Polyols from Microwave Liquefied Bagasse and Its Application to Rigid Polyurethane Foam
Abstract
:1. Introduction
2. Results and Discussion
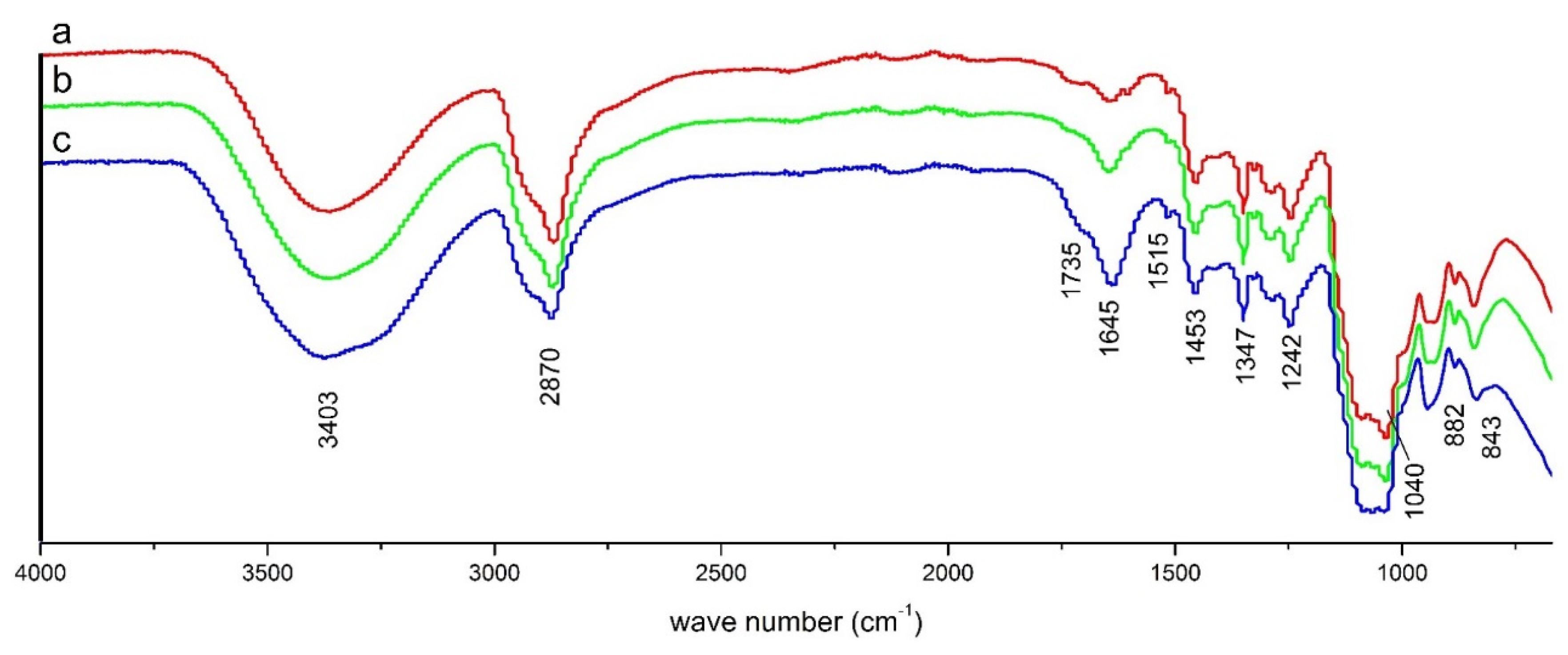
2.1. Liquefaction and Characteristics of LB

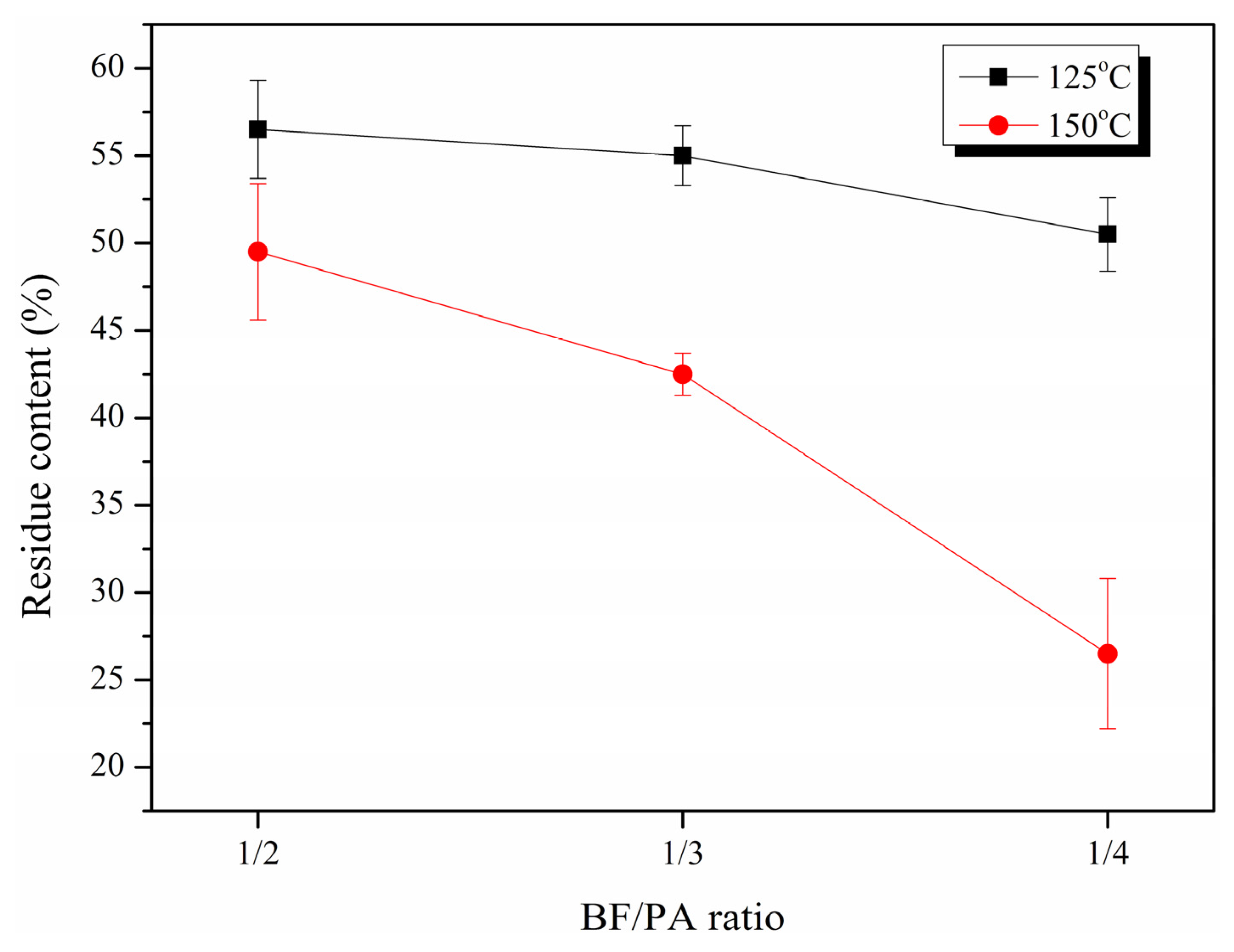

| BF/PA Ratio | Temperature (°C) | Mw | Mn |
|---|---|---|---|
| 1/2 | 125 | 790 ± 83 a | 1.86 ± 0.14 |
| 150 | 675 ± 57 | 1.76 ± 0.09 | |
| 1/3 | 125 | 742 ± 64 | 1.77 ± 0.10 |
| 150 | 600 ± 41 | 1.75 ± 0.13 | |
| 1/4 | 125 | 855 ± 73 | 1.91 ± 0.17 |
| 150 | 740 ± 36 | 1.74 ± 0.06 |
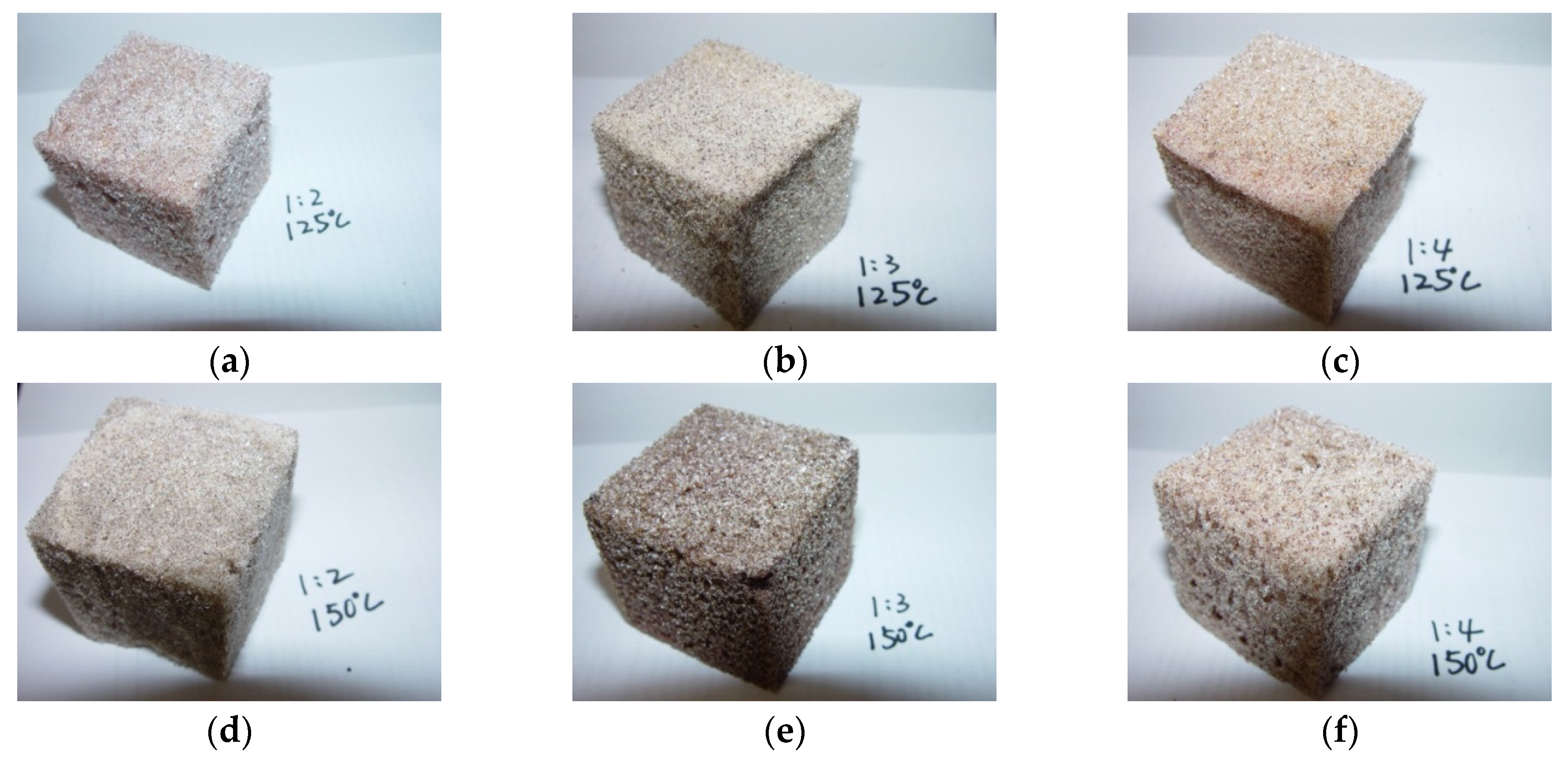
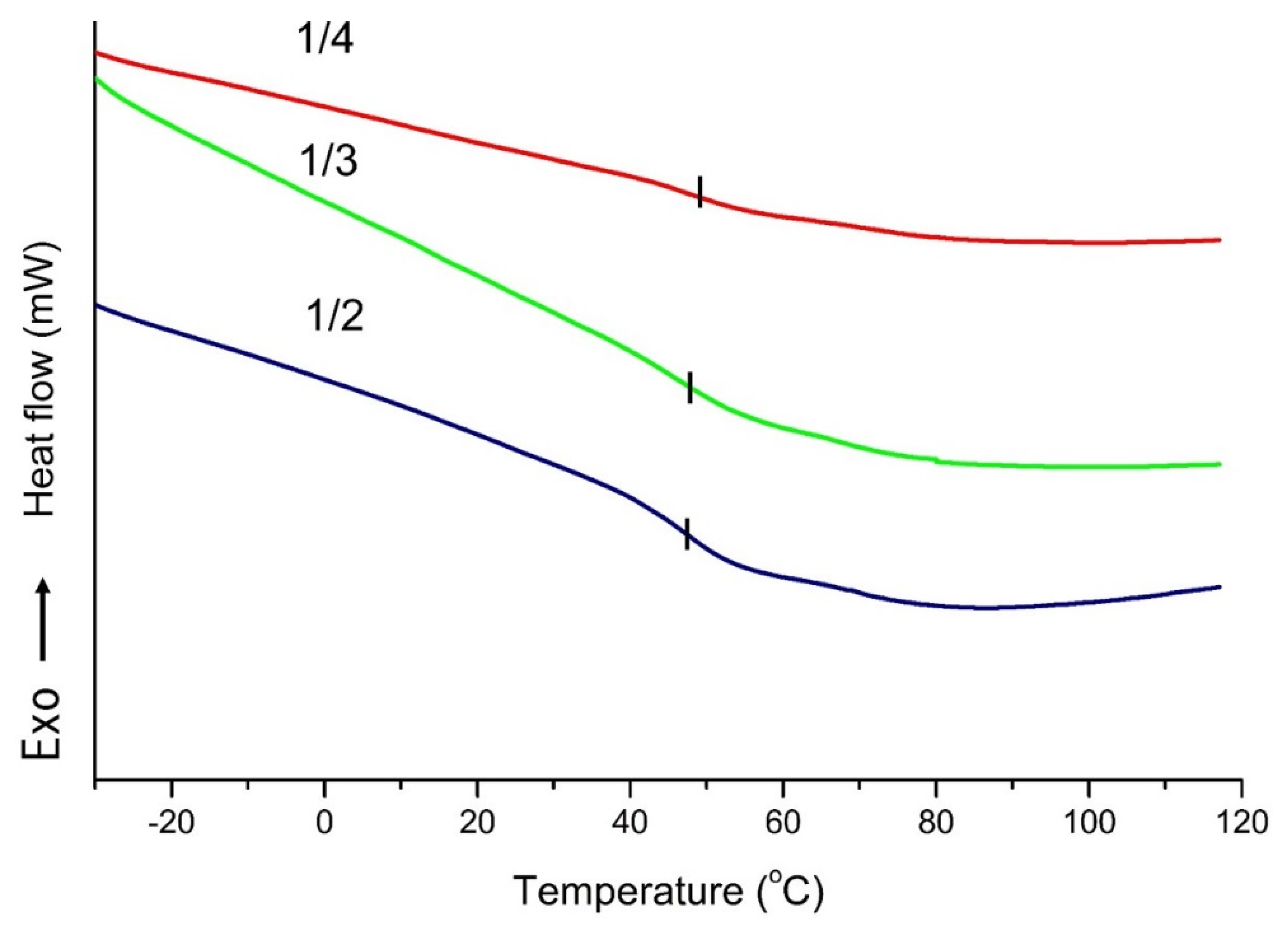
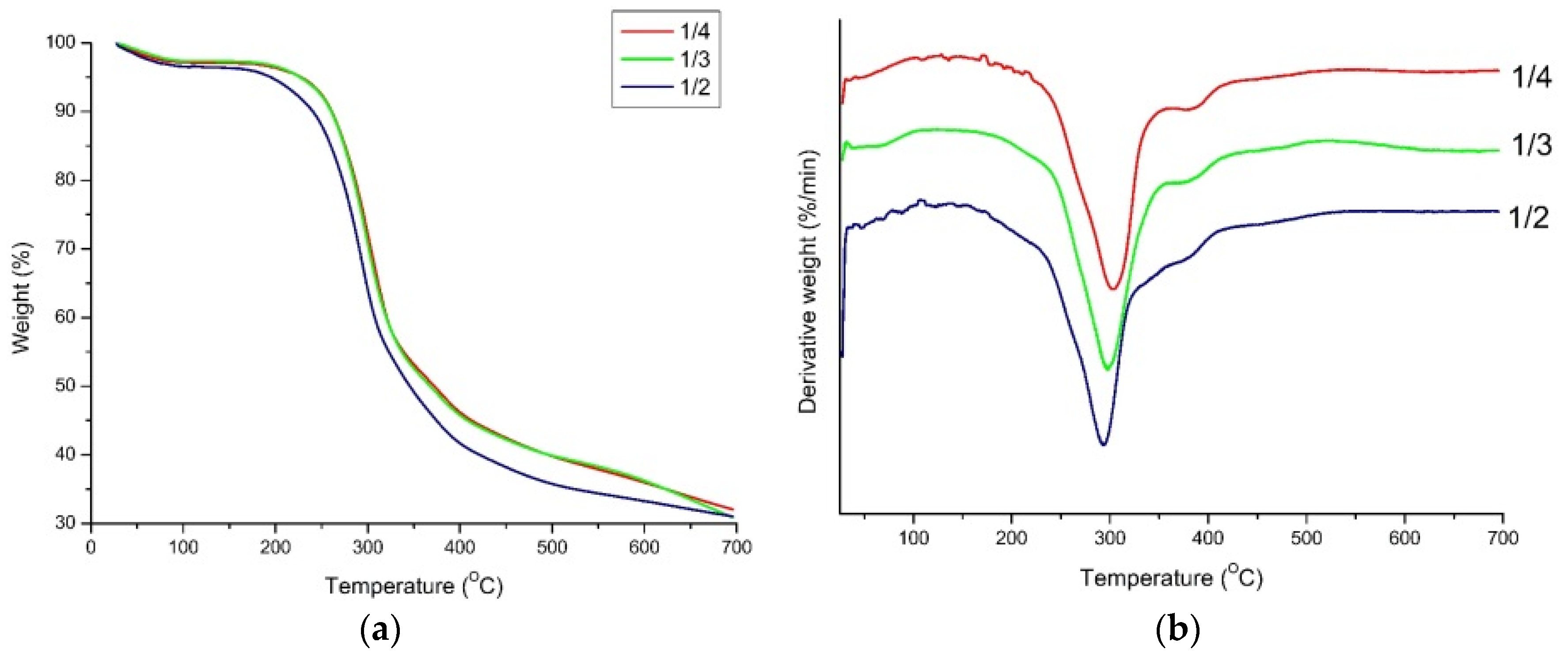
2.2. Characteristics of PU Foam
| BF/PA Ratio | Temperature (°C) | Density (g/cm−3) | CS a (Mpa) | MOE b (Mpa) |
|---|---|---|---|---|
| 1/2 | 125 | 0.031 ± 0.003 c | 0.19 ± 0.022 | 1.1 ± 0.045 |
| 150 | 0.032 ± 0.000 | 0.32 ± 0.017 | 2.0 ± 0.020 | |
| 1/3 | 125 | 0.032 ± 0.001 | 0.28 ± 0.027 | 1.2 ± 0.039 |
| 150 | 0.035 ± 0.002 | 0.33 ± 0.007 | 3.4 ± 0.061 | |
| 1/4 | 125 | 0.037 ± 0.005 | 0.34 ± 0.076 | 3.1 ± 0.033 |
| 150 | 0.043 ± 0.007 | 0.48 ± 0.051 | 5.1 ± 0.083 |
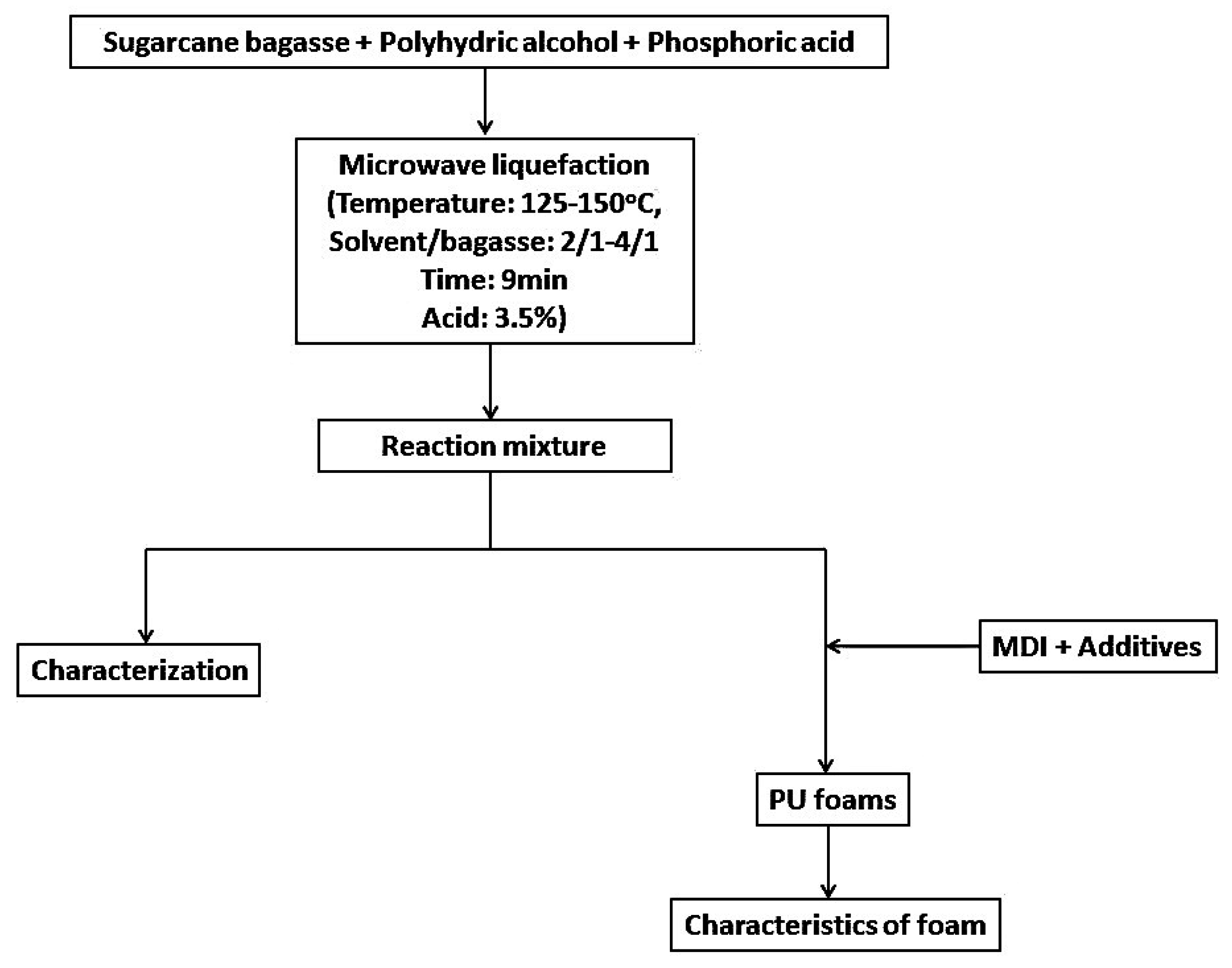
3. Experimental Section
3.1. Materials and Chemicals
