Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
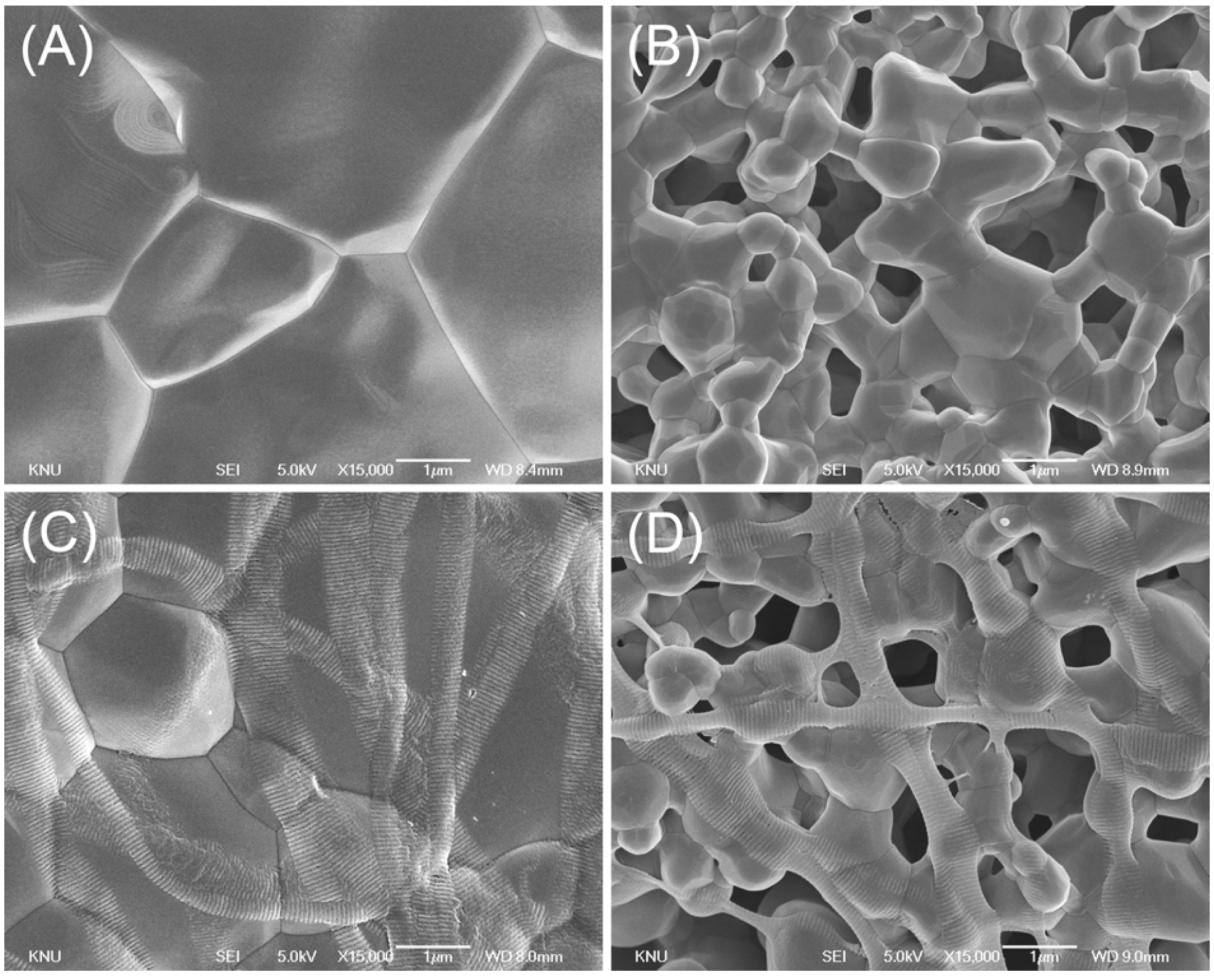
2.1. Surface Characterization
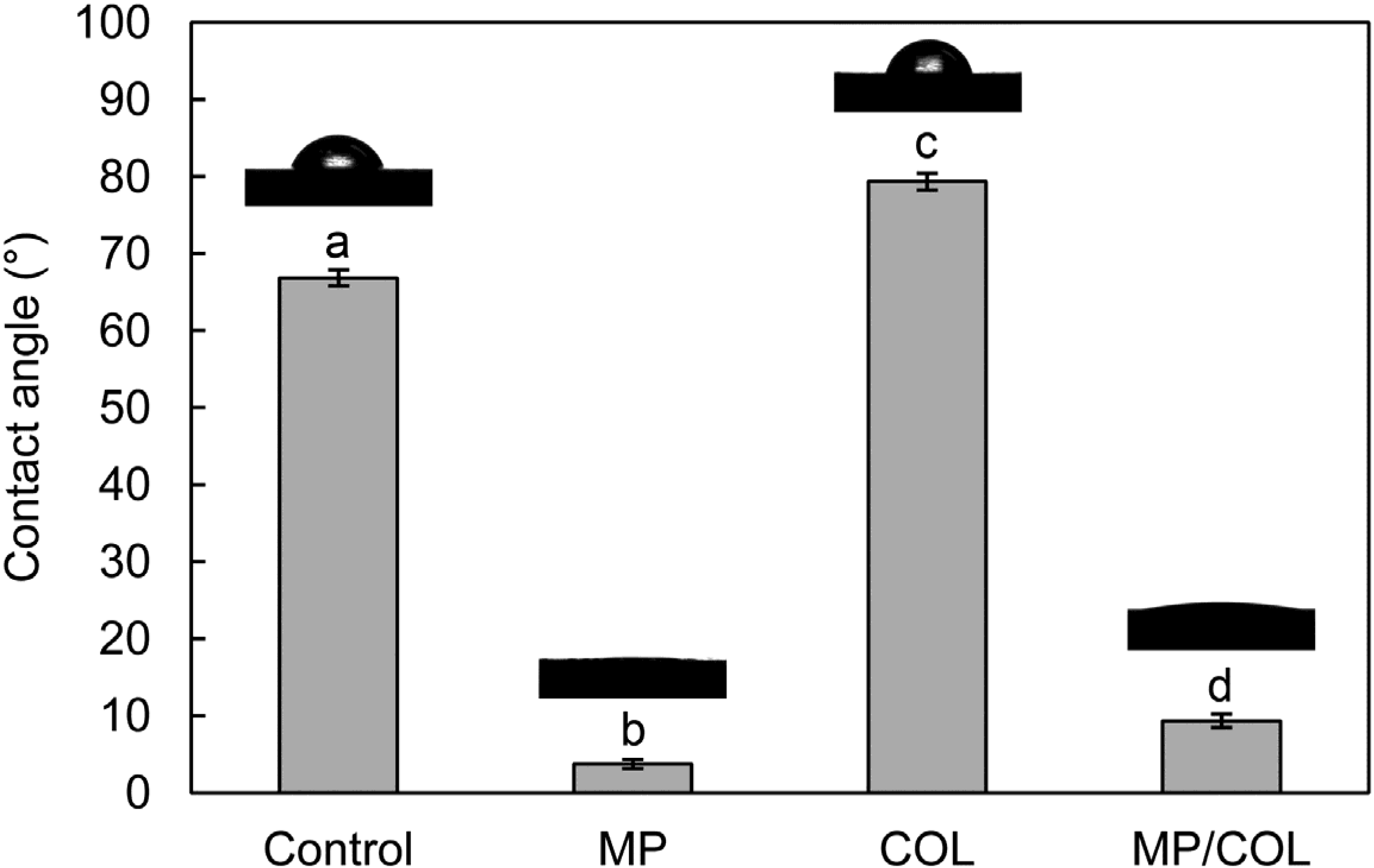
2.2. Surface Wettability
2.3. Quantitative Examination of the Type I Collagen Coatings

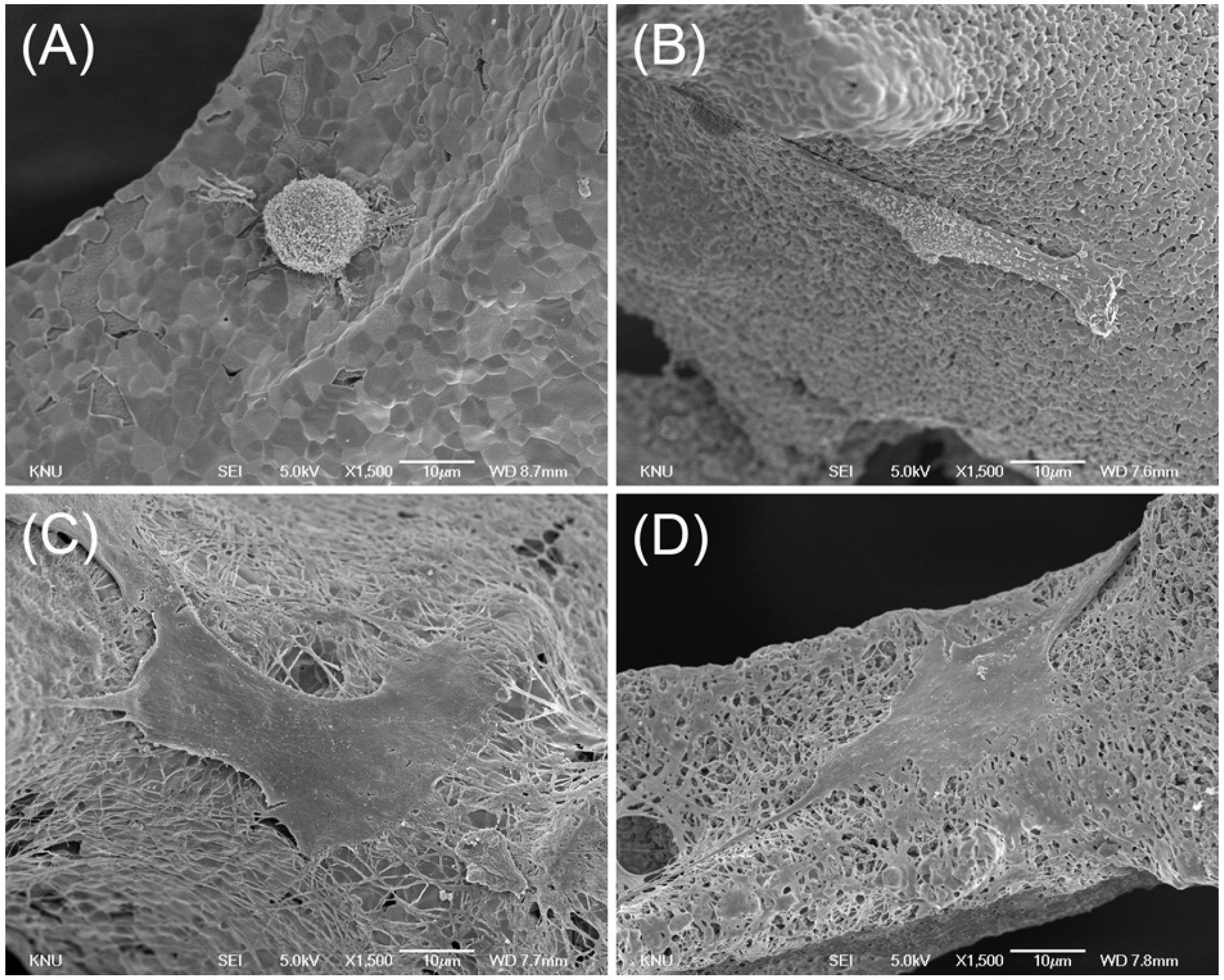
2.4. Cell Morphology

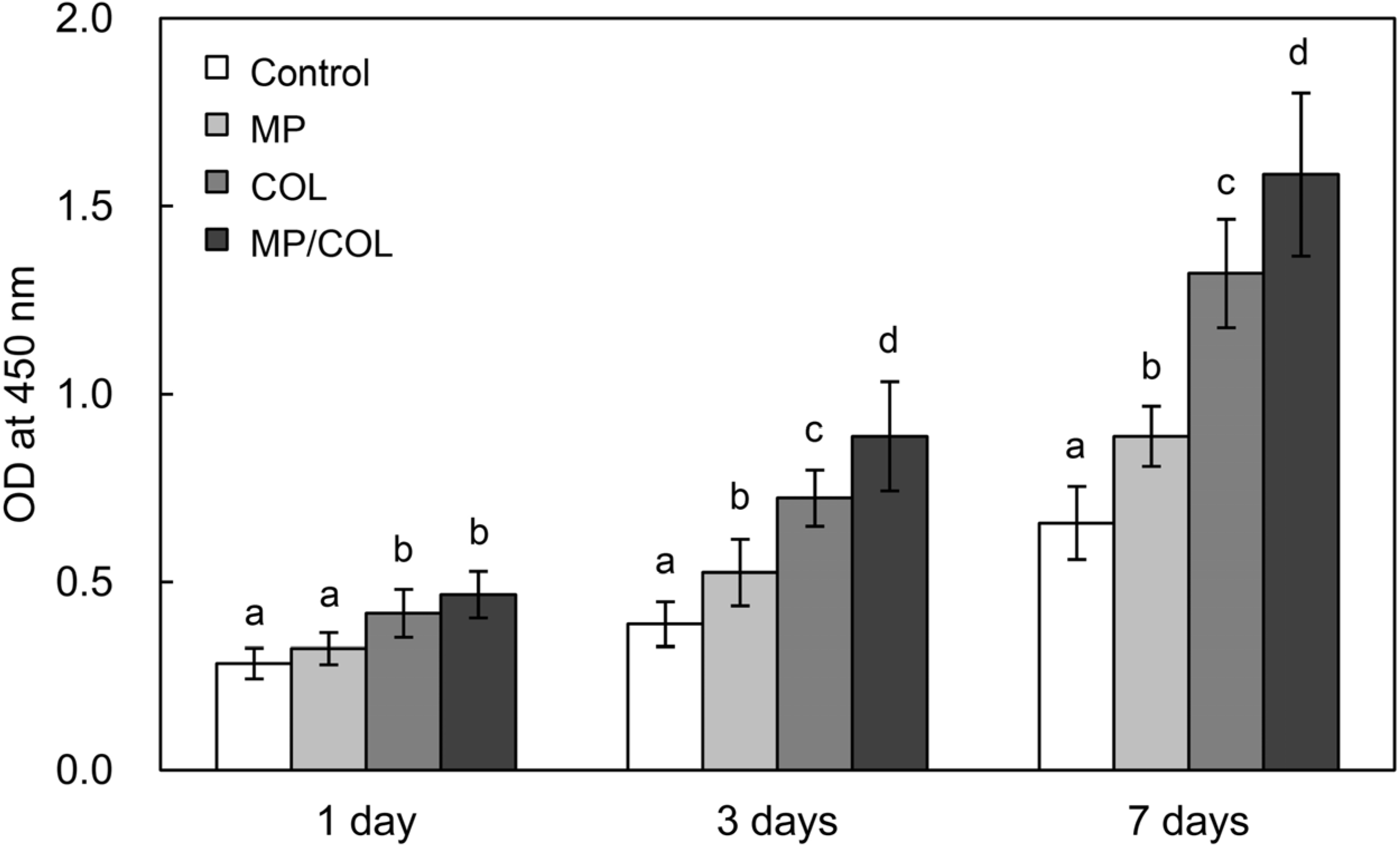
2.5. Cell Proliferation
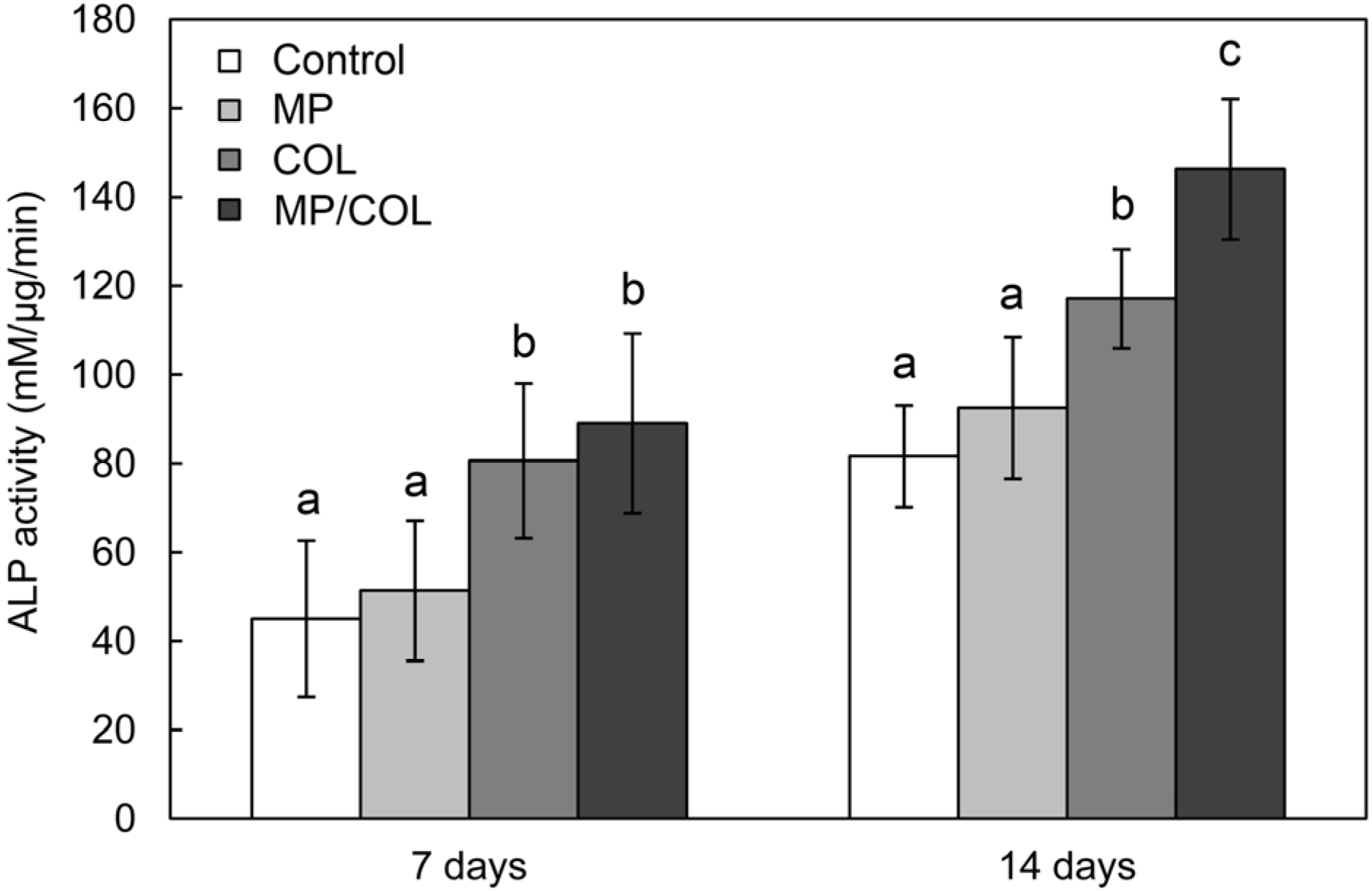
2.6. ALP Activity
3. Experimental Section
3.1. Preparation of the Unmodified Scaffolds
3.2. Surface Modification of the Scaffolds
3.3. SEM Examination
3.4. Surface Wettability
3.5. Quantitative Examination of Collagen Coating
3.6. Cell Culture
3.7. Cell Morphology
3.8. Cell Proliferation
3.9. ALP Activity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Van Lieshout, E.M.M.; van Kralingen, G.H.; El-Massoudi, Y.; Weinans, H.; Patka, P. Microstructure and biomechanical characteristics of bone substitutes for trauma and orthopaedic surgery. Bmc Musculoskel. Dis. 2011, 12. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G.; Einhorn, T.A.; Koval, K. Bone, grafts and bone graft substitutes in orthopedic trauma surgery—A critical analysis. J. Bone Joint Surg. Am. 2007, 89A, 649–658. [Google Scholar]
