Hybrid Membranes of PLLA/Collagen for Bone Tissue Engineering: A Comparative Study of Scaffold Production Techniques for Optimal Mechanical Properties and Osteoinduction Ability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Generation and Characterization of Scaffolds
| Material | Hydrolysis time (s) # | Carboxyl group concentration (mol/g) |
|---|---|---|
| PLLA | 0 | (3.7 ± 0.5) × 10−5 |
| 20 | (5.6 ± 0.9) × 10−5 | |
| 45 | (5.5 ± 1.0) × 10−5 | |
| 60 | (5.8 ± 0.7) × 10−5 * | |
| 180 | (5.9 ± 1.0) × 10−5 * |
| Material | Aminolysis time (min) # | Amine group concentration (mol/g) |
|---|---|---|
| PLLA | 0.5 | (1.2 ± 0.2) × 10−5 |
| 1 | (1.6 ± 0.2) × 10−5 | |
| 3 | (1.9 ± 0.2) × 10−5 ** | |
| 5 | (3.2 ± 0.2) × 10−5 *** |
| Material | Collagen concentration (wt%) | |
|---|---|---|
| Elemental analysis | Ninhydrin test | |
| PLLA | 0 | - |
| PC_hydrolysis | 1.4 ± 0.5 | 1.5 ± 0.2 |
| PC_aminolysis | 1.5 ± 0.6 | 0.30 ± 0.05 |
| PC_blend | 47 ± 1 | - |
| PC_cf_HFP | 71 ± 10 | - |
| PC_cf AA | 15 ± 6 | - |
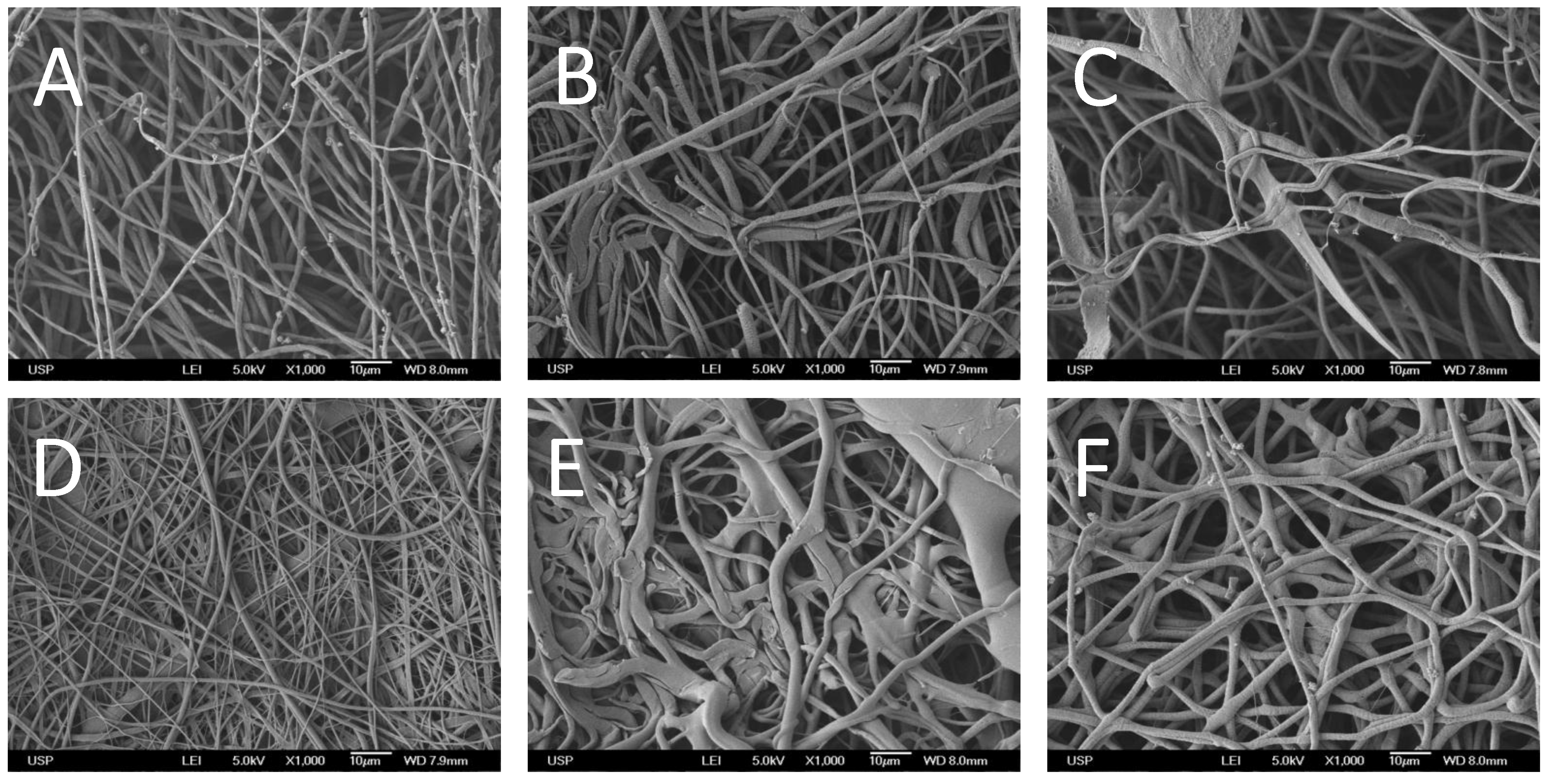
| Material | Elongation at break (%) | Tensile strength (MPa) | Young’s modulus 1 (GPa) |
|---|---|---|---|
| PLLA | 39 ± 8 | 18 ± 3 | 0.19 ± 0.03 |
| PC_hydrolysis | 14 ± 2 *** | 4.8 ± 0.8 * | 0.07 ± 0.01 |
| PC_aminolysis | 5.8 ± 0.3 *** | 7 ± 1 | 0.10 ± 0.02 |
| PC_blend | (5 ± 1) × 10 1 | (8 ± 1) × 10 1 *** | 1.0 ± 0.1 *** |
| PC_cs_HFP | 6 ± 1 *** | 38 ± 6 *** | 1.4 ± 0.2 *** |
| PC_cs_AA | 14 ± 2 *** | 24 ± 4 | 0.7 ± 0.1 *** |
2.2. Cell Culture

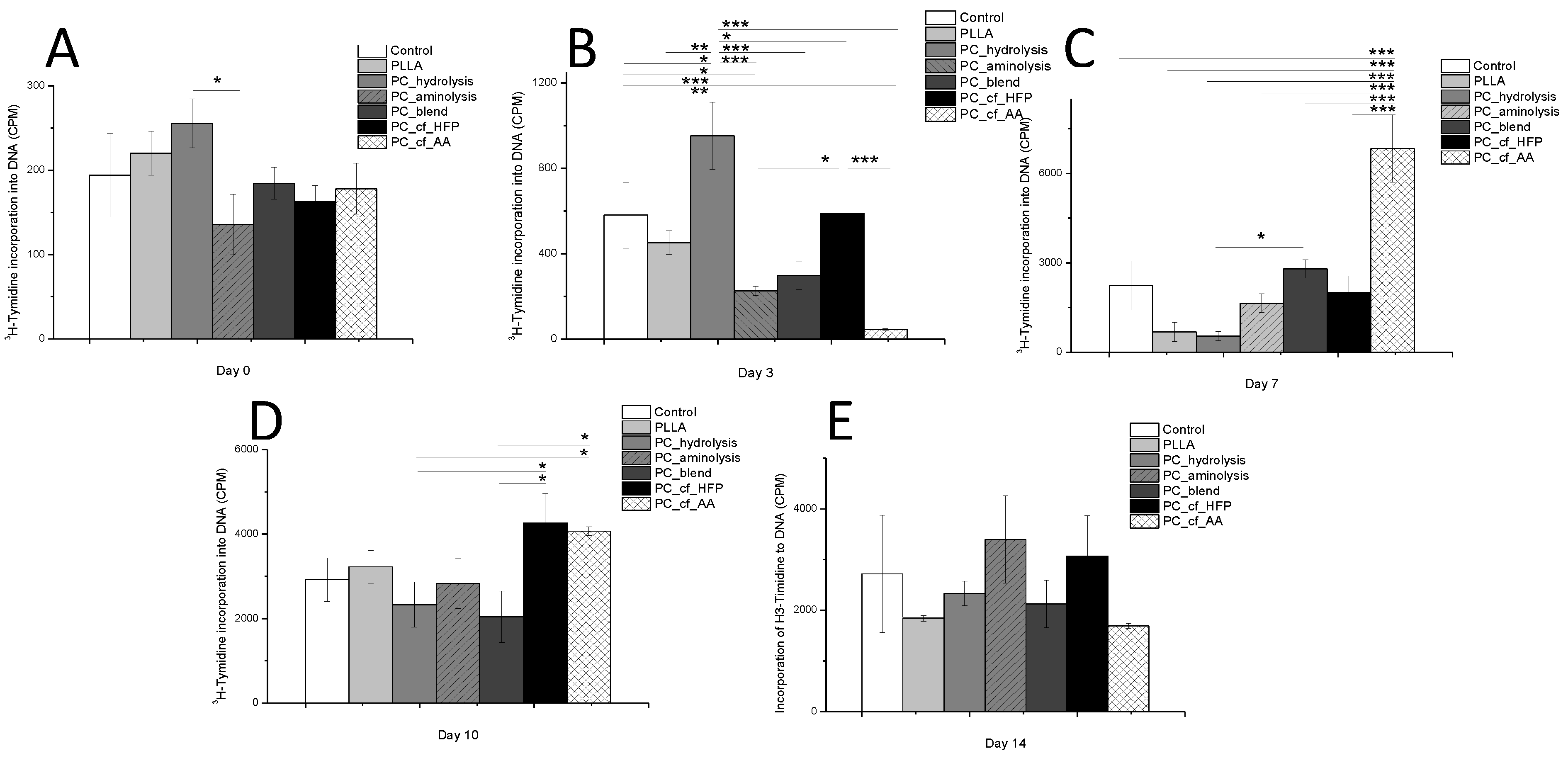
| Material | ALP (U/L) | |
|---|---|---|
| ODM | DMEM | |
| PLLA | 0.16 ± 0.05 | Not detected |
| PC_hydrolysis | 0.60 ± 0.08 | 0.15 ± 0.04 |
| PC_aminolysis | 0.5 ± 0.1 | Not detected |
| PC_blend | 1.2 ± 0.1 *** | 0.8 ± 0.1 |
| PC_Cf_HFP | 1.0 ± 0.2 *** | 0.34 ± 0.08 ** |
| PC_cf_AA | 1.6 ± 0.3 *** | Not detected |
| Control | 1.3 ± 0.1 *** | Not detected |
3. Experimental Section
3.1. Electrospinning of PLLA, Collagen and PLLA/Collagen Solutions
3.2. Scaffold Crosslinking
3.3. PLLA Functionalization and Collagen Immobilization on the Surface
3.4. Aminolysis, Hydrolysis and Collagen Quantification
