1. Introduction
The standard medical practice of soft tissue palpation is based on the qualitative assessment of stiffness of tissues. Human tissue lesions are generally correlated with the variation in elastic properties of tissues. In many cases, despite the difference in stiffness between a lesion and normal tissue, the detection and evaluation of a pathological lesion via palpation is difficult owing to its small size or its location. In general, lesions may or may not exhibit sonographic contrast, which would enable them to be ultrasonically detectable. For instance, tumors of the prostate or breast may be significantly stiffer than the embedding tissue, and yet be invisible or barely visible in standard ultrasound examinations. Diffuse diseases such as cirrhosis of the liver are known to significantly increase the stiffness of liver tissue; however, the tissue may appear normal in conventional ultrasound examination [
1].
Therefore, we can obtain new information related to the biomechanical properties of tissues for differentiating normal tissues from abnormal tissues by imaging tissue stiffness or a related parameter, such as strain under stress. In 1991, Ophir
et al. [
1] proposed a method to quantify strain information in biological tissues. A method capable of quantitatively imaging the stiffness of tissues with good resolution, sensitivity, and diminished speckle was presented in [
2]. Ultrasound elastography can generate numerous types of images referred to as elastograms. Ultrasound elastography is typically used to evaluate a mass as benign or malignant for lesions. Soft and hard materials exhibit large and low strain values, respectively, and large and low strain values are typically displayed as bright and dark regions, respectively. Ultrasound elastography is being increasingly used for assessing the biomechanical properties of tissues and has been applied to numerous organs and pathologies including many cancers, such as scirrhous carcinoma of the breast, liver cancer, and prostatic carcinoma [
3,
4,
5]. The different types of ultrasound elastography methods include compression ultrasound elastography, acoustic radiation force impulse (ARFI) technique, and shear wave elastography [
5,
6,
7].
Compression ultrasound elastography is the most commonly used method in which a constant stress is applied to the studied tissue [
5,
7,
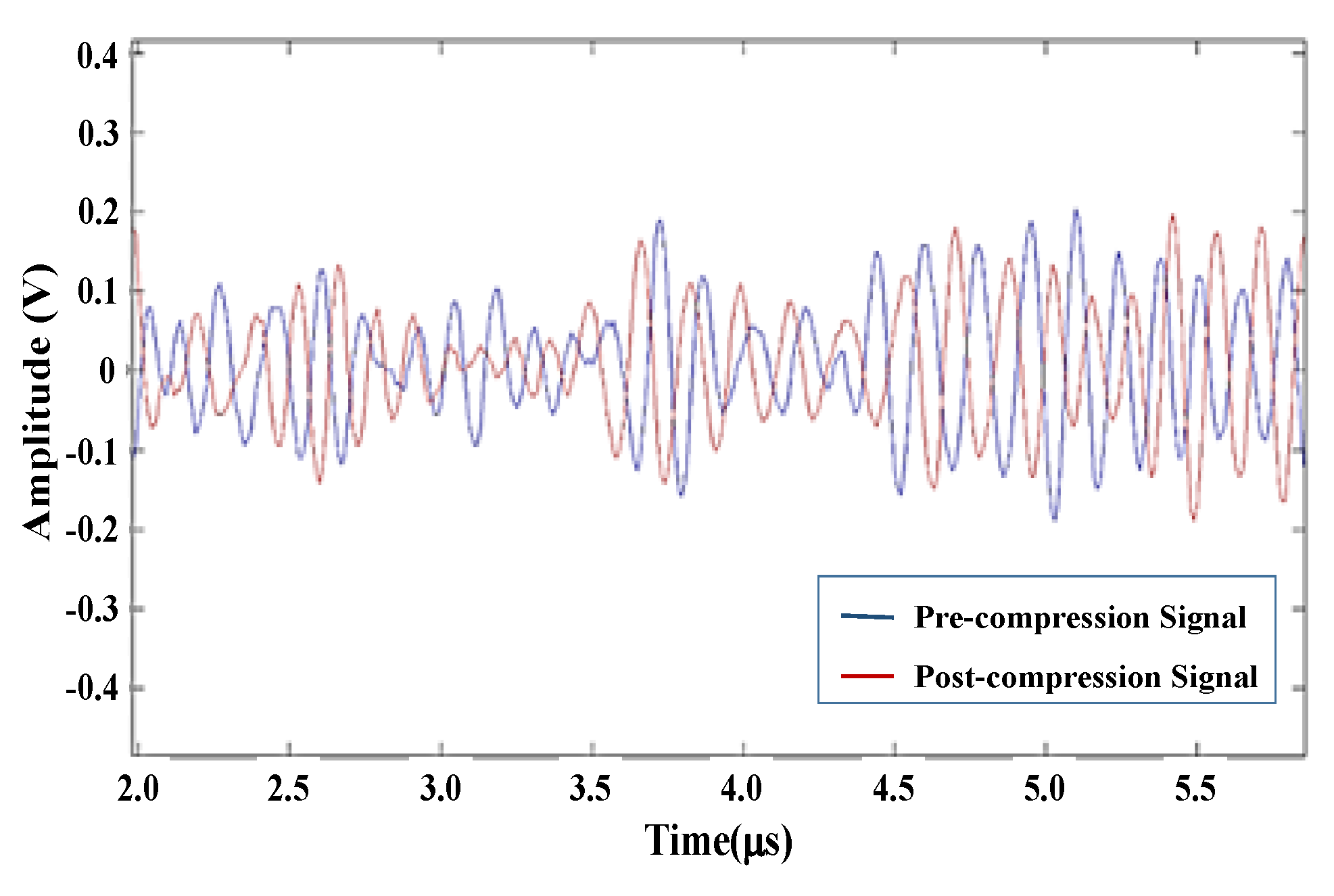
8]. The technique uses manual compression or a controlled stepper motor to move a transducer to generate stress on the tissue and measures tissue deformation to estimate the elasticity of the tissue. Displacement is estimated by comparing echoes before and after compression by correlation methods [
9]. The measured displacement, generated strain (ε), and stress can be used to compute the Young’s modulus, which is a more objective parameter of stiffness. This strain map is often referred to as an elastogram because the applied stress is unknown and only strain is displayed. Young’s modulus is the ratio of stress to strain within the elastic limit. Nightingale proposed the ARFI method in which acoustic radiation force is employed in place of transducer compression to generate force [
7]. Acoustic radiation force is a unidirectional force applied to absorbing or reflecting targets in the propagation path of an acoustic wave. Attenuation is a frequency-dependent phenomenon and is primarily caused by absorption in soft tissues. The momentum transfer from the acoustic wave generates a force that causes displacement of the tissue [
10,
11]. Sarvazyan
et al. [
12] proposed a method, which can be considered to be a precursor of elastography techniques, based on ultrasonic radiation pressure by combining radiation pressure or acoustic radiation force and the shear waves generated. This technique is referred to as shear wave elasticity imaging (SWEI). Thereafter, Nightingale
et al. validated the clinical feasibility of radiation force induced shear-wave imaging [
13]. When acoustic radiation force is applied to a given spatial volume for a short duration, transient shear waves that propagate away from the initial region of excitation and perpendicular to the push pulse are generated. In tissues, shear waves travel at a velocity of approximately 1–10 m/s and can be easily tracked using ultrasound [
10].