- Li, S.H.; de Wijn, J.R.; Li, J.P.; Layrolle, P.; de Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 6, 22–33. [Google Scholar]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Myoui, A.; Takaoka, K. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials 2005, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bignon, A.; Chouteau, J.; Chevalier, J. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J. Mater. Sci. Mater. Med. 2003, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med 2005, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Yuan, H.P.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Daculsi, G.; Saffarzadeh, A. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 2005, 36, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Legeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; Legeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Best, S.M.; Tanner, K.E.; Bonfield, W.; Revell, P.A. Mediation of bone ingrowth in porous hydroxyapatite bone graft substitutes. J. Biomed. Mater. Res. A 2004, 68A, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bartolo, P.J. Collagen surface modified poly (ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Fu, J.; Yang, S.; Hou, R.; Suo, J. Macroporous biphasic calcium phosphate scaffolds reinforced by poly-L-lactic acid/hydroxyapatite nanocomposite coatings forbone regeneration. Biochem. Eng. J. 2015, 98, 29–37. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nakayama, K.; Matsumoto, T. Differentiation and cell surface expression of transforming growth factor-beta receptors are regulated by interaction with matrix collagen in murine osteoblastic cells. J. Biol. Chem. 1996, 271, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 2000, 84, 207–213. [Google Scholar] [CrossRef]
- Roehlecke, C.; Witt, M.; Kasper, M. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs 2001, 168, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; Garcia, A.J. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J. Biomed. Mater. Res. A 2003, 65A, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Bataillon-Linez, P.; Huang, P. Surface analyses of micro-arc oxidized and hydrothermally treated titanium and effect on osteoblast behavior. J. Biomed. Mater. Res. A 2004, 68A, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pastrama, M.I.; Ding, Y.; Zheng, K.; Hellmich, C.; Boccaccini, A.R. Ultrasonic elasticity determination of 45S5 Bioglass®-based scaffolds: Influence of polymer coating and crosslinking treatment. J. Mech. Behav. Biomed. Mater. 2014, 40, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hum, J.; Luczynski, K.W.; Nooeaid, P.; Newby, P.; Lahayne, O.; Hellmich, C.; Boccaccini, A.R. Stiffness improvement of 45S5 Bioglass®-based scaffolds through natural and synthetic biopolymer coatings: An ultrasonic study. Strain 2013, 49, 431–439. [Google Scholar]
- Czenek, A.; Blanchard, R.; Dejaco, A.; Sigurjonsson, O.E.; Orlygsson, G.; Gargiulo, P. Quantitative intravoxel analysis of μCT-scanned resorbing ceramic biomaterials—Perspectives for computer-aided biomaterial design. J. Mater. Res. 2014, 29, 2757–2772. [Google Scholar] [CrossRef]