3.2. Liquefaction of Bagasse
3.3. Preparation of LB-PU Foam
3.4. Characteristics of LB
3.4.1. Acid Number
3.4.2. Hydroxyl Number
3.4.3. Gel Permeation Chromatography (GPC) Analysis
3.5. Characteristics of Polyurethane Foams
3.5.1. Differential Scanning Calorimeter (DSC)
3.5.2. Thermal Gravimetric Analysis (TGA)
3.5.3. Mechanical Properties of Foams
3.6. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, S.J.; Luo, X.L.; Li, Y.B. Polyols and polyurethane from the liquefaction of lignocellulosic biomass. ChemSusChem 2014, 7, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.; Coolman, R.; Mountziaris, T.J.; Huber, G.W. Catalytic fast pyrolysis of lignocellulosic biomass in a process development unit with continual catalyst addition and removal. Chem. Eng. Sci. 2014, 108, 33–46. [Google Scholar] [CrossRef]
- Pan, H. Synthesis of polymers from organic solvent liquefied biomass: A review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Liu, D.; Petrus, L. Qualitative analysis of products formed during the acid catalyzed liquefaction of bagasse in ethylene glycol. Bioresour. Technol. 2007, 98, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Han, G.P.; Wu, Q.L. Comparative properties of sugarcane rind and wood strands for structural composite manufacturing. For. Prod. J. 2004, 54, 283–288. [Google Scholar]
