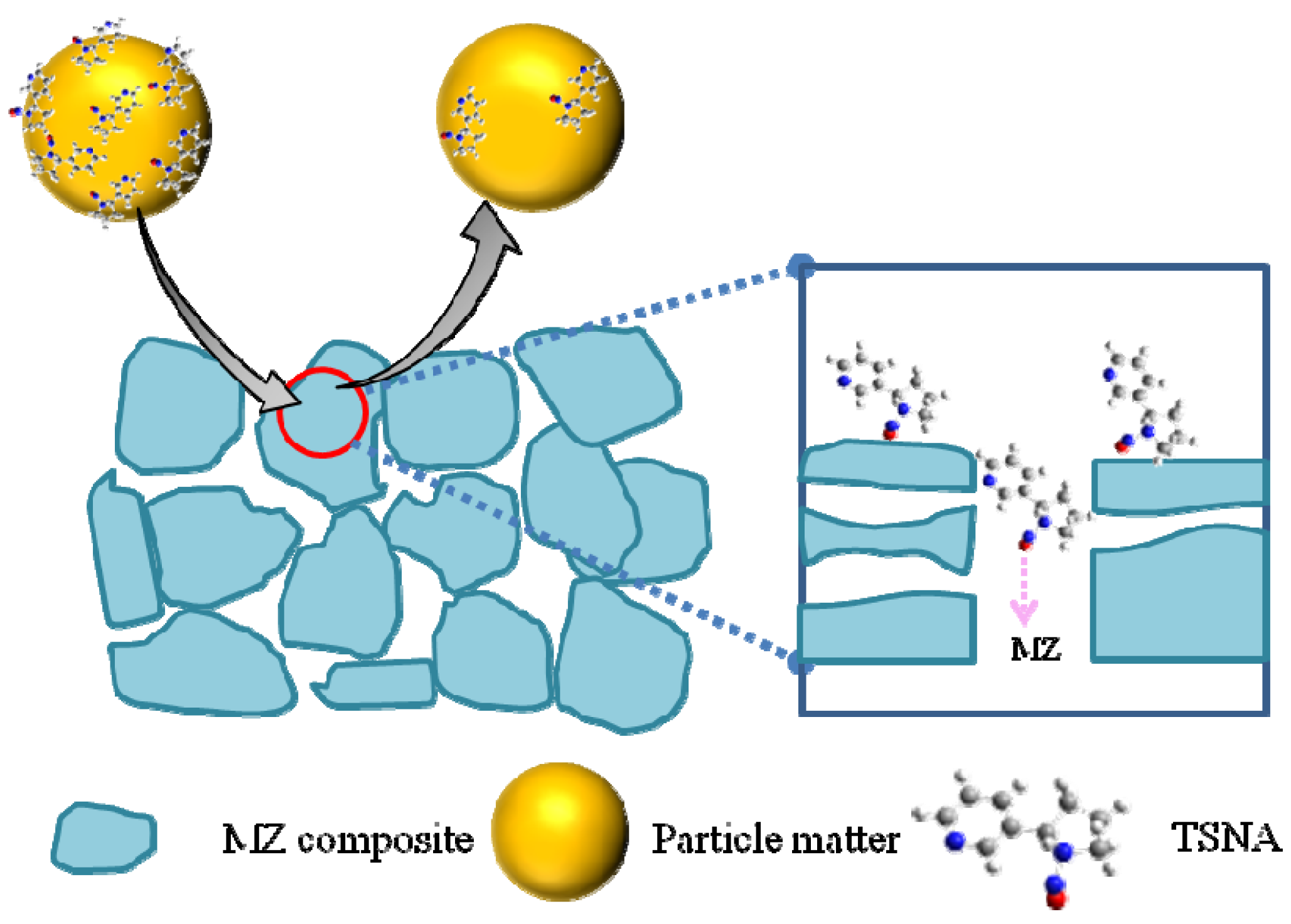
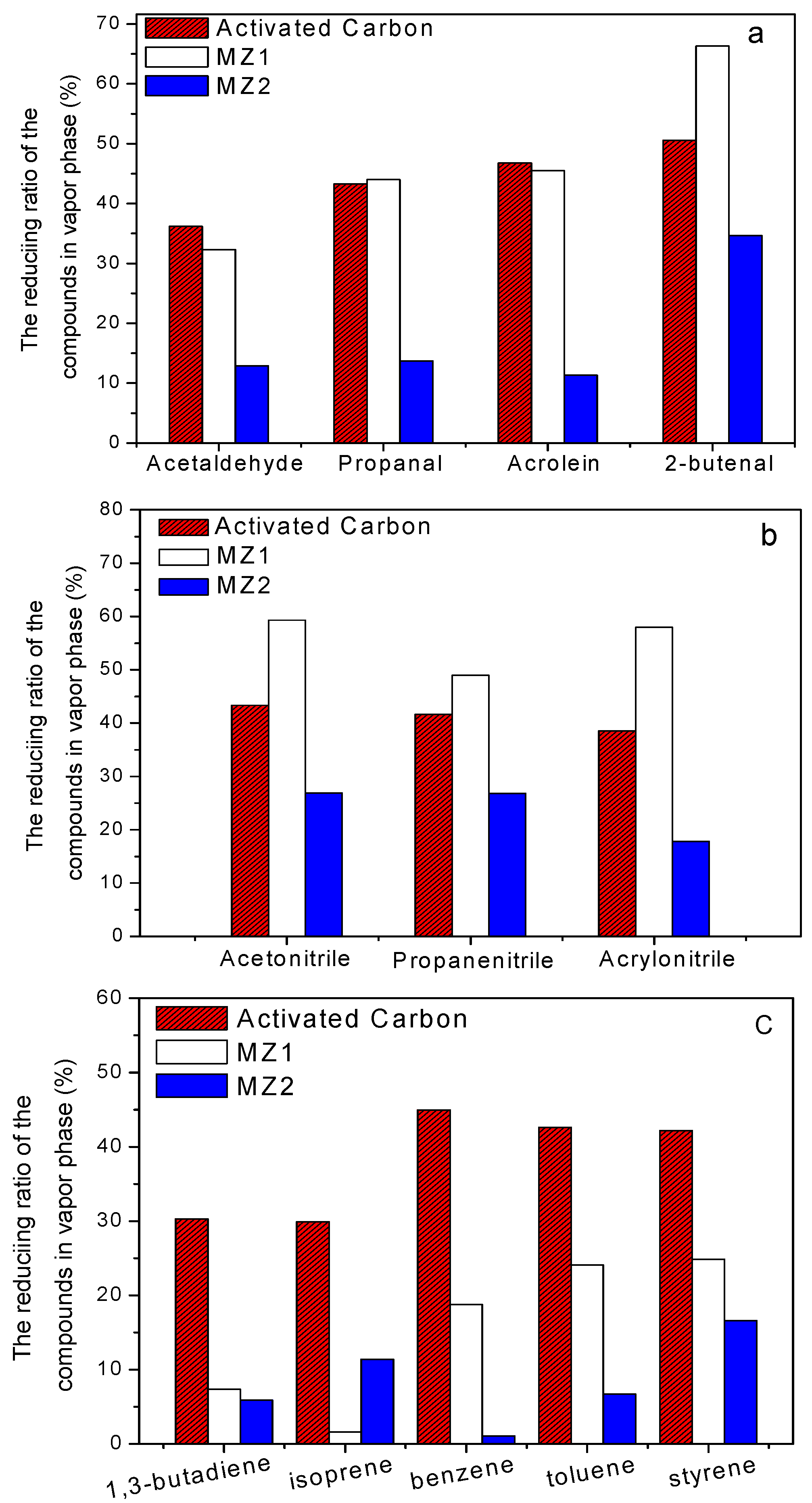
3. Experimental Section
3.1. Reagents and Sample Synthesis
3.2. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Enomoto, M.; Tierney, W.J.; Nozaki, K. Risk of human health by particulate matter as a source of air pollution—Comparison with tobacco smoking. J. Toxicol. Sci. 2008, 33, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.; Lu, A.H.; Schuth, F. The effect of carbon pore structure on the adsorption of cigarette smoke vapour phase compounds. Carbon 2009, 47, 1005–1011. [Google Scholar] [CrossRef]
- Baker, R.R. Smoke chemistry. In Tobacco: Production, Chemistry and Technology; Layten, D., Nielsen, M.T., Eds.; Blackwell Science: Oxford, UK, 1999; pp. 398–439. [Google Scholar]
- Keith, C.H. Particles size studies on tobacco smoke. Beitr. Tabakforsch. Int. 1982, 11, 123–131. [Google Scholar]
- Hayes, J.R.; Meckley, D.R.; Stavanja, M.S.; Nelson, P.R.; van Kampen, K.R.; Swauger, J.E. Effect of a flue-curing process that reduces tobacco specific nitrosamines on the tumor promotion in SENCAR mice by cigarette smoke condensate. Food Chem. Toxic. 2007, 45, 419–430. [Google Scholar] [CrossRef]
- Hecht, S.S.; Hoffmann, D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 1988, 9, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Yook, H.S.; Rhee, M.S.; Lee, C.H.; Cho, Y.J.; Byun, M.W. Application of gamma irradiation on breakdown of hazardous volatile N-nitrosamines. J. Food Sci. 2002, 67, 596–599. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Y.; Feng, G.; He, R.; Yang, W.; Li, D.; Zhou, X.; Zhu, L.; Tan, L. Addition of porphyrins to cigarette filters to reduce the levels of benzo[a]pyrene (B[a]P) and tobacco-specific N-nitrosamines (TSNA) in mainstream cigarette smoke. J. Agric. Food Chem. 2011, 59, 7172–7177. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.A.; Nielsen, P.T.; Rochelle, G.T. Decomposition of nitrosamines in CO2 capture by aqueous Piperazine or monoethanolamine. Environ. Sci. Technol. 2014, 48, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Huang, C.; Zhang, J.; Xie, W.; Xu, H.; Wei, M. Selectively reduction of tobacco specific nitrosamines in cigarette smoke by use of nanostructural titanates. Nanoscale 2013, 5, 5519–5523. [Google Scholar] [CrossRef] [PubMed]
