Properties of Chemically Combusted Calcium Carbide Residue and Its Influence on Cement Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Ingredient | Ca(OH)2 | CaCO3 | SiO2 | Fe2O3 | Al2O3 | LOI (loss on ignition) |
|---|---|---|---|---|---|---|
| Content (%) | 92 | 2.9 | 1.32 | 0.94 | 0.06 | 1.02 |
| Physical Properties | Specific Gravity | Retained on Sieve No. 325 (%) | BET * Surface Area (m2/g) | Median Particle Size, d50 (μm) |
|---|---|---|---|---|
| CCR | 2.92 | 3.50 | 7.05 | 9.05 |
| Chemical Composition (%) | OPC | SF |
|---|---|---|
| SiO2 | 22.52 | 94.00 |
| Al2O3 | 5.80 | 0.21 |
| Fe2O3 | 3.52 | 0.09 |
| SO3 | 2.54 | - |
| CaO | 62.08 | 0.12 |
| MgO | 1.55 | 0.33 |
| Na2O | 0.05 | - |
| K2O | 0.56 | 0.38 |
| LOI | 0.94 | 1.50 |
| Physical property | - | - |
| Specific gravity | 3.12 | 2.80 |
| Retained on sieve No. 325 (%) | 4.70 | 1.00 |
| BET surface area (m2/g) | 2.70 | 21.08 |
| Median particle size, d50 (μm) | 12.00 | 3.11 |
2.2. Synthesis of RCP