- Andrade, M.F.; Colodette, J.L. Dissolving pulp production from sugar cane bagasse. Ind. Crops Prod. 2014, 52, 58–64. [Google Scholar] [CrossRef]
- Yue, Y.Y.; Han, J.Q.; Han, G.P.; Zhang, Q.G.; French, A.D.; Wu, Q.L. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinity and percentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chumpoo, J.; Prasassarakich, P. Bio-oil from hydro-liquefaction of bagasse in supercritical ethanol. Energy Fuels 2010, 24, 2071–2077. [Google Scholar] [CrossRef]
- EI-barbary, M.H.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crops Prod. 2008, 27, 33–38. [Google Scholar]
- Liu, J.J.; Chen, F.G.; Qiu, M.H. Liquefaction of bagasse and preparation of rigid polyurethane foam from liquefaction products. J. Biobased Mater. Bioenergy 2009, 3, 401–407. [Google Scholar] [CrossRef]
- Yan, L.F.; Yang, N.K.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. CLEAN Soil Air Water 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Rafiqul, I.; Bai, L.G.; Yan, Y.J.; Li, T.C. Study on co-liquefaction of coal and bagasse by factorial experiment design method. Fuel Process. Technol. 2000, 68, 3–12. [Google Scholar] [CrossRef]
