Acid Denaturation Inducing Self-Assembly of Curcumin-Loaded Hemoglobin Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrophobic Characterization of Hemoglobin under Acid Condition
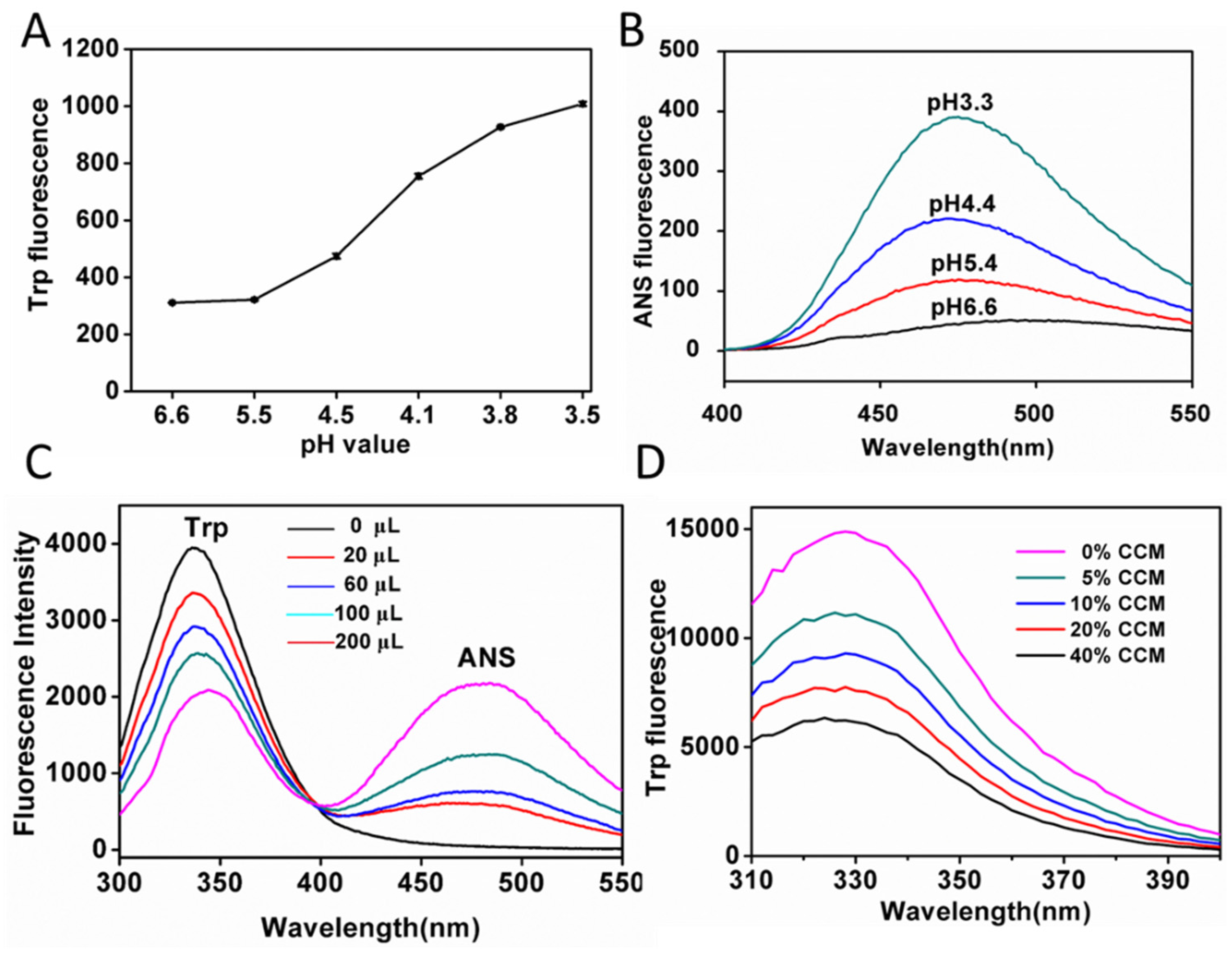
2.2. Preparation and Characterization of CCM-Hb-NPs

| CCM/Hb (w/w) | Particle Size (nm) | Zeta Potential (mV) | PDI | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| 0 | 15.2 | +13.39 | 0.102 | None | None |
| 10% | 74.7 | +17.63 | 0.146 | 5.02 | 47.7 |
| 20% | 82.5 | +22.40 | 0.153 | 14.40 | 75.0 |
| 30% | 92.5 | +20.08 | 0.156 | 18.20 | 70.0 |
| 40% | 104.6 | +18.99 | 0.194 | 20.90 | 59.6 |
2.3. Effect of pH on the Particle Size and Stability of Nanoparticles
| pH Value | Particle Size (nm) | Stability Time (h) |
|---|---|---|
| 6.6 | None | <0.1 |
| 5.6 | 199.5 | 8 |
| 4.1 | 82.5 | >168 |
| 2.8 | None | <0.1 |
2.4. CCM Extracted by Ethyl Acetate from CCM-Hb-NPs
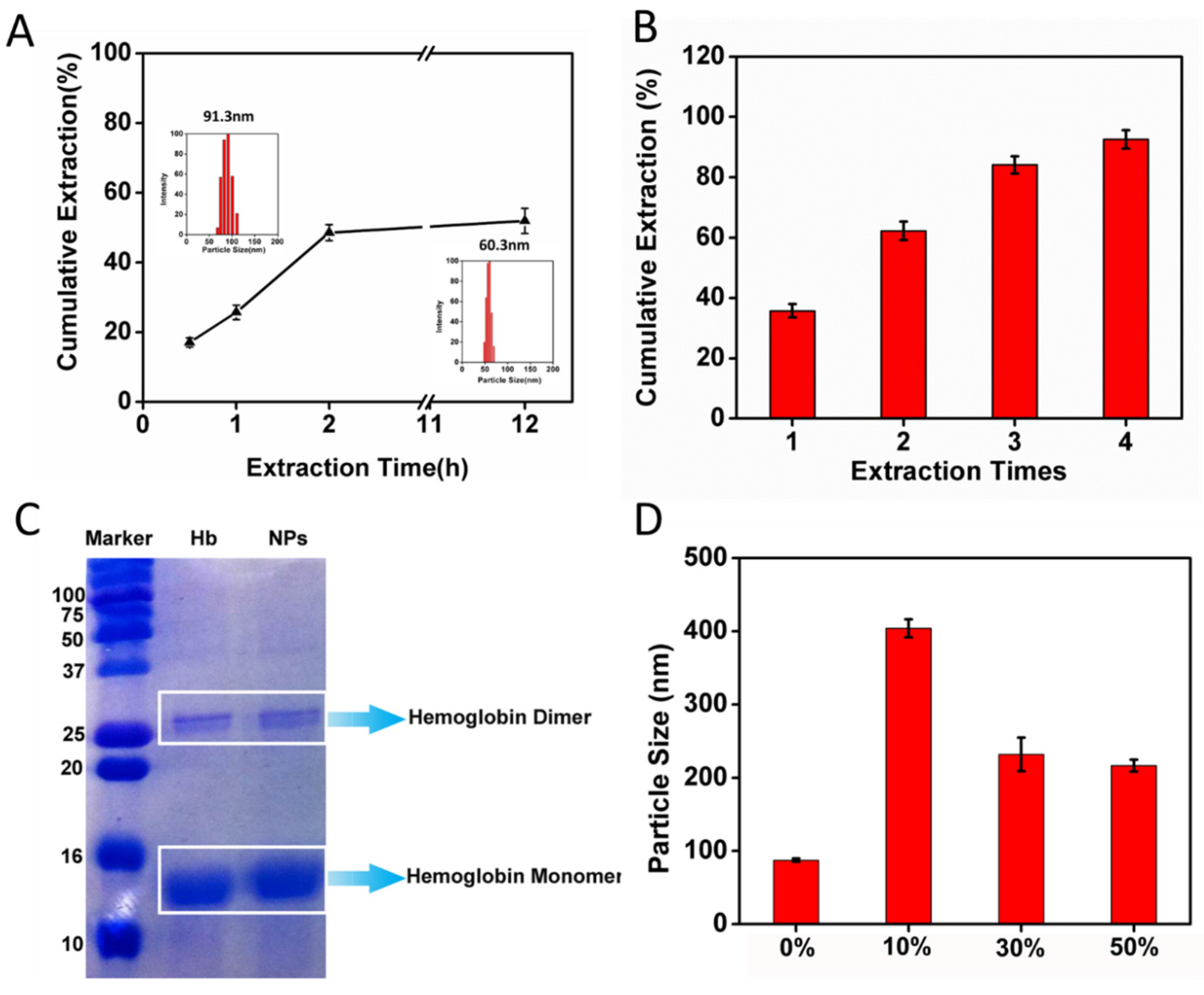
2.5. Size Change of CCM-Hb-NPs in Ethanol and SDS-PAGE of Nanoparticles
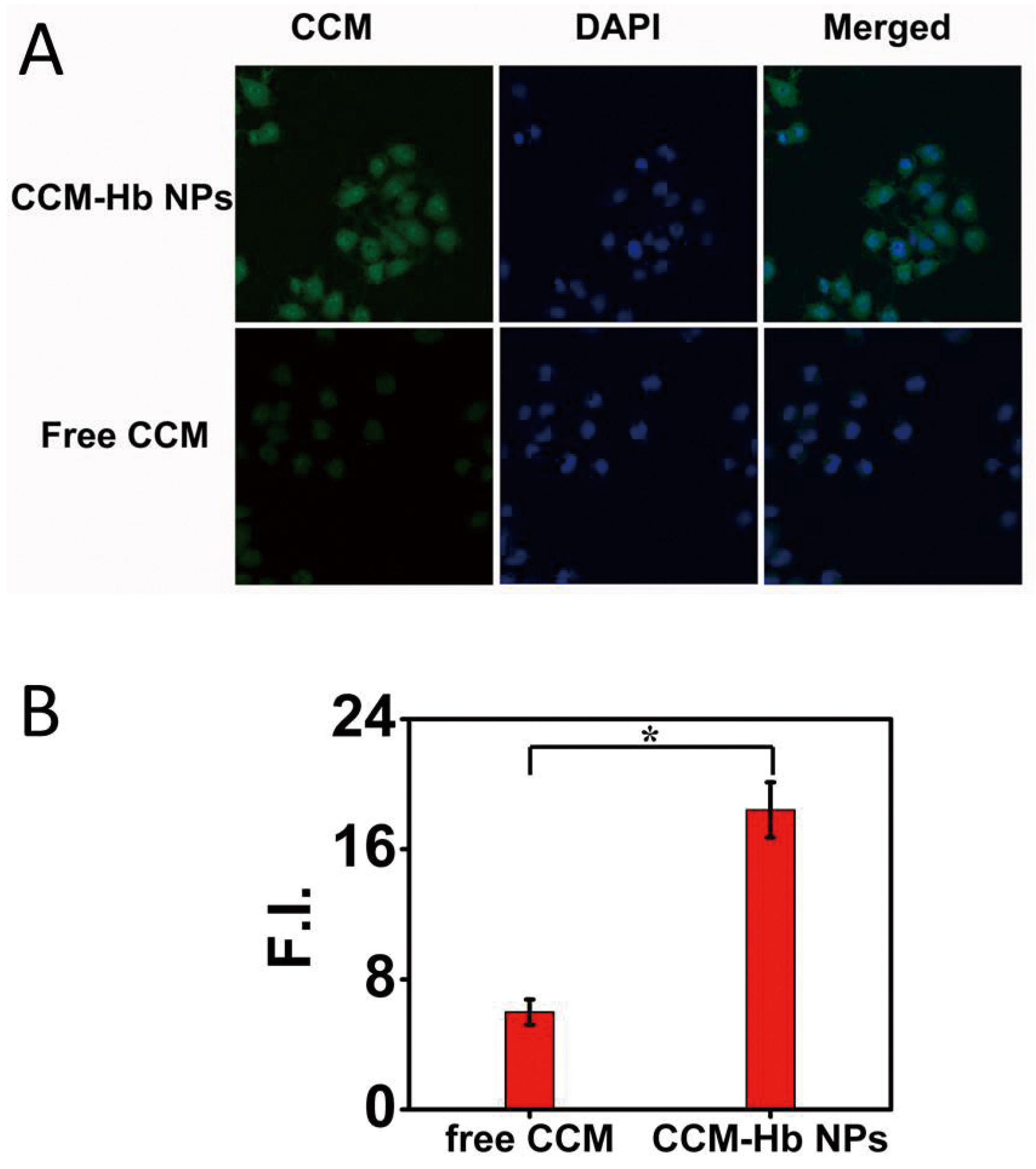
2.6. Cell Uptake Study
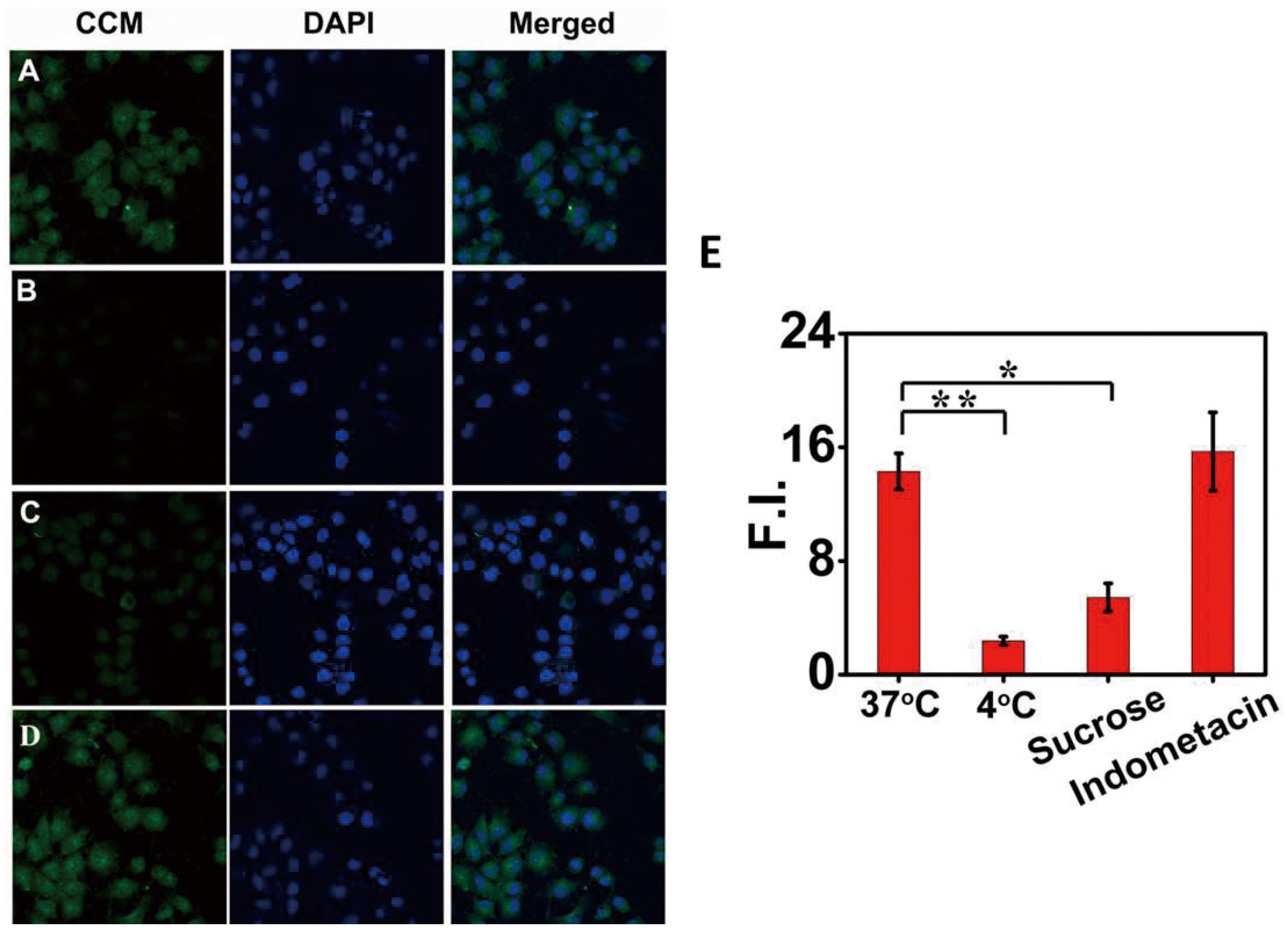
2.7. Cell Uptake Mechanism
2.8. In Vitro Cytotoxicity Assay

3. Materials and Methods
3.1. Materials
3.2. Preparation of CCM-Hb-NPs
3.3. Structure Change of Hemoglobin at Different pH Values
3.4. Size and Morphology of CCM-Hb-NPs