- Atawodi, S.E.; Richter, E. Bacterial reduction of N-oxides of tobacco specific nitrosamines (TSNA). Human Exp. Toxic. 1996, 15, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Meier, W.M.; Siegmann, K. Significant reduction of carcinogenic compounds in tobacco smoke by the use of zeolite catalyst. Microporous Mesoporous Mater. 1999, 33, 307–310. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zhu, J.H.; Ma, L.L.; Liu, L. Application of zeolite in the health science: Novel additive for cigarette to remove N-nitrosamines in smoke. Stud. Surf. Sci. Catal. 2002, 142, 1489–1492. [Google Scholar]
- Xu, Y.; Jiang, Q.; Cao, Y.; Wei, Y.L.; Yun, Z.Y.; Xu, J.H.; Wang, Y.; Zhou, C.F.; Shi, L.Y.; Zhu, J.H. The synthesis of novel porous functional materials for use as nitrosamine traps. Adv. Funct. Mater. 2004, 14, 1113–1123. [Google Scholar] [CrossRef]
- Lin, W.G.; Zhou, Y.; Gu, F.N.; Zhou, S.L.; Zhu, J.H. Catalytic degradation of tobacco-specific nitrosamines by ferric zeolite. Appl. Catal. B 2013, 129, 301–308. [Google Scholar] [CrossRef]
- Lin, W.G.; Wan, M.M.; Zhou, Y.; Gu, H.C.; Zhou, S.L.; Zhu, J.H. Novel selective catalyst derived from uniform clustered NaY zeolite microsphere. J. Mater. Chem. A 2013, 1, 6849–6857. [Google Scholar] [CrossRef]
- Lin, W.G.; Zhou, Y.; Cao, Y.; Zhou, S.L.; Wan, M.M.; Wang, Y.; Zhu, J.H. Applying heterogeneous catalysis to health care: In situ elimination of tobacco-specific nitrosamines (TSNA) in smoke by molecular sieves. Catal. Today 2013, 212, 52–61. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Gao, L.; Zhuang, T.T.; Zhu, J.H. Evaluation of zeolite additive to reduce the nitrosamines level of cigarette smoke. Inter. J. Chem. Eng. 2012, 5, 9–22. [Google Scholar]