- Rezende, C.A.; Lima, M.A.; Maziero, P.; Eduardo, R.D.; Garcia, W.; Polikarpov, L. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, T.; Chen, F.G. Fast liquefaction of bagasse in ethylene carbonate and preparation of xpoxy resin from the liquefied product. J. Appl. Polym. Sci. 2005, 98, 1961–1968. [Google Scholar] [CrossRef]
- Krzan, A.; Kunaver, M. Microwave heating in wood liquefaction. J. Appl. Polym. Sci. 2006, 101, 1051–1056. [Google Scholar] [CrossRef]
- Xiao, W.H.; Han, L.J.; Zhao, Y.Y. Comparative study of conventional and microwave-assisted liquefaction of corn stover in ethylene glycol. Ind. Crops Prod. 2011, 34, 1602–1606. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Producing lignin-based polyols through microwave-assisted liquefaction for rigid polyurethane foam production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef]
- Ouyang, X.P.; Zhu, G.D.; Huang, X.Z.; Qiu, X.Q. Microwave assisted liquefaction of wheat straw alkali lignin for the production of monophenolic compounds. J. Energy Chem. 2015, 24, 72–76. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bordado, J.C.; Mateus, M.M. Microwave-assisted Liquefaction of Cork—From an Industrial Waste to Sustainable Chemicals. Ind. Eng. Manag. 2015, 4, 173. [Google Scholar]