- Fritsch, A.; Dormieux, L.; Hellmich, C.; Sanahuja, J. Mechanical behavior of hydroxyapatite biomaterials: An experimentally validated micromechanical model for elasticity and strength. J. Biomed. Mater. Res. A 2009, 88A, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, A.; Hellmich, C.; Young, P. Micromechanics-derived scaling relations for poroelasticity and strength of brittle porous polycrystals. J. Appl. Mech. 2013, 80, 020905. [Google Scholar]
- Ye, X.Y.; Cai, S.; Xu, G.H.; Dou, Y.; Hu, H.T.; Ye, X.J. Preparation and in vitro evaluation of mesoporous hydroxyapatite coated β-TCP porous scaffolds. Mater. Sci. Eng. C 2013, 33, 5001–5007. [Google Scholar] [CrossRef]
- Muller, R.; Abke, J.; Schnell, E. Influence of surface pretreatment of titanium- and cobalt-based biomaterials on covalent immobilization of fibrillar collagen. Biomaterials 2006, 27, 4059–4068. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Hwang, Y.S.; Lee, J.E.; Han, C.D.; Park, J.C. Behavior of osteoblasts on a type I atelocollagen grafted ozone oxidized poly L-lactic acid membrane. Biomaterials 2001, 22, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: Effect of initial cell phenotype. Biomaterials 2005, 26, 6208–6216. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. A 2005, 72A, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ozawa, S.; Shih, J.H. Biomechanical evaluation of osseous implants having different surface topographies in rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ko, J.S.; Kim, H.M. The role of cell signaling defects on the proliferation of osteoblasts on the calcium phosphate apatite thin film. Biomaterials 2006, 27, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-H.; You, C.; Kim, K.-H. Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering. Materials 2015, 8, 1150-1161. https://doi.org/10.3390/ma8031150
Lee M-H, You C, Kim K-H. Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering. Materials. 2015; 8(3):1150-1161. https://doi.org/10.3390/ma8031150
Chicago/Turabian StyleLee, Mun-Hwan, Changkook You, and Kyo-Han Kim. 2015. "Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering" Materials 8, no. 3: 1150-1161. https://doi.org/10.3390/ma8031150