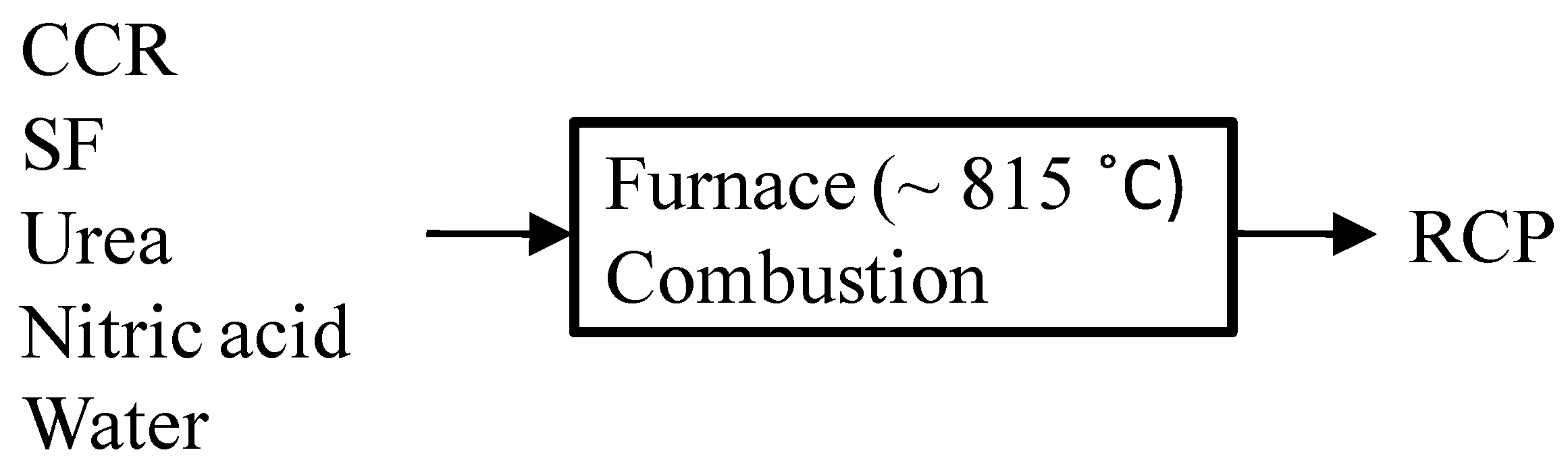
| Ingredients | CCR | SF | Urea | Nitric Acid | Water |
|---|---|---|---|---|---|
| Content (%) | 10.92 | 4.16 | 47.21 | 23.33 | 14.38 |
2.3. Mix Proportion
| Specimens | Mass (g) | |||||
|---|---|---|---|---|---|---|
| OPC | RCP | SF | SP | Sand | Water | |
| OPC | 100 | 0 | 0 | 1.6 | 100 | 21 |
| OPC–SF | 100 | 0 | 10 | 1.6 | 100 | 21 |
| OPC95/RCP5–SF | 95 | 5 | 10 | 1.6 | 100 | 21 |
2.4. Testing
3. Results and Discussion