3.5. Fluorescence Studies of Hemoglobin before and after Interacting with CCM
3.6. Fluorescence Resonance Energy Transfer from Hemoglobin to ANS at Acidic pH Value
3.7. Determination of pH-Dependent Particle Size and Stability
3.8. CCM Extracted by Ethyl Acetate in CCM-Hb-NPs
3.9. Size Change and Electrophoresis Gels of CCM-Hb-NPs
3.10. Cellular Uptake of CCM-Hb-NPs
3.11. Cell Uptake Mechanism of CCM-Hb-NPs
3.12. In Vitro Cytotoxicity
4. Conclusions
Supplementary Information
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maham, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.R.; Flenniken, M.L.; Gillitzer, E.; Harmsen, A.L.; Harmsen, A.G.; Jutila, M.A.; Douglas, T.; Young, M.J. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int. J. Nanomed. 2007, 2, 715–733. [Google Scholar]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Tang, X.; Cao, X.; Wu, J.; Yuan, A.; Qiao, Q.; Pan, J.; Hu, Y. Novel self-assembly endows human serum albumin nanoparticles with an enhanced antitumor efficacy. AAPS PharmSciTech 2014, 15, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Nab technology: A drug delivery platform utilizing endothelial gp60 receptor-based transport and tumour-derived SPARC for targeting. Drug Deliv. Rep. 2007, 16, 37–41. [Google Scholar]
- Perutz, M.F. Hemoglobin structure and respiratory transport. Sci. Am. 1978, 239, 92–125. [Google Scholar] [CrossRef] [PubMed]