3.5. Scanning Electron Microscopy
3.6. Elemental Analyses
3.7. Dynamic Mechanical Analysis (DMA)
3.8. Cell Proliferation
3.9. Alkaline Phosphatase Assay (ALP)
3.10. Cell Distribution on Scaffolds
3.11. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 2 October 1934. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Madihally, S.V. Next generation of electrosprayed fibers for tissue regeneration. Tissue Eng. Part B Rev. 2011, 17, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.I.; Xu, X.X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, C.; Gong, Y.; Ji, J.; Shen, J. Immobilization of natural macromolecules on poly-l-lactic acid membrane surface in order to improve its cytocompatibility. J. Biomed. Mater. Res. 2002, 63, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, B.; Beugeling, T.; Feijen, J.; Bantjes, K.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; San Antonio, J.D.; Antipova, O. Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res. 2011, 52, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, Y.; Shen, J. Endothelial cell functions in vitro cultured on poly(l-lactic acid) membranes modified with different methods. J. Biomed. Mater. Res. A 2004, 69, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, X.Q.; Liu, S.P.; Wang, H.S.; He, C.L. Fabrication and properties of PLLA-gelatin nanofibers by electrospinning. J. Appl. Polym. Sci. 2010, 114, 542–547. [Google Scholar]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Teng, W.K.; Chan, B.P.; Chew, S.Y. Photochemical crosslinked electrospun collagen nanofibers: Synthesis, characterization and neural stem cell interactions. J. Biomed. Mater. Res. A 2010, 95, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chu, B.; Hsiao, B.S. Mineralization of hydroxyapatite in electrospun nanofibrous poly(l-lactic acid) scaffolds. J. Biomed. Mater. Res. A 2006, 79, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of biomacromolecules onto aminolyzed poly(l-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.Y.; Hollister, S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 2009, 30, 4063–4069. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cheng, L.; Li, H.; Zhou, Y.; Zhang, Y.; Chang, J. Preparation of hydrophilic poly(l-lactide) electrospun fibrous scaffolds modified with chitosan for enhanced cell biocompatibility. Polymer 2012, 53, 2298–2305. [Google Scholar] [CrossRef]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wan, L.; Wu, J.; Gao, C. Microscale control over collagen gradient on poly(l-lactide) membrane surface for manipulating chondrocyte distribution. Colloids Surf. B Biointerfaces 2008, 67, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fitie, C.F.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Bramfeldt, H.; Vermette, P. Enhanced smooth muscle cell adhesion and proliferation on protein-modified polycaprolactone-based copolymers. J. Biomed. Mater. Res. A 2009, 88, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, X.; Liang, Q.; Xu, X.; Jing, X. Enzymatic degradation of poly(l-lactide) and poly(epsilon-caprolactone) electrospun fibers. Macromol. Biosci. 2004, 4, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, C.; Arecchi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, T.G. Biodegradable polymer nanocylinders fabricated by transverse fragmentation of electrospun nanofibers through aminolysis. Macromol. Rapid Commun. 2008, 29, 1231–1236. [Google Scholar] [CrossRef]
- Sun, M.; Downes, S. Physicochemical characterisation of novel ultra-thin biodegradable scaffolds for peripheral nerve repair. J. Mater. Sci. Mater. Med. 2009, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, C.R.; Coulais, C.; Guthold, M. The mechanical stress-strain properties of single electrospun collagen type I nanofibers. Acta Biomater. 2010, 6, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Bar-On, B.; Wagner, H.D. Mechanics of electrospun collagen and hydroxyapatite/collagen nanofibers. J. Mech. Behav. Biomed. Mater. 2012, 13, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Yang, F.; Gubler, M.J.; Ramakrishna, S.; Chan, C.K. Fabrication of mineralized polymeric nanofibrous composites for bone graft materials. Tissue Eng. Part A 2009, 15, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Kong, T.; Wang, L.; Liu, P.; Yang, W.; Chen, C. Regulation of endothelial cells migration on poly(d, l-lactic acid) films immobilized with collagen gradients. Colloids Surf. B Biointerfaces 2010, 79, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.N.; Chai, C.; Lee, P.C.; Tang, Y.N.