3.1. Characterization of Raw Materials

3.2. Characterization of RCP Powder
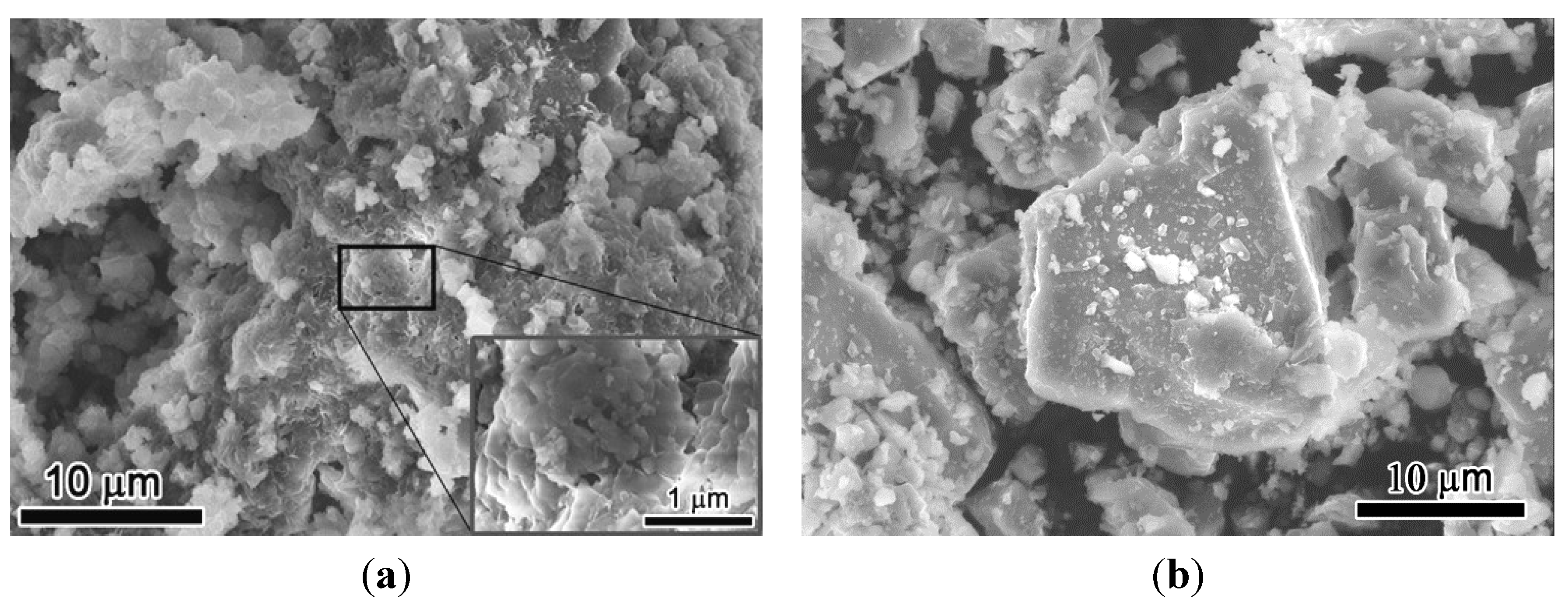

3.3. Mineralogical Analysis of RCP

| Composition | 2CaO·SiO2 | Ca(OH)2 | CaO | SiO2 | Ca3Si3O8(OH)2 |
|---|---|---|---|---|---|
| Fraction (%) | 40.6 | 34.2 | 9.3 | 2.3 | 13.6 |
3.4. Hydration Reactivity of RCP Paste
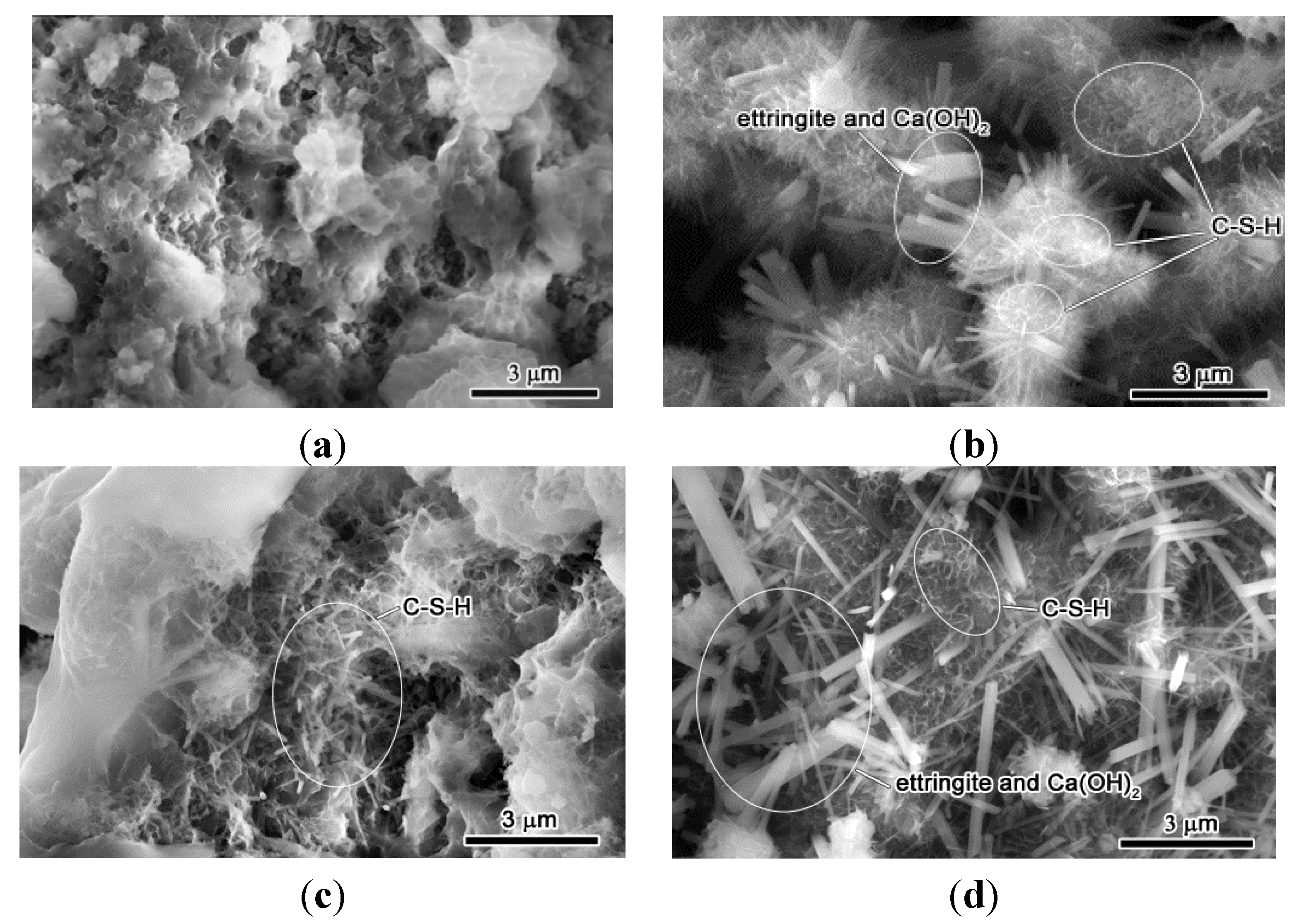
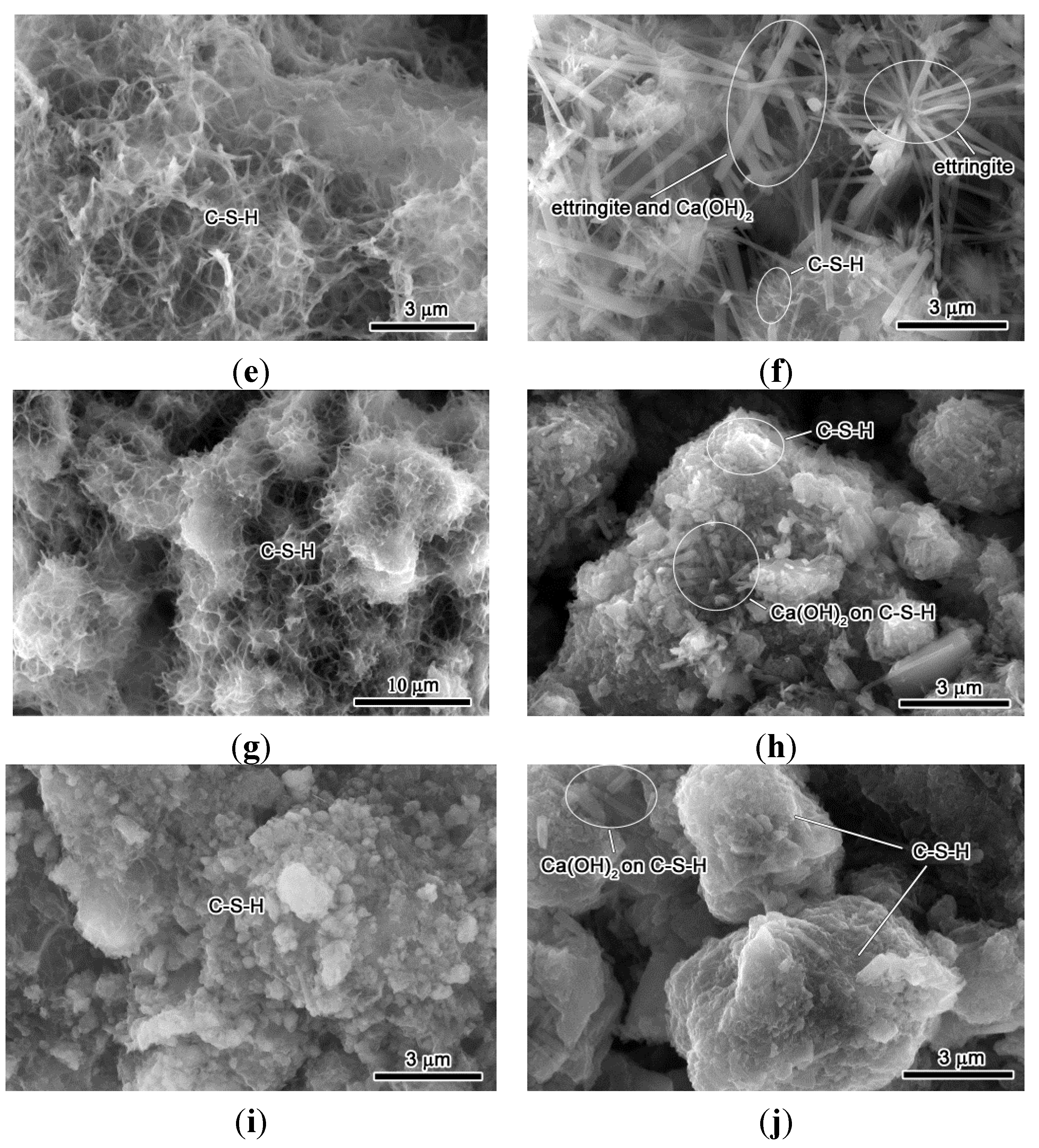
3.5. Initial and Final Setting Time
| Specimen | Initial Setting Time (h) | Final Setting Time (h) |