- Shaeffer, J.R.; McDonald, M.J.; Turci, S.M.; Dinda, D.M.; Bunn, H.F. Dimer-monomer dissociation of human hemoglobin A. J. Biol. Chem. 1984, 259, 14544–14547. [Google Scholar] [PubMed]
- Buehler, P.W.; D’Agnillo, F.; Schaer, D.J. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol. Med. 2010, 16, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Mozzarelli, A.; Ronda, L.; Faggiano, S.; Bettati, S.; Bruno, S. Haemoglobin-based oxygen carriers: Research and reality towards an alternative to blood transfusions. Blood Transfus. 2010, 8, S59–S68. [Google Scholar] [PubMed]
- Quirolo, K. Hemoglobin-based oxygen carriers: A long road from bench to bedside. Pediatr. Hematol. Oncol. 2007, 24, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Silverman, T.A.; Weiskopf, R.B. Planning Committee and the Speakers. Hemoglobin-based Oxygen Carriers Current Status and Future Directions. Anesthesiology 2009, 111, 946–963. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.; Biessels, P.; Ng, N.F.; Woods, C.; Bell, D.N.; Adamson, G. Synthesis and characterization of a hemoglobin-ribavirin conjugate for targeted drug delivery. Bioconjug. Chem. 2006, 17, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Palmer, A.F. Development of a dichloroacetic acid-hemoglobin conjugate as a potential targeted anti-cancer therapeutic. Biotechnol. Bioeng. 2011, 108, 1413–1420. [Google Scholar] [CrossRef]