- Pan, H.; Zheng, Z.F.; Hse, C.Y. Microwave-assisted liquefaction of wood with polyhydric alcohols and its application in preparation of polyurethane (PU) foams. Eur. J. Wood Wood Prod. 2012, 70, 461–470. [Google Scholar] [CrossRef]
- Xie, J.L.; Qi, J.Q.; Hse, C.Y.; Shupe, T.F. Effect of lignin derivatives in the bio-polyols from microwave liquefied bamboo on the preparation of polyurethane foams. Bioresources 2013, 9, 578–588. [Google Scholar] [CrossRef]
- Xiao, W.H.; Niu, W.J.; Yi, F.; Liu, X.; Han, L.J. Influence of crop residue types on microwave-assisted liquefaction performance and products. Energy Fuels 2013, 27, 3204–3208. [Google Scholar] [CrossRef]
- Dolomanova, V.; RAUHE, J.C.M.; Jensen, L.R.; Pyrz, R.; Timmons, A.B. Mechanical properties and morphology of nano-reinforced rigid PU foam. J. Cell. Plast. 2011, 47, 81–93. [Google Scholar] [CrossRef]
- Xu, J.M.; Jiang, J.C.; Hse, C.Y.; Shupe, T.F. Preparation of polyurethane foams using fractionated products in liquefied wood. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Gao, L.L.; Liu, Y.H.; Lei, H.W.; Peng, H.; Ruan, R. Preparation of semirigid polyurethane foam with liquefied bamboo residues. J. Appl. Polym. Sci. 2010, 116, 1694–1699. [Google Scholar] [CrossRef]