|---|---|---|
| OPC | 2.45 | 3.58 |
| OPC–SF | 4.20 | 5.28 |
| OPC95/RCP5–SF | 5.13 | 6.67 |
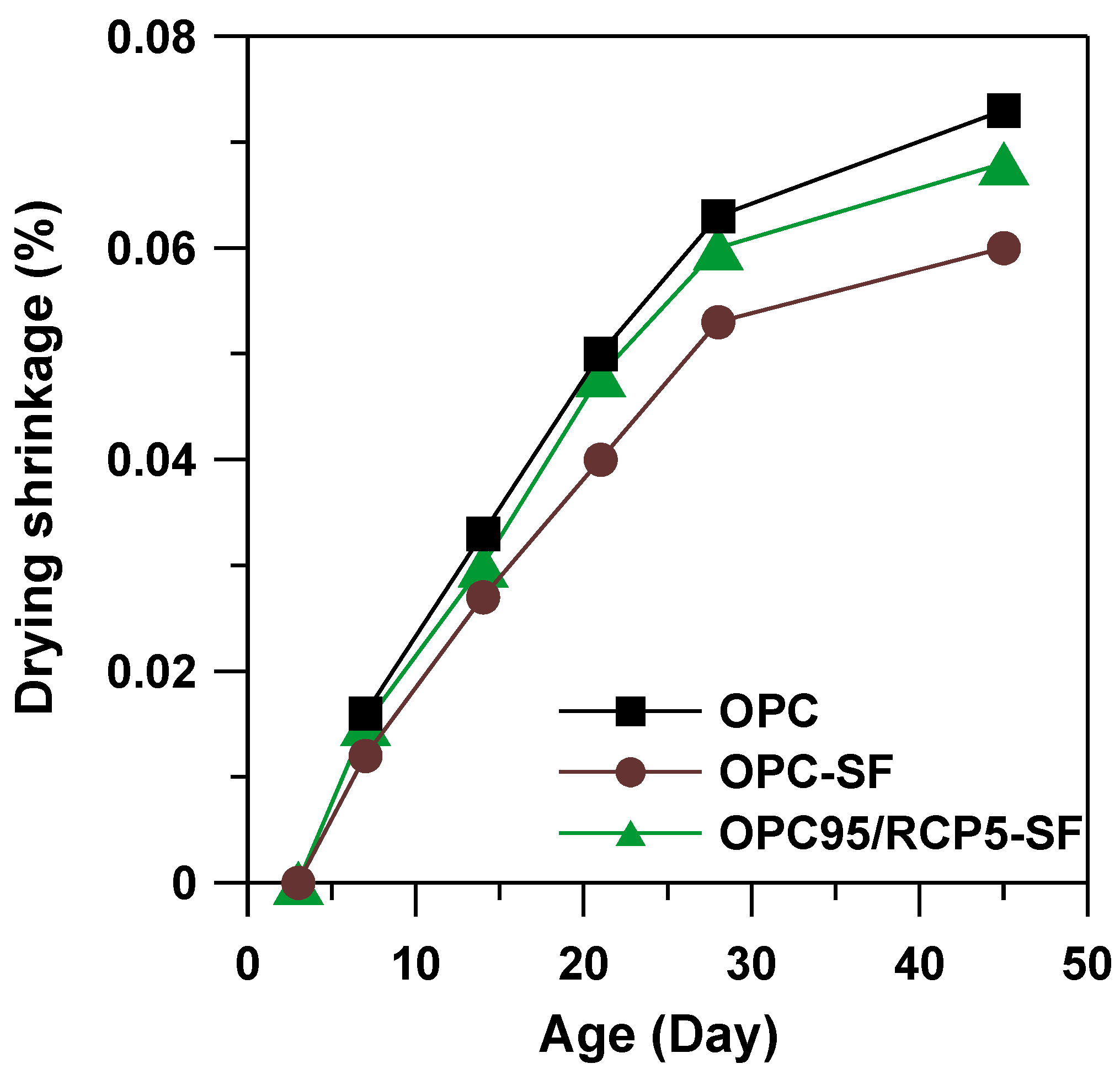
3.6. Drying Shrinkage
3.7. Compressive Strength

4. Conclusions
- In comparison to OPC, RCP is synthesized at quite a low temperature (approximately 800 °C). This shows the potential to reduce the energy consumption. Moreover, it would increase the sustainable value of waste material instead of sending them to landfills.