Ultrasound elastography is being increasingly used by clinicians to detect lesions or cancers in soft tissues. However, poor quality of elasticity imaging may degrade its value in clinical applications [
14]. Imaging quality is determined by the visibility of small lesions and is limited by decorrelation noise [
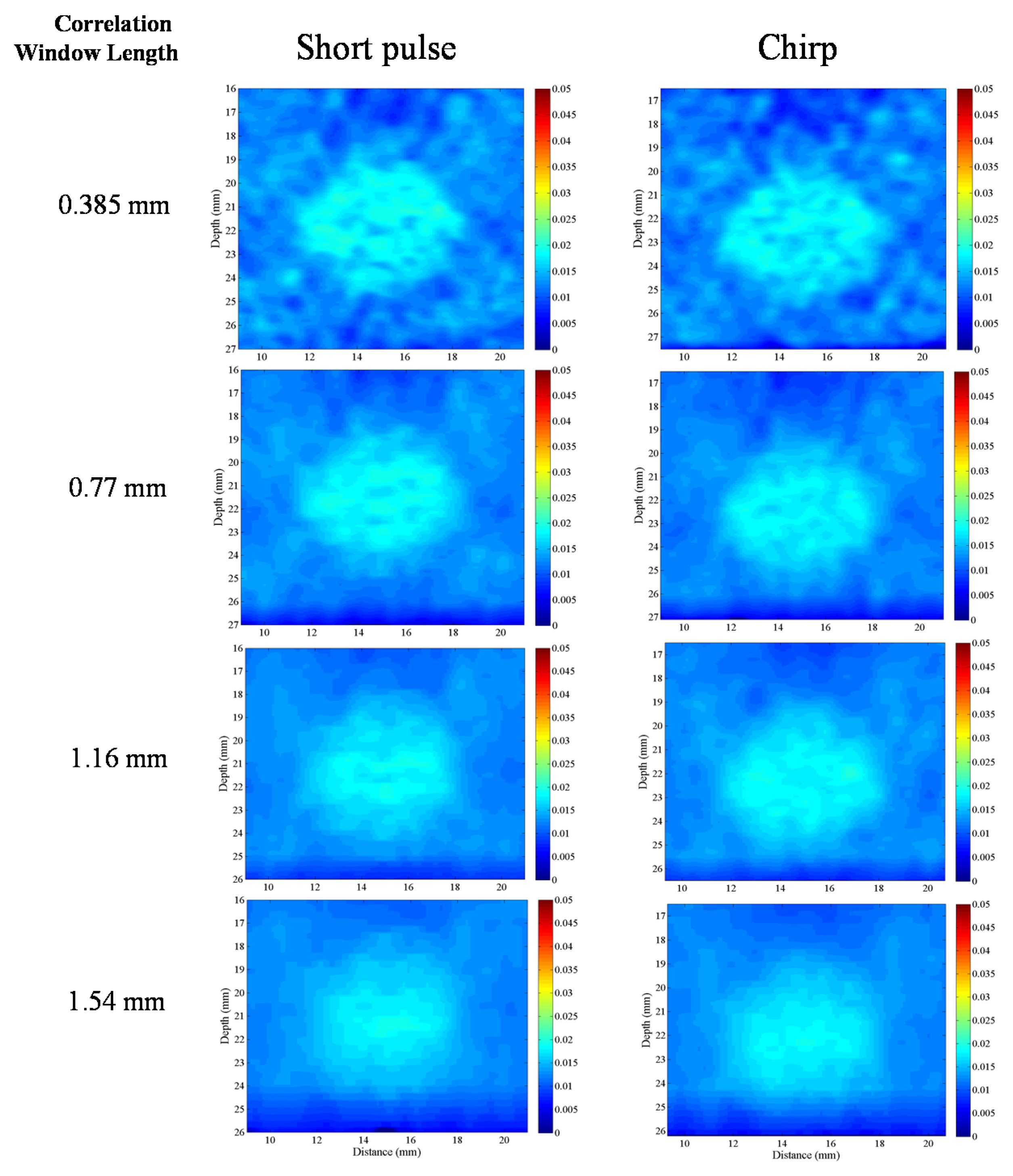
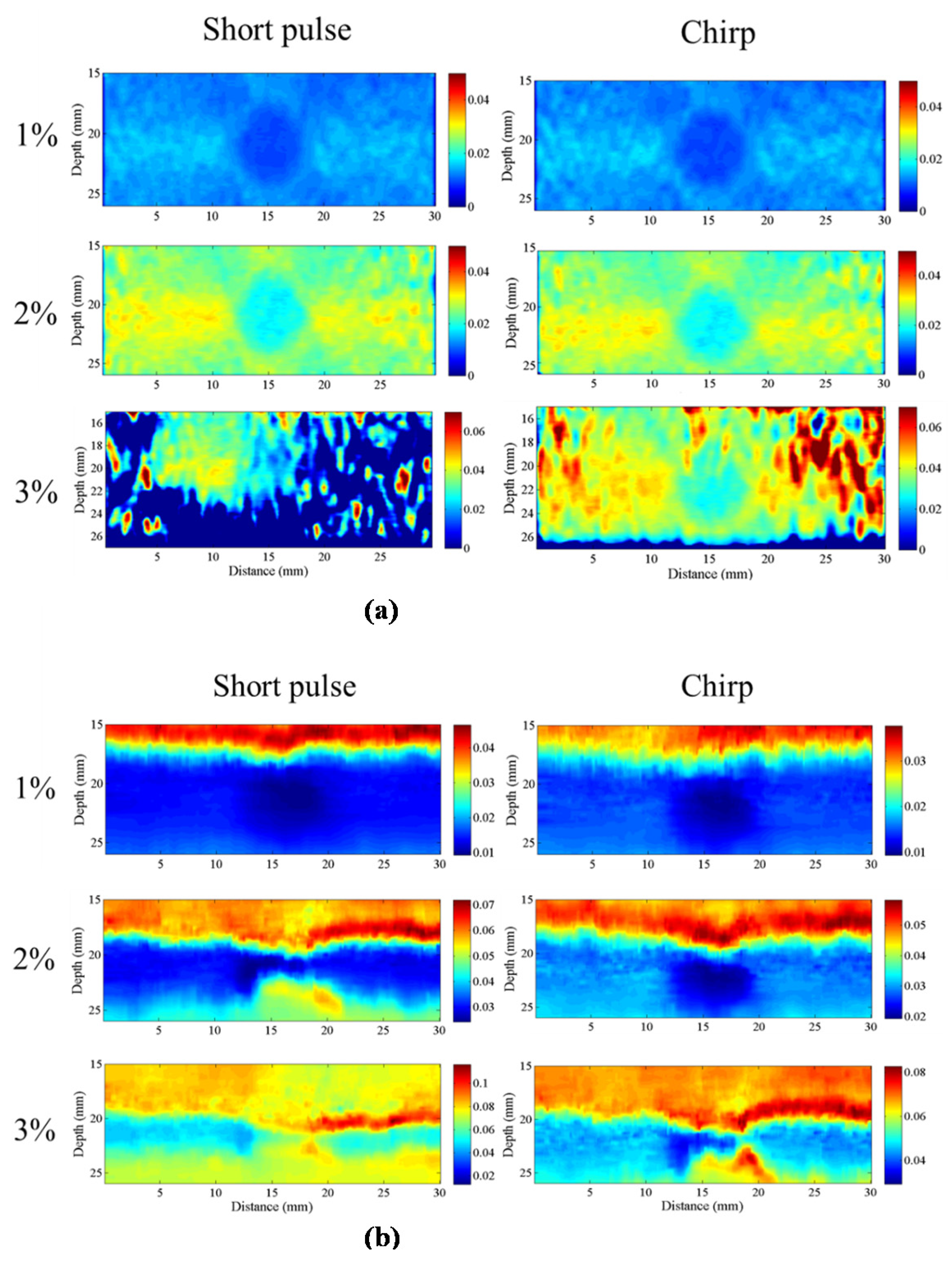
15]. Elastographic signal-to-noise ratio (SNRe), one of the major quality metrics of elasticity imaging, is affected by numerous factors such as echo signal-to-noise ratio (eSNR), applied strain, attenuation, penetration depth, and axial resolution. In an ultrasound system, the energy of the trigger signal affects eSNR and penetration depth, whereas the bandwidth of the signal influences axial resolution. A low eSNR reduces the uniformity between continuous echo frames acquired to estimate correlation-based displacement. Therefore, large displacement errors are generated and appear in strain images as a high-intensity noise; this is referred to as the decorrelation noise. In this situation, longer correlation window lengths reduce decorrelation noise for small tissue deformations at the cost of slightly-reduced axial resolution [
16]. Therefore, the performance of elasticity imaging can be improved by increasing the energy of the trigger signal. In ultrasound imaging systems, the energy of the signal can be increased in two ways. First, when the peak power of the trigger signal is increased, commercial ultrasound systems increase peak power of trigger signal to the maximum value that is close to the mechanical index (MI) specification. Second, the average power of the trigger signal can be increased by lengthening the duration of the trigger. Therefore, a coded excitation signal can increase the average power of the trigger signal without affecting the power amplitude to realize a better eSNR.