- Chen, F.G.; Lu, Z.M. Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Hu, S.J.; Li, Y.B. Polyols and polyurethane foams from base-catalyzed liquefaction of lignocellulosic biomass by crude glycerol: Effects of crude glycerol impurities. Ind. Crops Prod. 2014, 57, 188–194. [Google Scholar] [CrossRef]
- Xu, J.M.; Jiang, J.C.; Hse, C.Y.; Shupe, T.F. Renewable chemical feedstocks from integrated liquefaction processing of lignocellulosic materials using microwave energy. Green Chem. 2012, 14, 2821–2830. [Google Scholar] [CrossRef]
- Lee, S.H.; Teramoto, Y.; Shiraishi, N. Acid-catalyzed liquefaction of waste paper in the presence of phenol and its application to novolak-type phenolic resin. J. Appl. Polym. Sci. 2002, 83, 1473–1481. [Google Scholar] [CrossRef]
- Hoareau, W.; Trindade, W.G.; Siegmund, B.; Castellan, A.; Frollini, E. Sugar cane bagasse and curaualignins oxidized by chlorine dioxide and reacted with furfuryl alcohol: Characterization and stability. Polym. Degrad. Stab. 2004, 86, 567–576. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Sun, J.X.; Cao, F.C.; Sun, X.F.; Sun, R.C. Comparative study of hemicellulose isolated with alkaline peroxide from lignocellulosic materials. J. Wood Chem. Technol. 