- Bombick, D.W.; Bombick, B.R.; Ayres, P.A.; Putnam, K.; Avalos, J.; Borgerding, M.F.; Doolittle, D.J. Evaluation of the genotoxic and cytotoxic potential of mainstream whole smoke and smoke condensate from a cigarette containing a novel carbon filter. Fundam. Appl. Toxicol. 1997, 39, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, J.H.; Ma, L.L.; Ji, A.; Wei, Y.L.; Shang, X.Y. Removing nitrosamines from main-stream smoke of cigarette by zeolite. Microporous Mesoporous Mater. 2003, 60, 125–138. [Google Scholar] [CrossRef]
- Shen, B.; Ma, L.L.; Zhu, J.H.; Xu, Q.H. Decomposition of N-nitrosamines over zeolites. Chem. Lett. 2000, 4, 380–381. [Google Scholar] [CrossRef]
- Gu, F.N.; Wei, F.; Yang, J.Y.; Lin, N.; Lin, W.G.; Wang, Y.; Zhu, J.H. A new strategy to synthesize hierarchical mesoporous zeolites. Chem. Mater. 2010, 22, 2442–2450. [Google Scholar] [CrossRef]
- Zhou, C.F.; Wang, Y.M.; Xu, J.H.; Zhuang, T.T.; Wang, Y.; Wu, Z.Y.; Zhu, J.H. New efficient Al-containing SBA-15 materials for removing nitrosamines in mild conditions. Stud. Surf. Sci. Catal. 2005, 156, 907–916. [Google Scholar]
- Yue, M.B.; Zhuang, T.T.; Dong, X.; Sun, L.B.; Zhu, J.H. Directly transforming as-synthesized MCM-41 to mesoporous MFI zeolite. J. Mater. Chem. 2008, 18, 2044–2050. [Google Scholar] [CrossRef]
- Ishihara, A.; Kimura, K.; Owaki, A.; Inui, K.; Hashimoto, T.; Nasu, H. Catalytic cracking of VGO by hierarchical ZSM-5 zeolite containing mesoporous silica-aluminas using a Curie point pyrolyzer. Catal. Commun. 2012, 28, 163–167. [Google Scholar] [CrossRef]
- Ishihara, A.; Kawaraya, D.; Sonthisawate, T.; Kimura, K.; Hashimoto, T.; Nasu, H. Catalytic cracking of soybean oil by hierarchical zeolite containing mesoporous silica-aluminas using a Curie point pyrolyzer. J. Mol. Catal. A Chem. 2015, 396, 310–318. [Google Scholar] [CrossRef]
- Ishihara, A.; Inui, K.; Hashimoto, T.; Nasu, H. Preparation of hierarchical b and Y zeolite-containing mesoporous silica-aluminas and their properties for catalytic cracking of n-dodecane. J. Catal. 2012, 295, 81–90. [Google Scholar] [CrossRef]