- The BET surface area of the RCP was quite similar to that of OPC. This indicates that a more porous structure exists in RCP than in OPC, which increases the surface area of RCP. RCP consisted of 40.6% 2CaO·SiO2, 34.2% residual Ca(OH)2, and 13.6% Ca3Si3O8(OH)2. These components can potentially improve the content of hydraulic reactive constituents in cement.
- From SEM micrographs and in comparison to OPC mix, RCP was found to retard the hydration reaction due to the slow-reacting nature of 2CaO·SiO2. The results are consistent with the delayed initial and final setting time results.
- The drying shrinkage of OPC95/RCP5–SF mix reduced by 7% in comparison to the OPC mortar at the age of 45 days. Moreover, at the age of 45 days, the compressive strength of OPC95/RCP5–SF mortar mix was found to be 111 MPa, which is higher than that of OPC–SF mortar by 8% and OPC mortar by 10%, respectively. An optimization of the OPC replacement by RCP will be considered in our future research so as to enhance the compressive strength of the cementitious system.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hardjito, D.; Antoni; Wibowo, G.M.; Christianto, D. Pozzolanic activity assessment of LUSI (Lumpur SIdoarjo) mud in semi high volume pozzolanic mortar. Materials 2012, 5, 1654–1660. [Google Scholar] [CrossRef]
- Kocak, Y.; Nas, S. The effect of using fly ash on the strength and hydration characteristics of blended cements. Constr. Build. Mater. 2014, 46, 106–111. [Google Scholar]
- Baldino, N.; Gabriele, D.; Lupi, F.R.; Seta, L.; Zinno, R. Rheological behaviour of fresh cement pastes: Influence of synthetic zeolites, limestone and silica fume. Cem. Concr. Res. 2014, 63, 38–45. [Google Scholar] [CrossRef]
- Ouyang, D.; Xu, W.T.; Lo, T.Y.; Sham, J.F.C. Increasing mortar strength with the use of activated kaolin by-products from paper industry. Constr. Build. Mater. 2011, 25, 1537–1545. [Google Scholar] [CrossRef]
- Xu, W.T.; Lo, T.Y.; Memon, S.A. Microstructure and reactivity of rich husk ash. Constr. Build. Mater. 2012, 29, 541–547. [Google Scholar] [CrossRef]
- Makaratat, N.; Jaturapitakkul, C.; Namarak, C.; Sata, V. Effects of binder and CaCl2 contents on the strength of calcium carbide residue-fly ash concrete. Cem. Concr. Compos. 2011, 33, 436–443. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, B.; Zhou, W.J.; Hu, S.Y.; Chen, D.J.; Griffy-Brown, C. CO2 emissions in calcium carbide industry: An analysis of China’s mitigation potential. Int. J. Greenh. Gas Control 2011, 5, 1240–1249. [Google Scholar] [CrossRef]
- Wang, Y.L.; Dong, S.J.; Liu, L.L.; Cui, S.P. Using calcium carbide slag as one of calcium-containing raw materials to produce cement clinker. Energy Environ. Mater. 2013, 171, 743–744. [Google Scholar]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P. Microstructure of calcium carbide residue-ground fly ash paste. J. Mater. Civ. Eng. 2011, 23, 298–304. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P. Calcium carbide residue-ground fly ash mixture as a new material to encapsulate zinc. In Proceedings of the 2009 International Conference on Chemical, Biological and Environmental Engineering, Singapore, 9–11 October 2009.