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials 2006, 27, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Kuboki, Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; Zaharias, R.; Stanford, C. Osteoblast integrin adhesion and signaling regulate mineralization. J. Dent. Res. 2001, 80, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Boudriot, U.; Wack, C.; Leifeld, I.; Grabedunkel, C.; Dersch, R.; Rudisile, M.; Wendorff, J.H.; Greiner, A.; Paletta, J.R.; et al. Influence of nanofibers on the growth and osteogenic differentiation of stem cells: A comparison of biological collagen nanofibers and synthetic plla fibers. J. Mater. Sci. Mater. Med. 2009, 20, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. A 2010, 94, 1312–1320. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, F.; Bentini, R.; Burrows, M.C.; Carreira, A.C.O.; Kossugue, P.M.; Sogayar, M.C.; Catalani, L.H. Hybrid Membranes of PLLA/Collagen for Bone Tissue Engineering: A Comparative Study of Scaffold Production Techniques for Optimal Mechanical Properties and Osteoinduction Ability. Materials 2015, 8, 408-423. https://doi.org/10.3390/ma8020408
Gonçalves F, Bentini R, Burrows MC, Carreira ACO, Kossugue PM, Sogayar MC, Catalani LH. Hybrid Membranes of PLLA/Collagen for Bone Tissue Engineering: A Comparative Study of Scaffold Production Techniques for Optimal Mechanical Properties and Osteoinduction Ability. Materials. 2015; 8(2):408-423. https://doi.org/10.3390/ma8020408
Chicago/Turabian StyleGonçalves, Flávia, Ricardo Bentini, Mariana C. Burrows, Ana C. O. Carreira, Patricia M. Kossugue, Mari C. Sogayar, and Luiz H. Catalani. 2015. "Hybrid Membranes of PLLA/Collagen for Bone Tissue Engineering: A Comparative Study of Scaffold Production Techniques for Optimal Mechanical Properties and Osteoinduction Ability" Materials 8, no. 2: 408-423. https://doi.org/10.3390/ma8020408