- Zhou, C.F.; Cao, Y.; Zhuang, T.T.; Huang, W.; Zhu, J.H. Capturing volatile nitrosamines in gas stream by zeolites: Why and how. J. Phys. Chem. C 2007, 111, 4347–4357. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, F.; Quo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voort, B.; Ravikovitch, P.I.; de Jong, K.P.; Benjelloun, M.; van Bavel, E.; Janssen, A.H.; Neimark, A.V.; Weckhuysen, B.M.; Vansant, E.F. A new templated ordered structure with combined micro- and mesopores and internal silica nanocapsules. J. Phys. Chem. B 2002, 106, 5873–5877. [Google Scholar] [CrossRef]
- Laha, S.C.; Mukherjee, P.; Sainkar, S.R.; Kumar, R. Cerium containing MCM-41-type mesoporous materials and their acidic and redox catalytic properties. J. Catal. 2002, 207, 213–223. [Google Scholar] [CrossRef]
- Vidya, K.; Dapurkar, S.E.; Selvam, P.; Badamali, S.K.; Kumar, D.; Gupta, N.M. Encapsulation, characterization and catalytic properties of uranyl ions in mesoporous molecular sieves. J. Mol. Catal. A: Chem. 2002, 181, 91–97. [Google Scholar] [CrossRef]
- Orlovi, A.M.; Janakovi, D.T.; Drmani, S.A.; Marinkovi, Z.; Skala, D.U. Alumina/silica aerogel with zinc chloride as an alkylation catalyst. J. Serb. Chem. Soc. 2001, 66, 685–695. [Google Scholar]
- Zhou, C.F.; Wang, Y.M.; Cao, Y.; Zhuang, T.T.; Huang, W.; Chun, Y.; Zhu, J.H. Solvent-free surface functionalizing SBA-15 as versatile trap of nitrosamines. J. Mater. Chem. 2006, 16, 1520–1528. [Google Scholar] [CrossRef]
- Zhou, C.F.; Yun, Z.Y.; Xu, Y.; Wang, Y.M.; Chen, J.; Zhu, J.H. Adsorption and room temperature degradation of N-nitrosodiphenylamine on zeolites. New J. Chem. 2004, 28, 807–814. [Google Scholar] [CrossRef]
- Kamaloo, E.; Deskins, N.A.; Kazantzis, N.; Thompson, R.W. Molecular modeling of adsorbed NDMA and water in MFI zeolites. Microp. Mesop. Mater. 2013, 182, 198–206. [Google Scholar] [CrossRef]
- Roohia, H.; Akbarib, F. Adsorption of parent nitrosamine on the nanocrystaline H-zeolite: A theoretical study. Appl. Surf. Sci. 2010, 256, 7575–7582. [Google Scholar] [CrossRef]