Since the elastic properties of soft tissues are an important diagnostic indicator in clinics, palpation is one of the most commonly used diagnostic methods. However, palpation is limited to the inspection of superficial tissue, and the diagnosis is subjective with low sensitivity. The disadvantages of assessing tissue elasticity via palpation can be overcome by using ultrasound elastography. However, the eSNR is diminished because of the attenuation of ultrasonic energy by soft tissues, thus resulting in reduction of elastography qualities such as SNRe and elastographic contrast-to-noise ratio (CNRe). Chirp-coded pulse excitation has been proposed for improving the SNRe and CNRe of ultrasound elastography. However, the elongated chirp-coded pulses decrease the axial resolution of an ultrasound image. Therefore, pulse compression technique can be employed to improve the axial resolution of images. However, the effects of chirp-coded pulse modulated with different window functions and different strain analysis algorithms on elastography qualities are still not clearly known.
The aim of this study is to develop an ultrasound elasticity imaging system with chirp-coded excitation for assessing the biomechanical properties of tissue-mimicking phantom. Further, we analyzed the correlation between the qualities of elastography and a chirp-coded pulse modulated with different window functions and the effects of different strain analysis algorithms on the qualities of elastography.
2. System Architecture
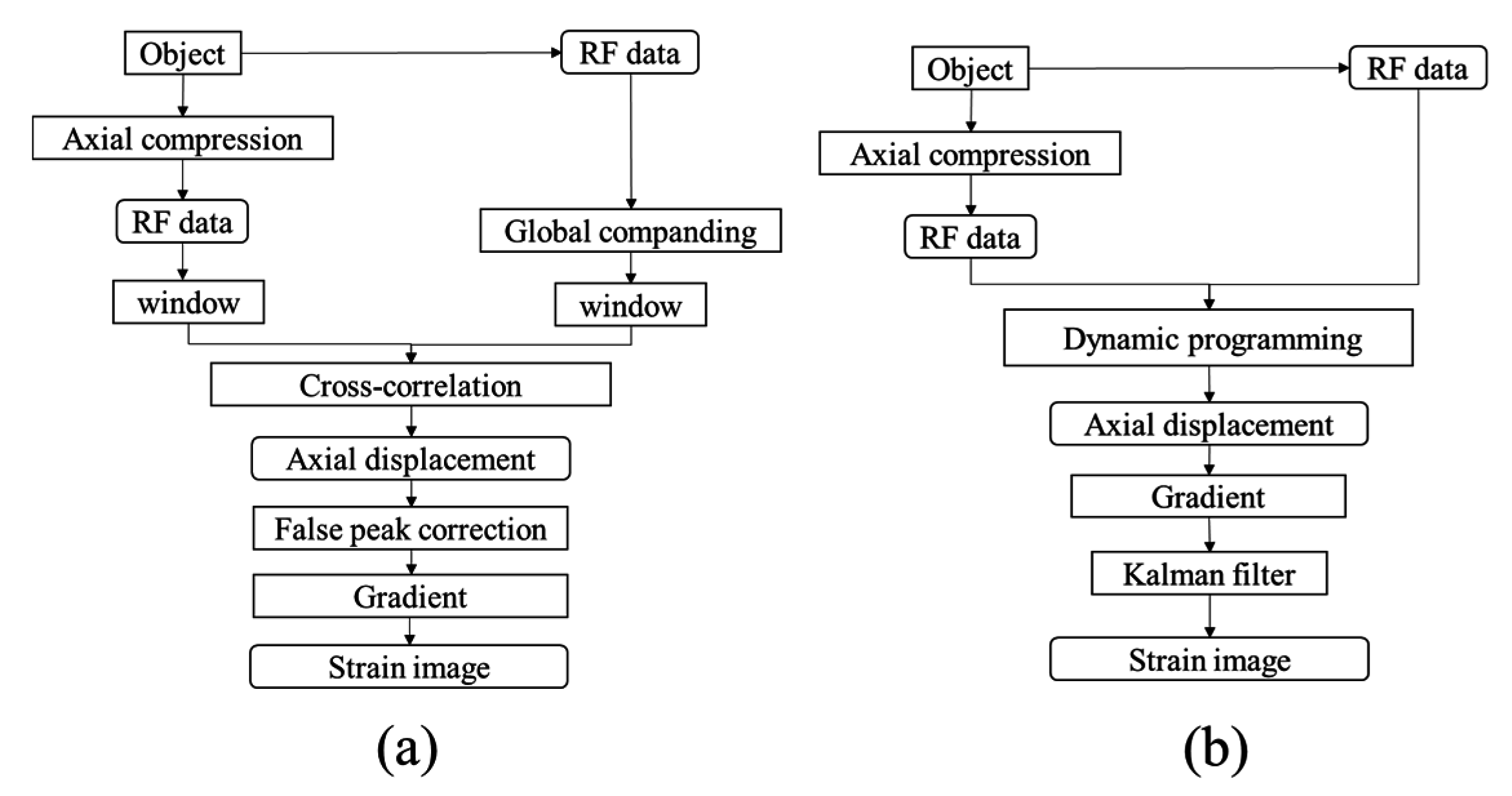
In this study, an ultrasound elastography system was developed for quantitatively assessing the elasticity properties of tissues and phantoms. The schematic diagram of the proposed ultrasound elastography system with two types of excitation signals is shown in
Figure 1. First, a 7.5-MHz single-element focused transducer (Model V320; Panametrics, Waltham, MA, USA) was excited by an ultrasonic pulser (Model 5058PR; Panametrics). Subsequently, a chirp waveform was generated using the MATLAB program and used as the trigger signal input for the arbitrary waveform generator (AWG; Model AFG3252; Tektronix Inc., Beaverton, OR, USA) connected to a radio-frequency power amplifier (Model 25A250A; Amplifier Research, Souderton, PA, USA). The echoes were then amplified by the receiver (Model 5073PR; Panametrics).
Figure 1.
Block diagram of ultrasound elasticity imaging system.
Figure 1.
Block diagram of ultrasound elasticity imaging system.