2005, 24, 239–262. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Liu, C.F.; Sun, R.C. Comparative study of alkali-and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chaikumpollert, O.; Methacanon, P.; Suchiva, K. Structure elucidation of hemicellulose from Vetiver grass. Carbohydr. Polym. 2004, 57, 191–196. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues-wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Hse, C.Y.; Shupe, T.F.; Pan, H. Liquefaction behaviors of bamboo residues in a glycerol-based solvent using microwave energy. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.F.; Sun, R.C. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicellulose, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, S.Q. Effect of the addition of wood flours on the properties of rigid polyurethane foam. J. Appl. Polym. Sci. 2009, 113, 2902–2909. [Google Scholar] [CrossRef]
- Wang, T.P.; Li, D.; Wang, L.J.; Yin, J.; Chen, X.D.; Mao, Z.H. Effects of CS/EC ratio on structure and properties of polyurethane foams prepared from untreated liquefied corn stover with PAPI. Chem. Eng. Res. Des. 2008, 86, 416–421. [Google Scholar] [CrossRef]
- Zhang, H.R.; Pang, H.; Zhang, L.; Chen, X.D.; Liao, B. Biodegradability of polyurethane foam from liquefied wood based polyols. J. Polym. Environ. 2013, 21, 329–334. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Koizumi, A.; Doi, S.; Tamura, Y.; Ono, H. Wood species effects on the characteristics of liquefied wood and the properties of polyurethane films prepared from the liquefied wood. Biomass Bioenerg. 2001, 21, 381–390. [Google Scholar] [CrossRef]
- Japanese Industrial Standard. Rigid Cellular Plastics Determination of Compression Properties; JIS K 7220; Japanese Industrial Standards Committee: Tokyo, Japan, 2006.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhai, X.; Hse, C.Y.; Shupe, T.F.; Pan, H. Polyols from Microwave Liquefied Bagasse and Its Application to Rigid Polyurethane Foam. Materials 2015, 8, 8496-8509. https://doi.org/10.3390/ma8125472
Xie J, Zhai X, Hse CY, Shupe TF, Pan H. Polyols from Microwave Liquefied Bagasse and Its Application to Rigid Polyurethane Foam. Materials. 2015; 8(12):8496-8509. https://doi.org/10.3390/ma8125472
Chicago/Turabian StyleXie, Jiulong, Xianglin Zhai, Chung Yun Hse, Todd F. Shupe, and Hui Pan. 2015. "Polyols from Microwave Liquefied Bagasse and Its Application to Rigid Polyurethane Foam" Materials 8, no. 12: 8496-8509. https://doi.org/10.3390/ma8125472