- Yun, Z.Y.; Zhu, J.H.; Xu, Y.; Xu, J.H.; Wu, Z.Y.; Wei, Y.L.; Zhou, Z.P. An in situ FTIR investigation on the adsorption of nitrosamines in zeolites. Microporous Mesoporous Mater. 2004, 72, 127–135. [Google Scholar] [CrossRef]
- Gao, L.; Cao, Y.; Zhou, S.L.; Zhuang, T.T.; Wang, Y.; Zhu, J.H. Eliminating carcinogenic pollutants in environment: Reducing the tobacco specific nitrosamines level of smoke by zeolite-like calcosilicate. J. Hazard. Mater. 2009, 169, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, L.; Gu, F.N.; Yang, J.Y.; Yang, J.; Wei, F.; Wang, Y.; Zhu, J.H. Novel functional mesoporous material derived from 3D net-linked SBA-15. Chem. Euro. J. 2009, 15, 6748–6757. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, W.G.; Wan, M.M.; Yang, J.; Zhu, J.H. Novel selective adsorbent derived from hierarchical rockery-like MCM-41 monolith. J. Mater. Chem. 2012, 22, 23633–23641. [Google Scholar] [CrossRef]
- Sun, X.D.; Lin, W.G.; Wang, L.J.; Zhou, B.; Lv, X.L.; Wang, Y.; Zheng, S.J.; Wang, W.M.; Tong, Y.G.; Zhu, J.H. Liquid adsorption of tobacco specific N-nitrosamines by zeolite and activated carbon. Microporous Mesoporous Mater. 2014, 200, 260–268. [Google Scholar] [CrossRef]
- Li, Y.Y.; Han, K.K.; Lin, W.G.; Wan, M.M.; Wang, Y.; Zhu, J.H. Fabrication of a new MgO/C sorbent for CO2 capture at elevated temperature. J. Mater. Chem. A 2013, 1, 12919–12925. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.Y.; Cao, Y.; Yue, M.B.; Yang, J.; Zhu, J.H. Hierarchical Composites to Reduce N-Nitrosamines in Cigarette Smoke. Materials 2015, 8, 1325-1340. https://doi.org/10.3390/ma8031325
Li YY, Cao Y, Yue MB, Yang J, Zhu JH. Hierarchical Composites to Reduce N-Nitrosamines in Cigarette Smoke. Materials. 2015; 8(3):1325-1340. https://doi.org/10.3390/ma8031325
Chicago/Turabian StyleLi, Yan Yan, Yi Cao, Ming Bo Yue, Jing Yang, and Jian Hua Zhu. 2015. "Hierarchical Composites to Reduce N-Nitrosamines in Cigarette Smoke" Materials 8, no. 3: 1325-1340. https://doi.org/10.3390/ma8031325