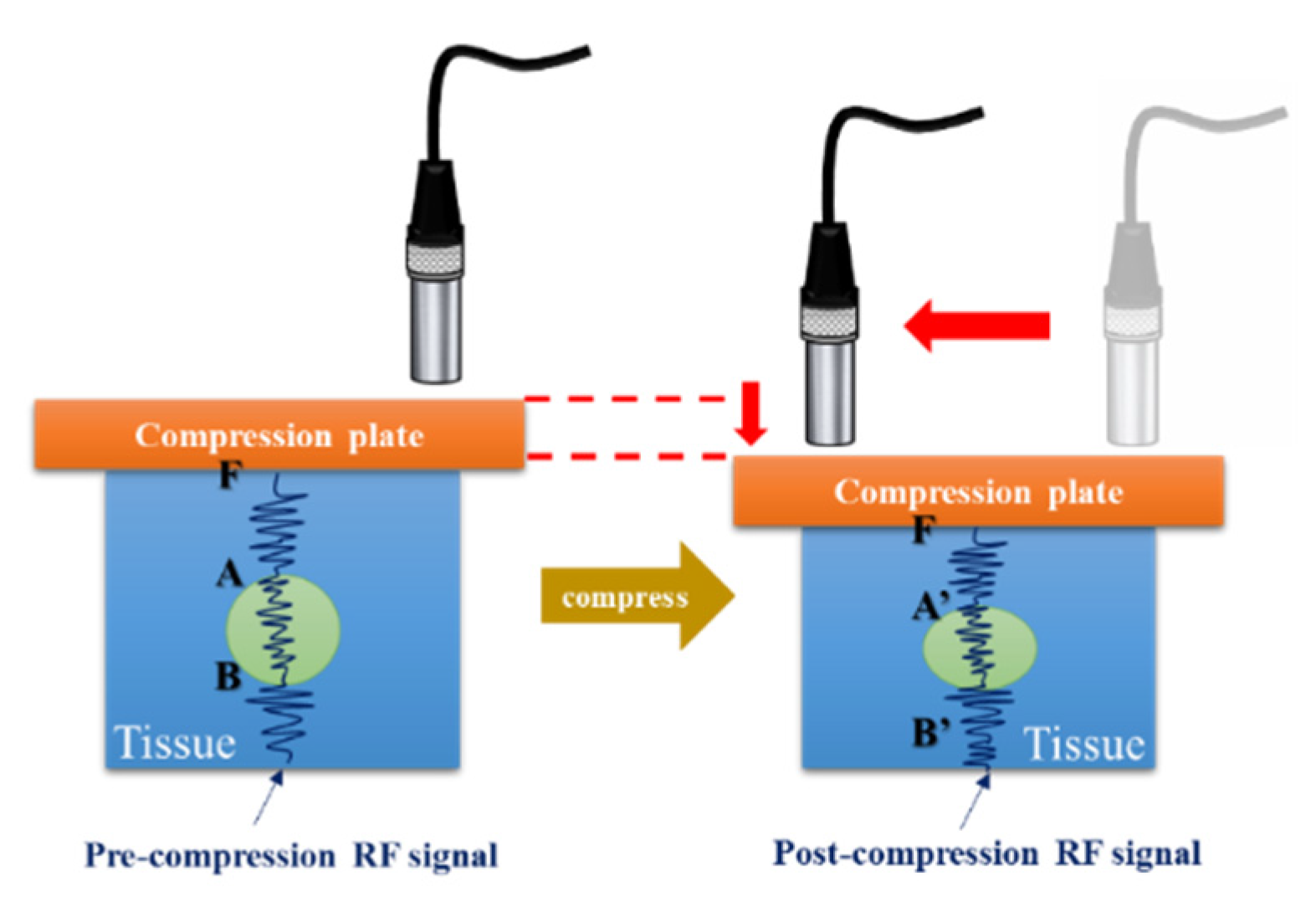
The received signals were digitized by an 8-bit analog-to-digital converter (Model PCI5152; National Instruments, Austin, TX, USA) at 2 GS/s sampling rate housed in a personal computer. The LabVIEW program was used to control the motor, acquire the data, and process the signals. Additionally, a three-axis step motor was used to move the ultrasonic probe and the indentation system containing the load cell (Model SMT S-Type; Interface Inc., Scottsdale, AZ, USA) and the compression plate (Polymethylpentene (PMP/TPX; Plastics Industry Development Center, Tainan City, Taiwan)), as shown in
Figure 2a. The load cell was connected in series with a compression plate to record the corresponding force response. The indentation system was combined with a fixture and fixed on a
Z-axis motor, and the ultrasonic probe was fixed on an
X-axis motor, as shown in
Figure 2b.
Figure 2.
(a) Load cell and compression plate and (b) indentation system.
Figure 2.
(a) Load cell and compression plate and (b) indentation system.
5. Conclusions
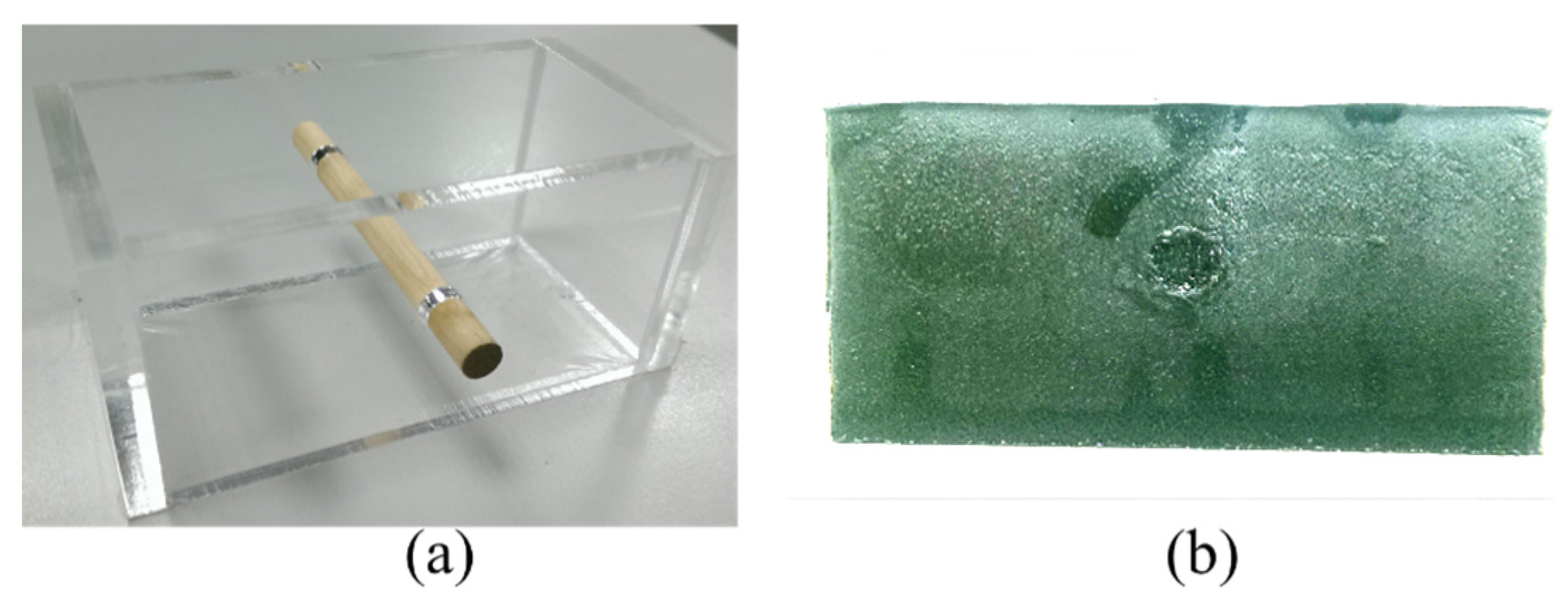
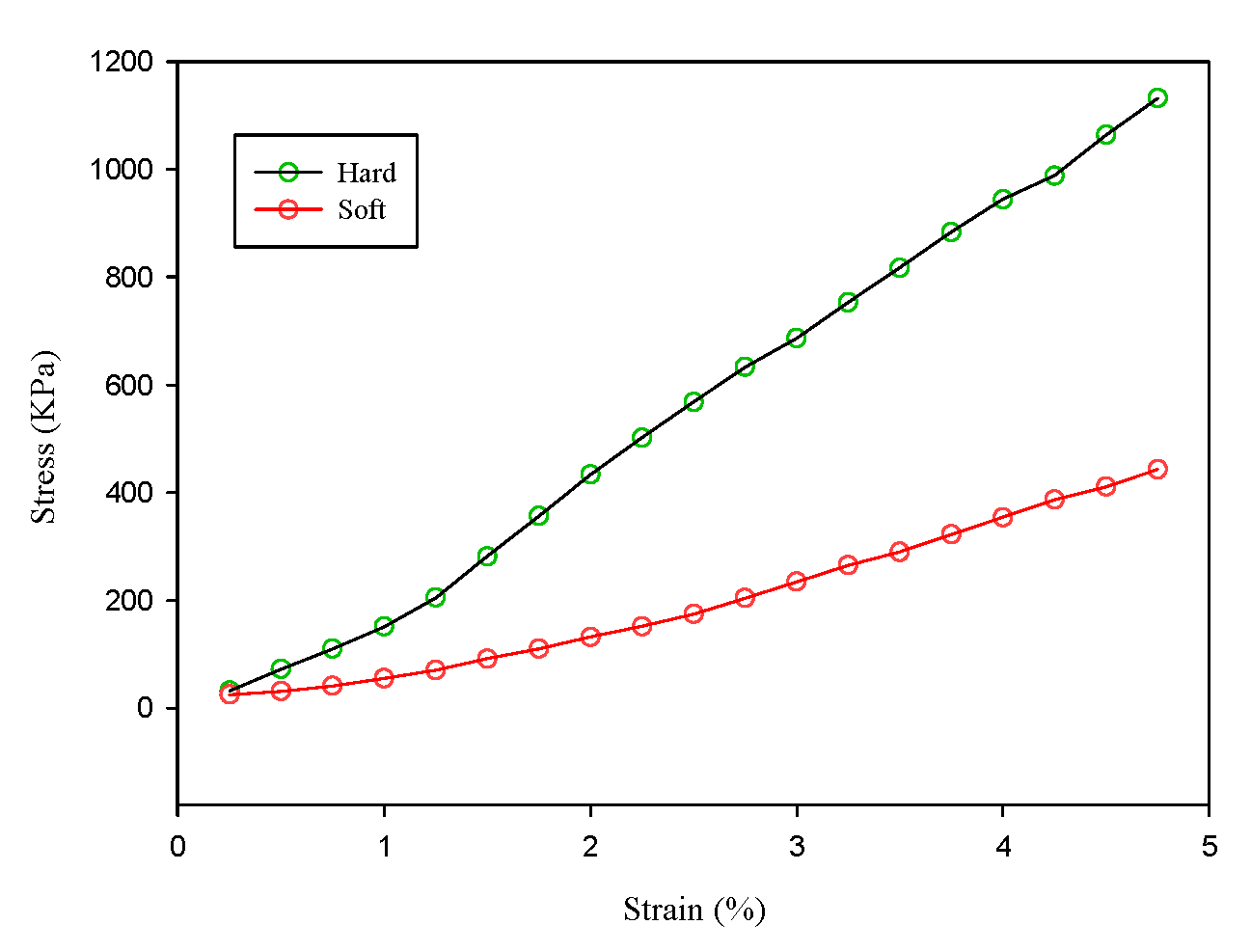
In this study, we proposed an ultrasound elastography system with chirp-coded excitation for evaluating the elasticity of a phantom (Young’s modulus of the background materials and cylindrical inclusion were 13.27 and 26.86 kPa, respectively) and investigating the strain performance of CC and AD algorithms. In order to minimize side lobes without degrading axial resolution, a mismatched compression filter was used to decode the echo signals.