- Gong, G.M.; Zhi, F.; Wang, K.; Tang, X.; Yuan, A.; Zhao, L.; Ding, D.; Hu, Y. Fabrication of a nanocarrier system through self-assembly of plasma protein and its tumor targeting. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Xu, Y.; Zhou, Y.; Meng, Z.; Ren, G.; Zhao, Y.; Zhang, X.; Wu, J.; Hu, Y. Molecular switch for the assembly of lipophilic drug incorporated plasma protein nanoparticles and in vivo image. Biomacromolecules 2012, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Gong, G.M.; Su, H.; Liu, W.; Wang, Z.; Li, L. Self-assembly of plasma protein through disulfide bond breaking and its use as a nanocarrier for lipophilic drugs. Polym. Chem. 2014, 5, 4871–4874. [Google Scholar] [CrossRef]
- Field, E.O.; O’Brien, J.R. Dissociation of human haemoglobin at low pH. Biochem. J. 1955, 60, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Hultin, H.O. Changes in trout hemoglobin conformations and solubility after exposure to acid and alkali pH. J. Agric. Food Chem. 2004, 52, 3633–3643. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Girigoswami, A. A fluorimetric and circular dichroism study of hemoglobin—Effect of pH and anionic amphiphiles. J. Colloid Interface Sci. 2006, 296, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Abbasi, A.Z.; Pfeiffer, C.; Hussain, S.Z.; Khalid, Z.M.; Gil, P.R.; Parak, W.J.; Hussain, I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 102, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Coombes, A.G.; Davies, M.C.; Davis, S.S.; Illum, L. Preparation of sub-100 nm human serum albumin nanospheres using a pH-coacervation method. J. Drug Target 1993, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G. Acid-induced unfolding of flounder hemoglobin: Evidence for a molten globular state with enhanced pro-oxidative activity. J. Agric. Food Chem. 2002, 50, 7669–7676. [Google Scholar] [CrossRef] [PubMed]
- Ali, V.; Prakash, K.; Kulkarni, S.; Ahmad, A.; Madhusudan, K.P.; Bhakuni, V. 8-anilino-1-naphthalene sulfonic acid (ANS) induces folding of acid unfolded cytochrome c to molten globule state as a result of electrostatic interactions. Biochemistry 1999, 38, 13635–13642. [Google Scholar] [CrossRef] [PubMed]
- Gryczynski, Z.; Lubkowski, J.; Bucci, E. Heme-Protein Interactions in Horse Heart Myoglobin at Neutral Ph and Exposed to Acid Investigated by Time-Resolved Fluorescence in the Picosecond to Nanosecond Time Range. J. Biol. Chem. 1995, 270, 19232–19237. [Google Scholar] [PubMed]
- Johnson, J.D.; El-Bayoumi, M.A.; Weber, L.D.; Tulinsky, A. Interaction of alpha-chymotrypsin with the fluorescent probe 1-anilinonaphthalene-8-sulfonate in solution. Biochemistry 1979, 18, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Y.; Zhao, S.; Shao, T.; Cheng, Y. Human serum albumin (HSA) nanoparticles stabilized with intermolecular disulfide bonds. Chem. Commun. 