- Makaratat, N.; Jaturapitakkul, C.; Laosamathikul, T. Effects of calcium carbide residue-fly ash binder on mechanical properties of concrete. J. Mater. Civ. Eng. 2010, 22, 1164–1170. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A.; Cholaphatsorn, A. Strength development in silty clay stabilized with calcium carbide residue and fly ash. Soils Found. 2013, 53, 477–486. [Google Scholar] [CrossRef]
- Amnadnua, K.; Tangchirapat, W.; Jaturapitakkul, C. Strength, water permeability, and heat evolution of high strength concrete made from the mixture of calcium carbide residue and fly ash. Mater. Des. 2013, 51, 894–901. [Google Scholar] [CrossRef]
- Jaturapitakkul, C.; Roongreung, B. Cementing material from calcium carbide residue-rice husk ash. J. Mater. Civ. Eng. 2003, 15, 470–475. [Google Scholar] [CrossRef]
- Krammart, P.; Tangtermsirikul, S. Properties of cement made by partially replacing cement raw materials with municipal solid waste ashes and calcium carbide waste. Constr. Build. Mater. 2004, 18, 579–583. [Google Scholar] [CrossRef]
- Schorr, R.J.; Sengupta, S.; Helferich, R.L.; Gordon, G.M.; Rautaray, D. Cement and Methods of Preparing Cement. Available online: http://www.freepatentsonline.com/y2010/0175588.html (accessed on 15 November 2014).
- Dham, M.; Rushing, T.S.; Helferich, R.; Marth, T.; Sengupta, S.; Revur, R.; Weiss, C.A.; Cummins, T.K. Enhancement of reactive powder concrete via nanocement integration. Transp. Res. Rec. 2010, 2142, 18–24. [Google Scholar] [CrossRef]
- Energy Efficiency and CO2 Emissions: Prospective Scenarios for the Cement Industry. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/15313/1/reqno_jrc59826_as_published_jrc59826_jrc-2010-energy_efficiency_and_co2_emissions_prospective_scenarios_for_the_ce%5B1%5D.pdf (accessed on 9 February 2015).
- Bilodeau, A.; Malhotra, M. High-volume fly ash system: Concrete solution for sustainable development. ACI Mater. J. 2000, 97, 41–48. [Google Scholar]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM C305; ASTM International: West Conshohocken, PA, USA, 2006.
- Standard Specification for Flow Table for Use in Tests of Hydraulic Cement; ASTM C230; ASTM International: West Conshohocken, PA, USA, 2003.
- Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance; ASTM C403; ASTM International: West Conshohocken, PA, USA, 1999.
- Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement; ASTM C596; ASTM International: West Conshohocken, PA, USA, 2009.
- Biricik, H.; Sarier, N. Comparative study of the characteristics of nano silica-, silica fume- and fly ash-incorporated cement mortars. Mater. Res. 2014, 17, 570–582. [Google Scholar] [CrossRef]
- Franus, W. SEM investigation of microstructures in hydration products of portland cement. Available online: http://zeolity.wszia.edu.pl/documents/2014/12/sem-investigation-of-microstructures-in-hydration-products-of-portland-cement-in-english.pdf (accessed on 3 February 2015).
- Bensted, J.; Barnes, P. Structure and Performance of Cements; Spon Press: New York, NY, USA, 2002; pp. 540–542. [Google Scholar]
- Rao, G.A. Role of water-binder ratio on the strength development in mortars incorporated with silica fume. Cem. Concr. Res. 2001, 31, 443–447. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Li, Z.; Bai, J.; Memon, S.A.; Dong, B.; Fang, Y.; Xu, W.; Xing, F. Properties of Chemically Combusted Calcium Carbide Residue and Its Influence on Cement Properties. Materials 2015, 8, 638-651. https://doi.org/10.3390/ma8020638
Sun H, Li Z, Bai J, Memon SA, Dong B, Fang Y, Xu W, Xing F. Properties of Chemically Combusted Calcium Carbide Residue and Its Influence on Cement Properties. Materials. 2015; 8(2):638-651. https://doi.org/10.3390/ma8020638
Chicago/Turabian StyleSun, Hongfang, Zishanshan Li, Jing Bai, Shazim Ali Memon, Biqin Dong, Yuan Fang, Weiting Xu, and Feng Xing. 2015. "Properties of Chemically Combusted Calcium Carbide Residue and Its Influence on Cement Properties" Materials 8, no. 2: 638-651. https://doi.org/10.3390/ma8020638