Development of a chirp coded excitation ultrasound system is developed by using chirp signal as the ultrasound trigger signal in order to achieve better signal-to-noise ratio and deeper penetration depth.
Table 6 shows that the echo signal of short pulse is less than the echo signal of chirp-coded excitation. According to experiment results and discussion, there are a 15 dB SNR improvement and a 1–2 mm penetration depth improvement by chirp-coded excitation, pulse compression, the appropriate receiver and the handmade expander of noise reduction and SNR enhancement.
Table 6.
The relationship between the pulse type and echo peak amplitude.
Table 6.
The relationship between the pulse type and echo peak amplitude.
| Pulse Type | Peak Frequency (MHz) | Echo Peak Amplitude (V) |
|---|
| Unipolar Pulse | 25 | 0.96 |
| Bipolar Pulse | 25 | 1.04 |
| Coded excitation (Chirp) | 25 | 2.08 |
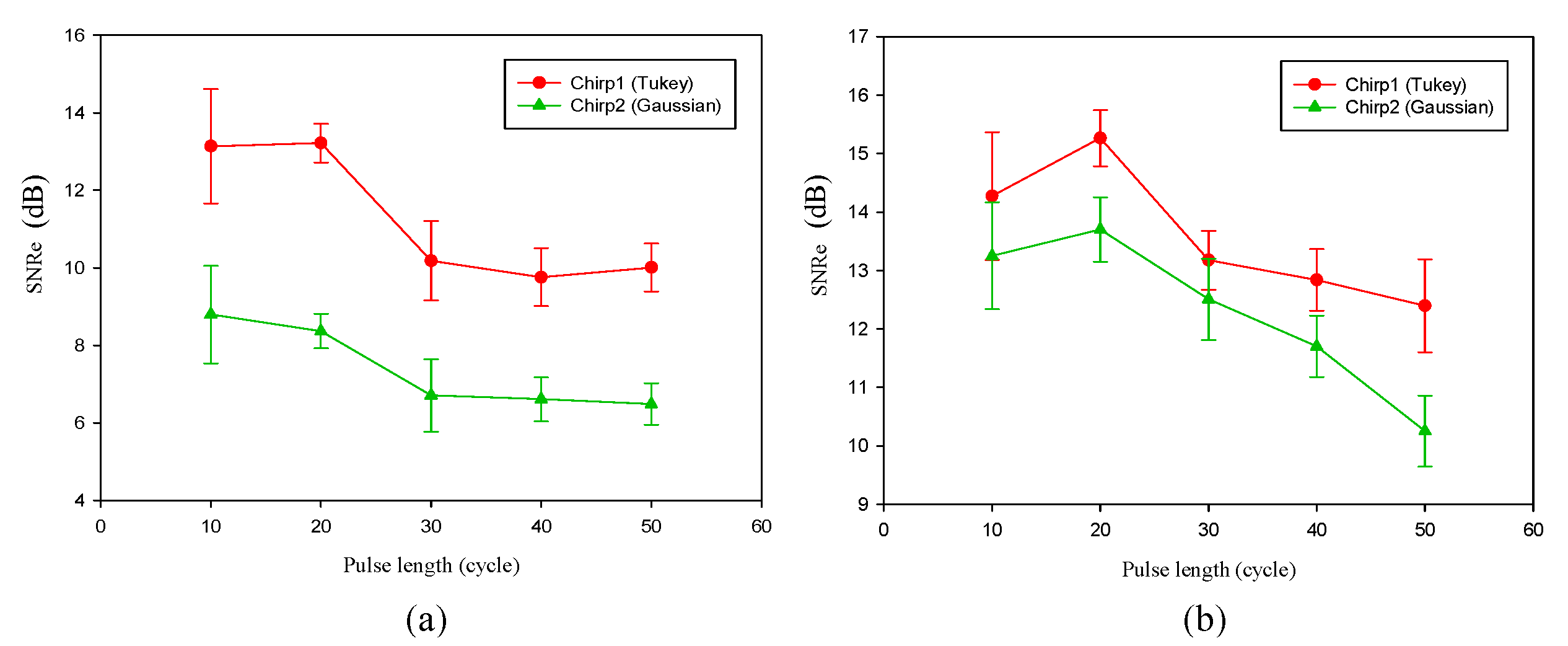
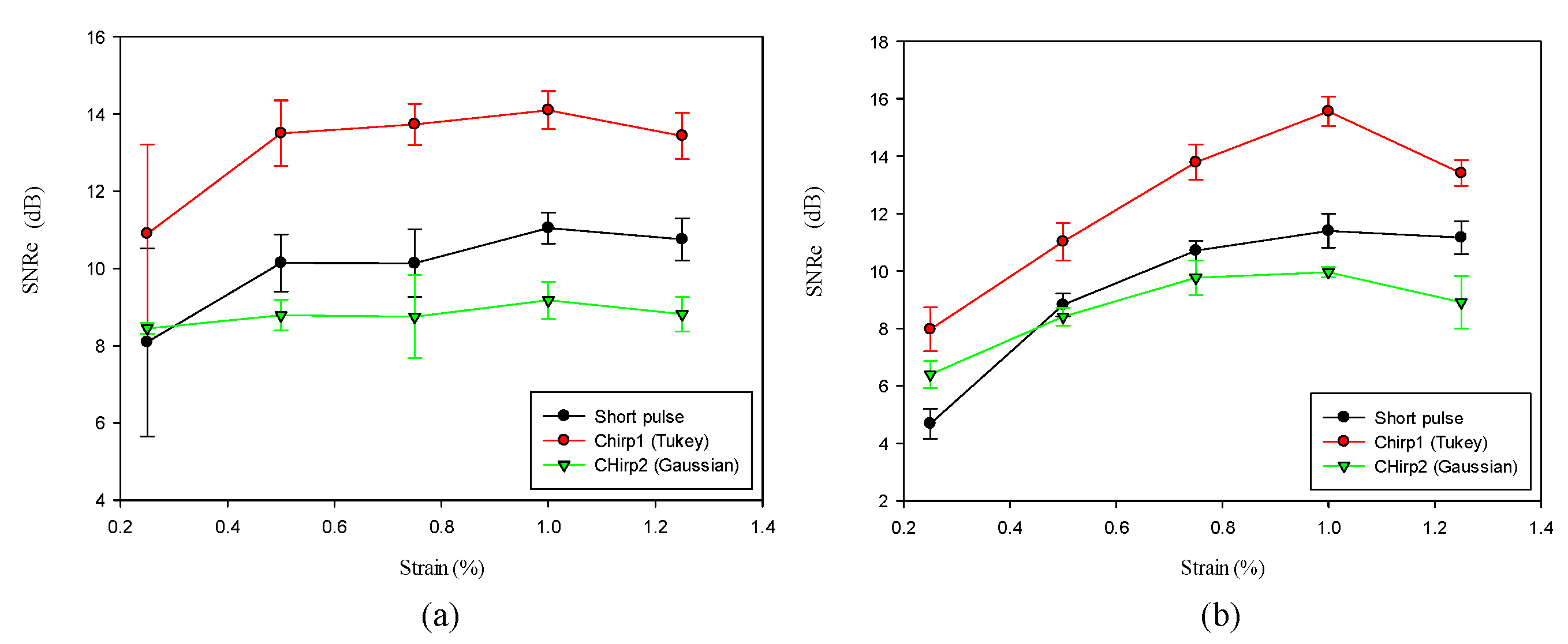
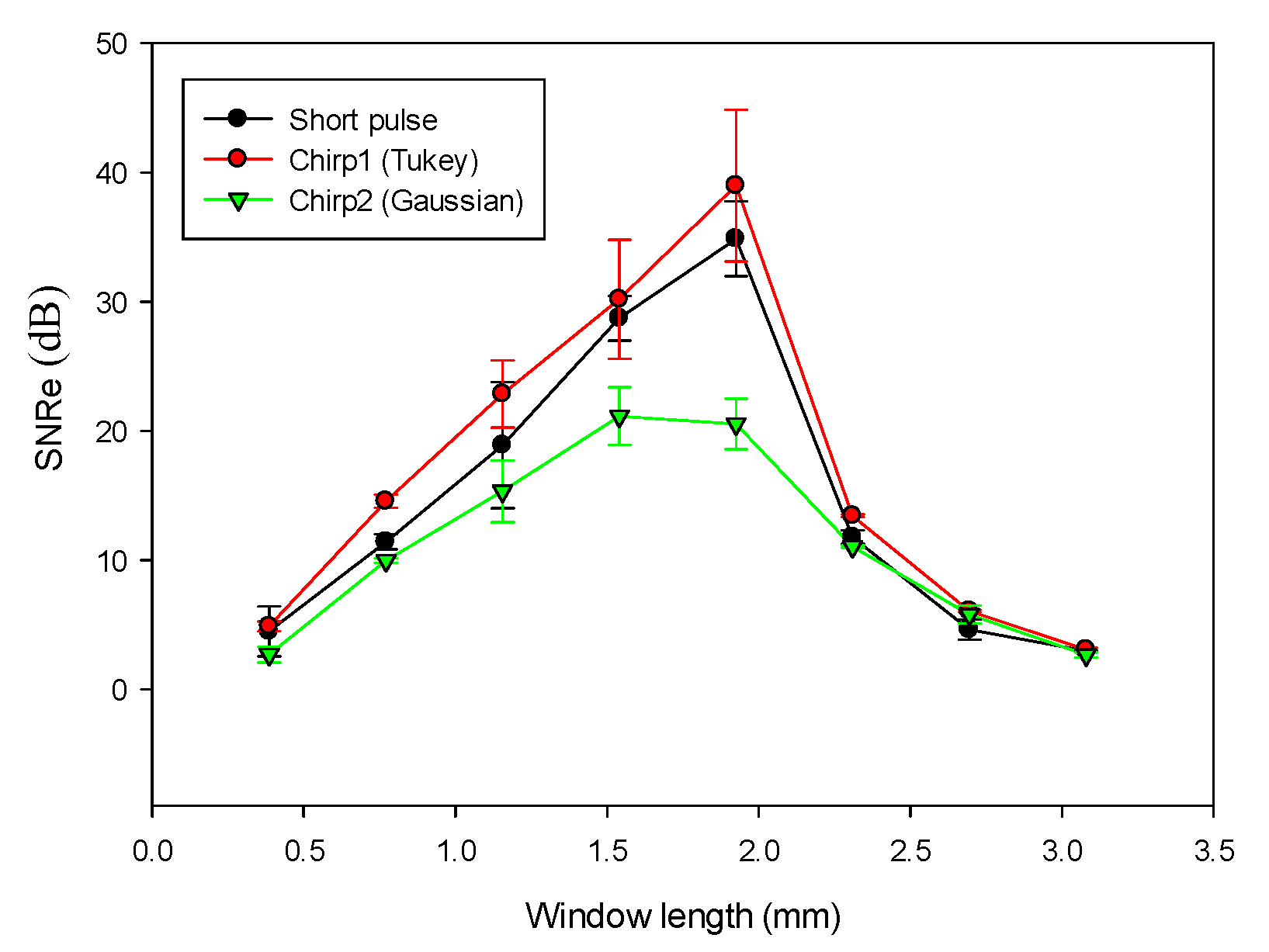
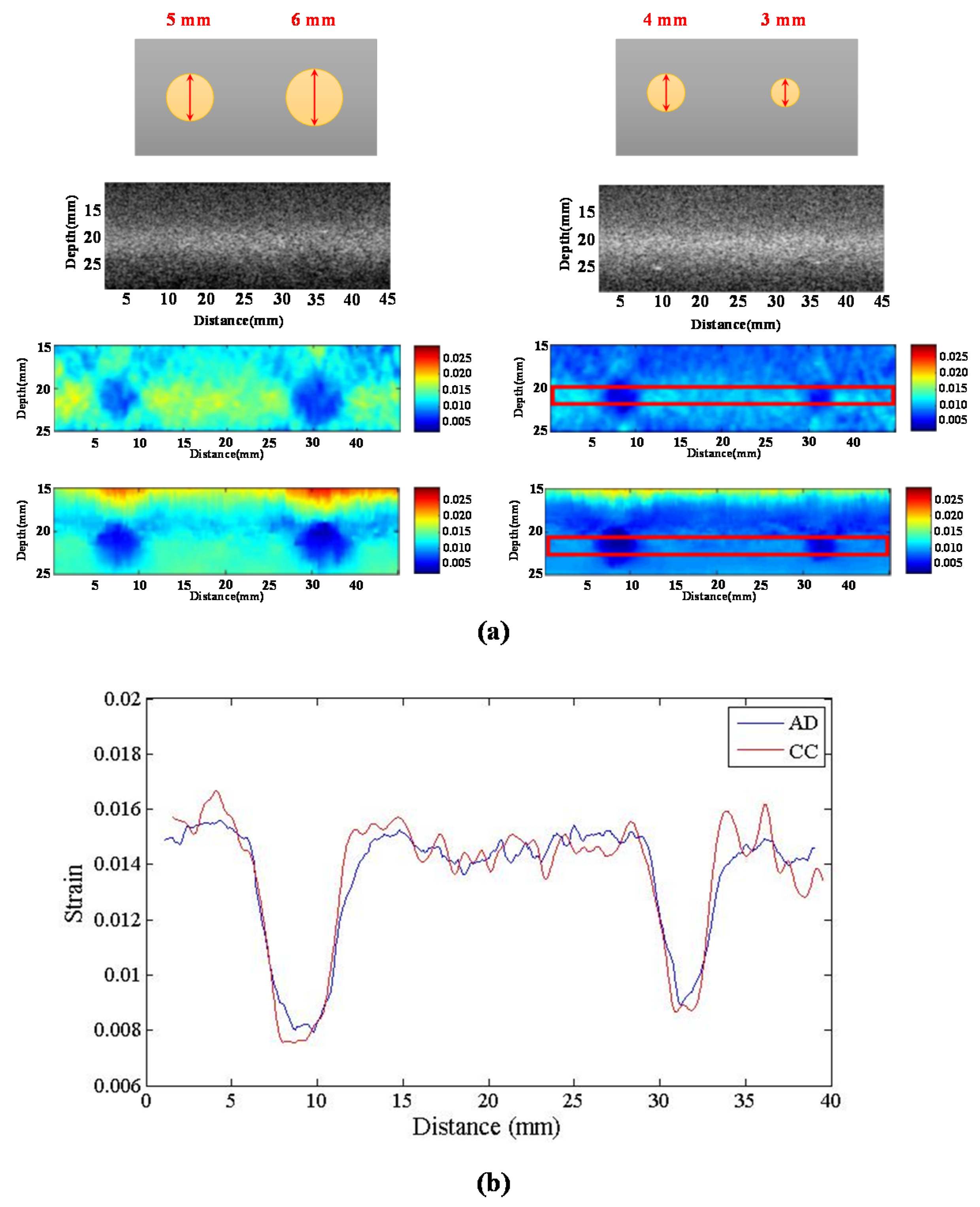
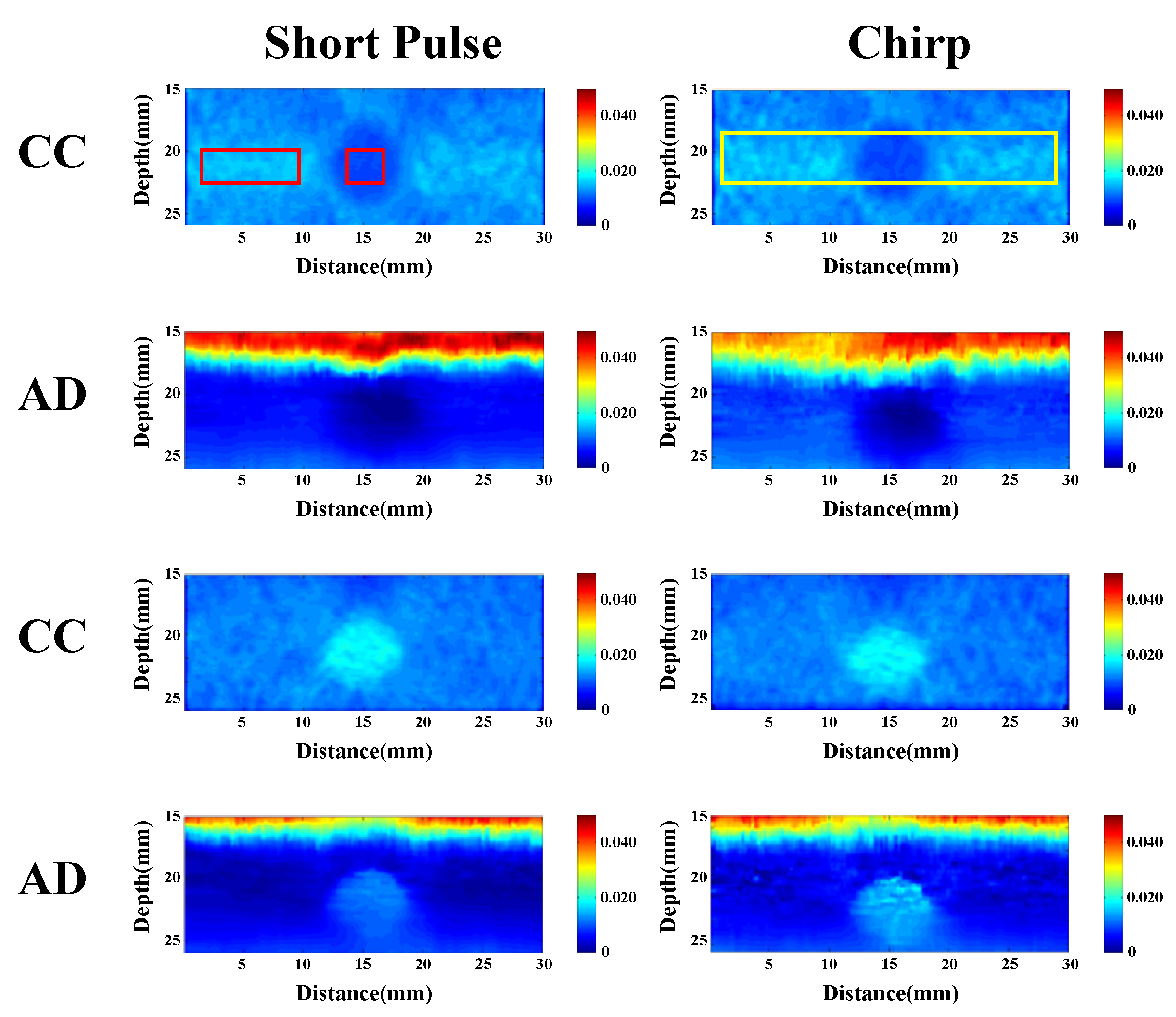
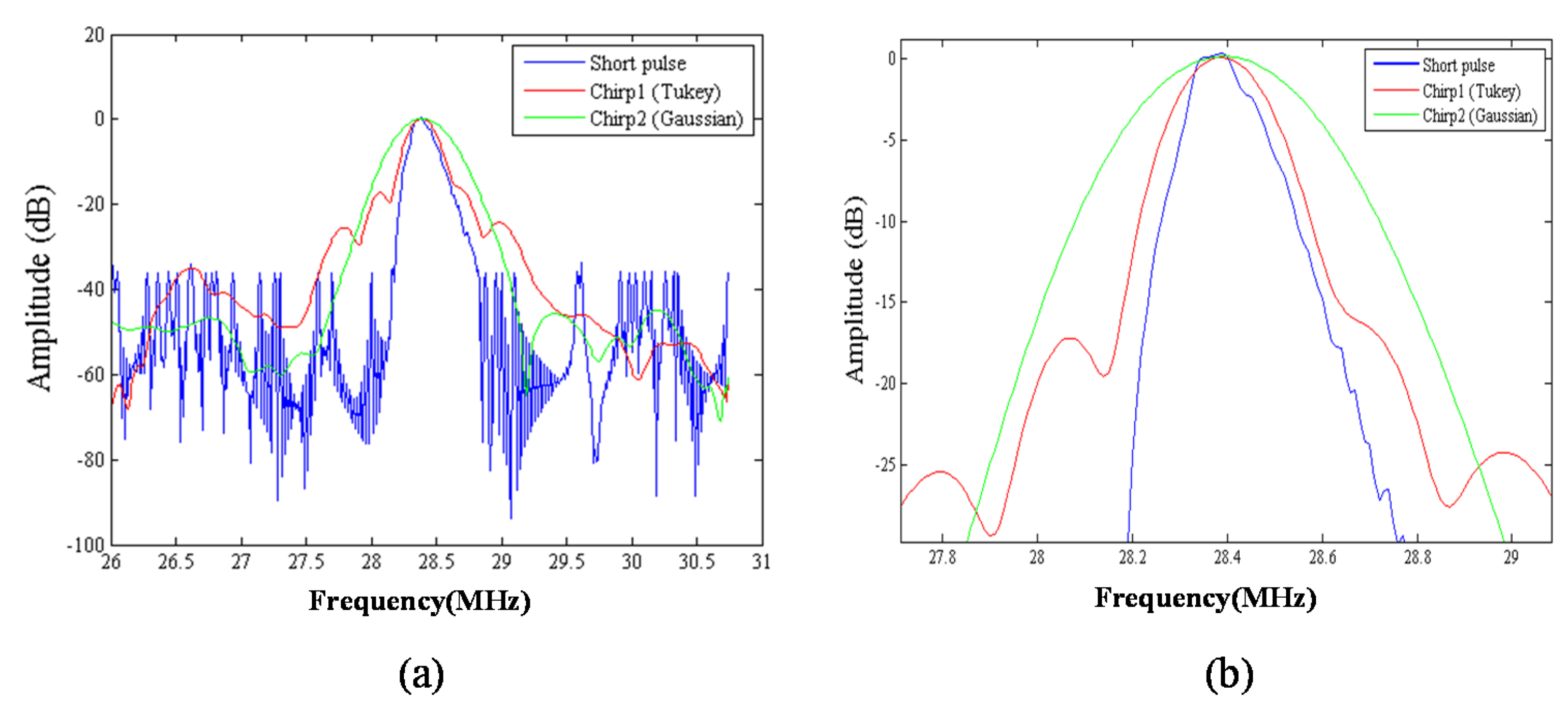
We compared the performance of different chirp schemes with the conventional short pulse. The chirp with Gaussian window, which is common and suitable for B-mode imaging, was found to exhibit poor performance of SNRe than both the short pulse and the chirp with Tukey window because the width of the main lobe was not been considered for evaluating the optimal chirp scheme for B-mode imaging. Thereafter, the effects of different factors such as chirp pulse length, applied strain, and correlation window length on strain imaging were investigated. Optimal parameters of the three pulses were the same for both the CC and AD algorithms.