2013, 49, 2234–2236. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Dong, H.; Jia, X.; Guo, W.; Lu, H.; Yang, Y.; Ju, H.; Zhang, X.; Hu, Y. Functionalized Graphene Oxide Mediated Adriamycin Delivery and miR-21 Gene Silencing to Overcome Tumor Multidrug Resistance in Vitro. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Atsumi, N.; Jacobs, E.E., Jr.; Austen, W.G.; Vlahakes, G.J. Ultrapure, stroma-free, polymerized bovine hemoglobin solution: Evaluation of renal toxicity. J. Surg. Res. 1989, 47, 407–411. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, J.; Hu, W.; Zhang, Y.; Zhi, F.; Zhou, Z.; Wu, J.; Hu, Y. Acid Denaturation Inducing Self-Assembly of Curcumin-Loaded Hemoglobin Nanoparticles. Materials 2015, 8, 8701-8713. https://doi.org/10.3390/ma8125486
Wang K, Wang J, Hu W, Zhang Y, Zhi F, Zhou Z, Wu J, Hu Y. Acid Denaturation Inducing Self-Assembly of Curcumin-Loaded Hemoglobin Nanoparticles. Materials. 2015; 8(12):8701-8713. https://doi.org/10.3390/ma8125486
Chicago/Turabian StyleWang, Kaikai, Juan Wang, Wenwen Hu, Yifan Zhang, Feng Zhi, Zaigang Zhou, Jinhui Wu, and Yiqiao Hu. 2015. "Acid Denaturation Inducing Self-Assembly of Curcumin-Loaded Hemoglobin Nanoparticles" Materials 8, no. 12: 8701-8713. https://doi.org/10.3390/ma8125486