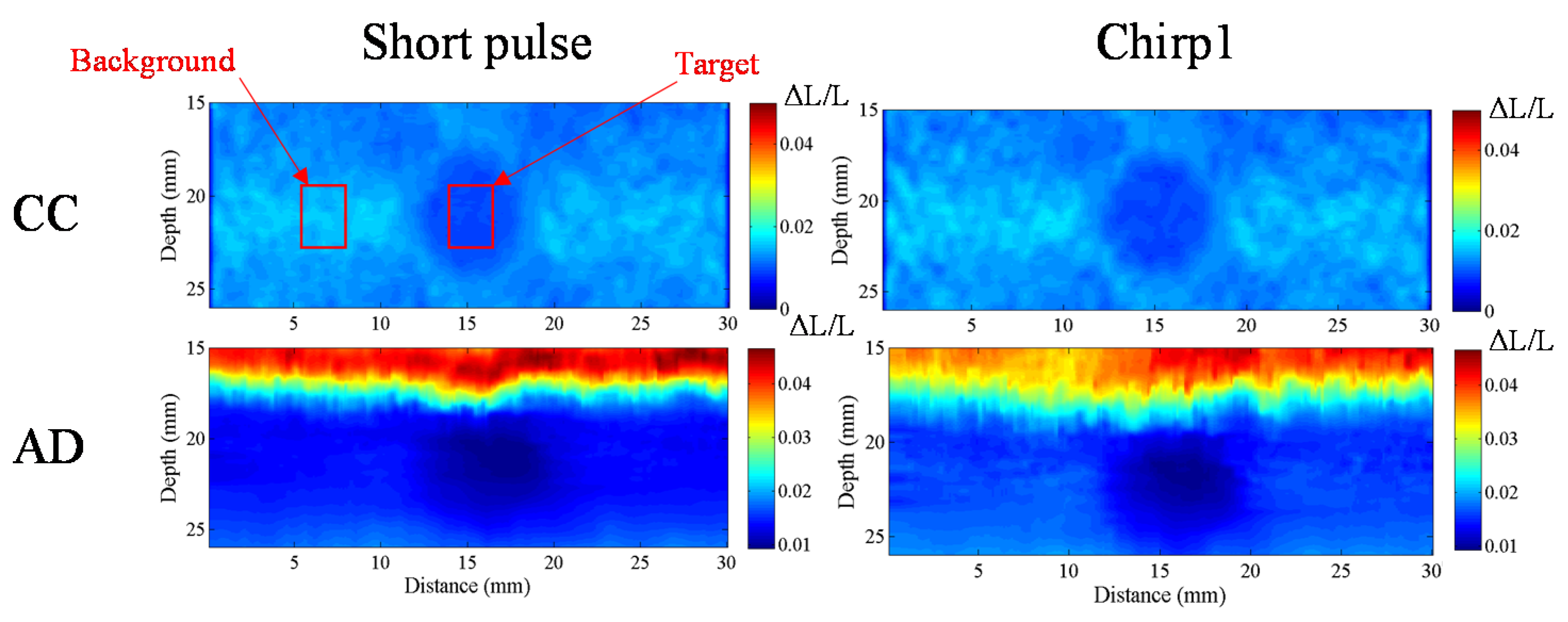
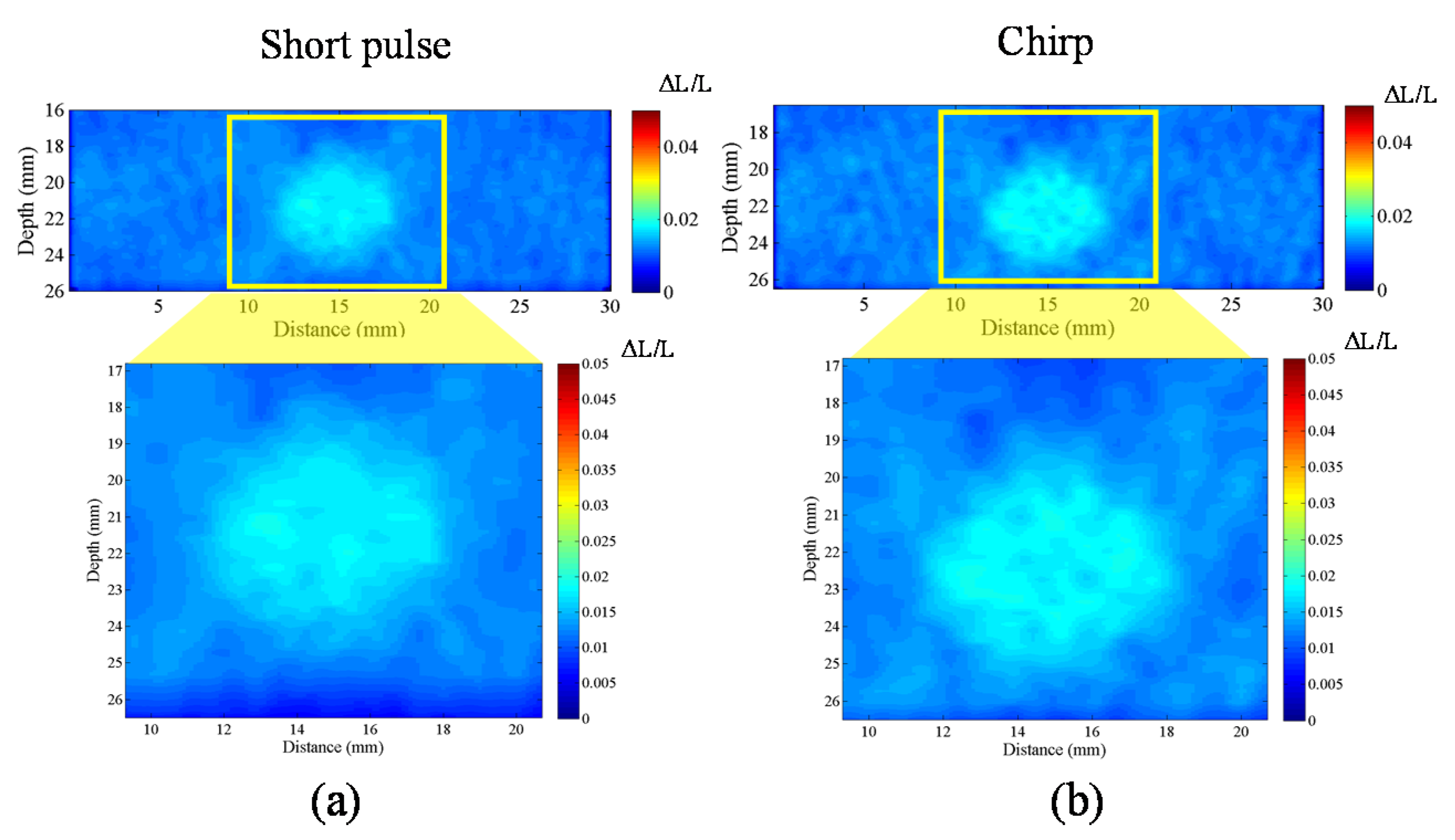
The homogenous phantom experimental results show that the SNRe of elastography measured using a short pulse is 11 dB. The SNRe values measured using a 20-cycle chirp-coded ultrasound system modulated using CC and AD algorithms were 15 and 13 dB, respectively. The CNRe of the image obtained using the chirp-coded pulse can be improved by 4.1 dB when compared with that obtained using the short pulse. The results validate that a chirp with Tukey window has better lesion detectability than a short pulse. Additionally, the Young’s modulus values of the cylindrical inclusion analyzed using the CC and AD algorithms were 25.52 and 22.72 kPa, respectively. These results show that the ultrasound elasticity imaging system with chirp-coded excitation modulated by a Tukey window can acquire highly accurate and high quality elastography images. In the future, the ultrasound elasticity imaging system will be used on human subjects to validate the feasibility of collecting
in vivo data [
29,
30,
31].