Electrical Properties and Interfacial Studies of HfxTi1–xO2 High Permittivity Gate Insulators Deposited on Germanium Substrates
Abstract
:1. Introduction
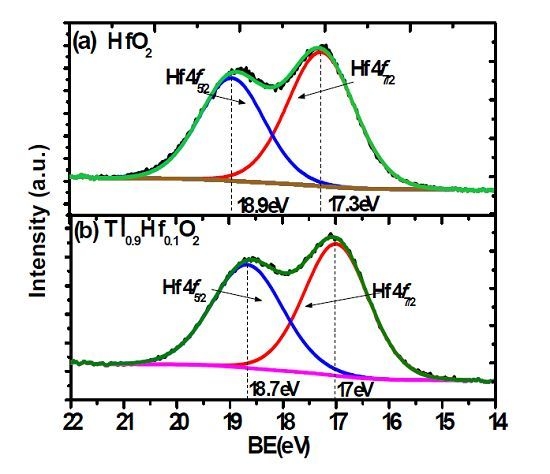
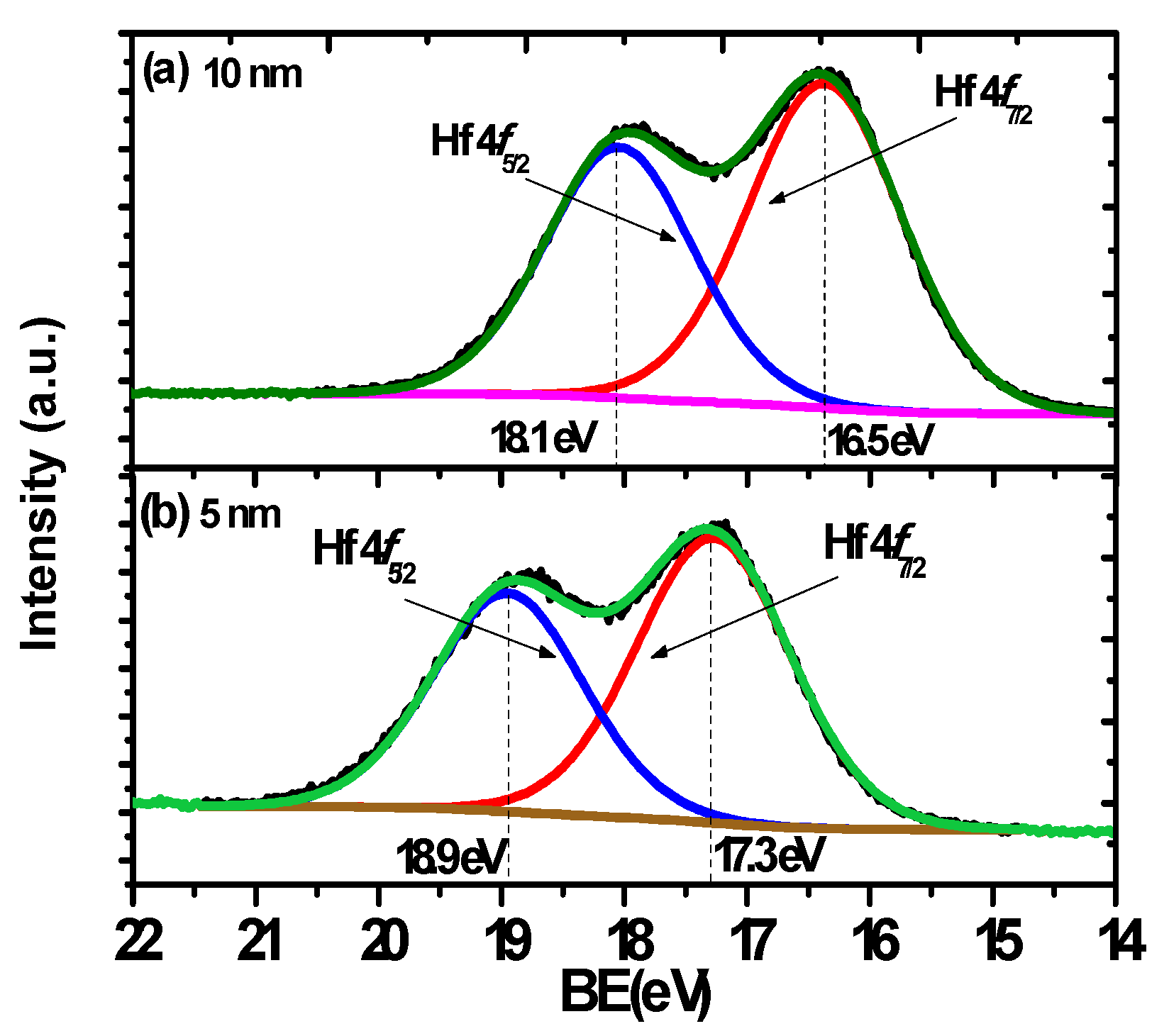
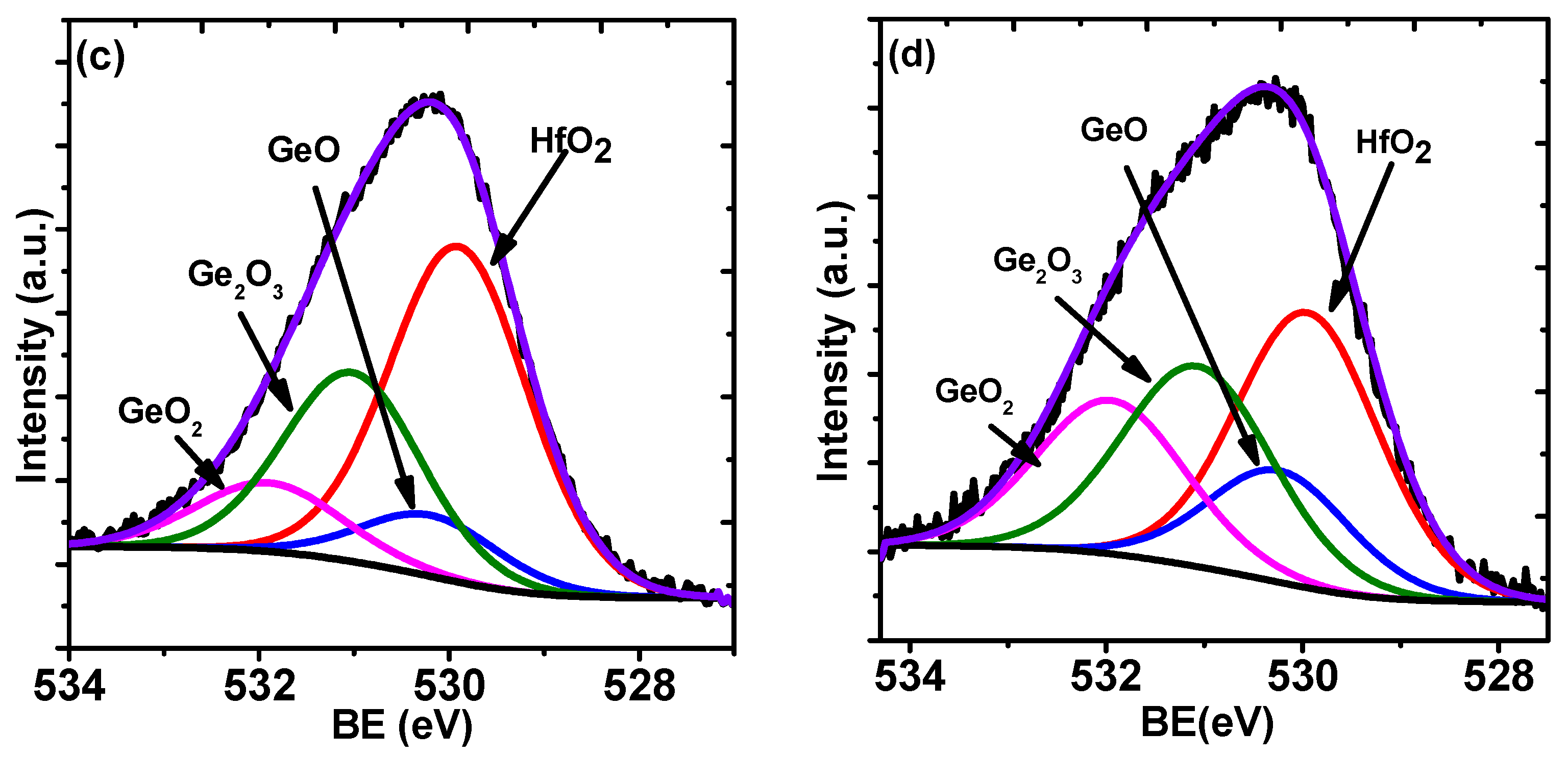
2. Results and Discussion


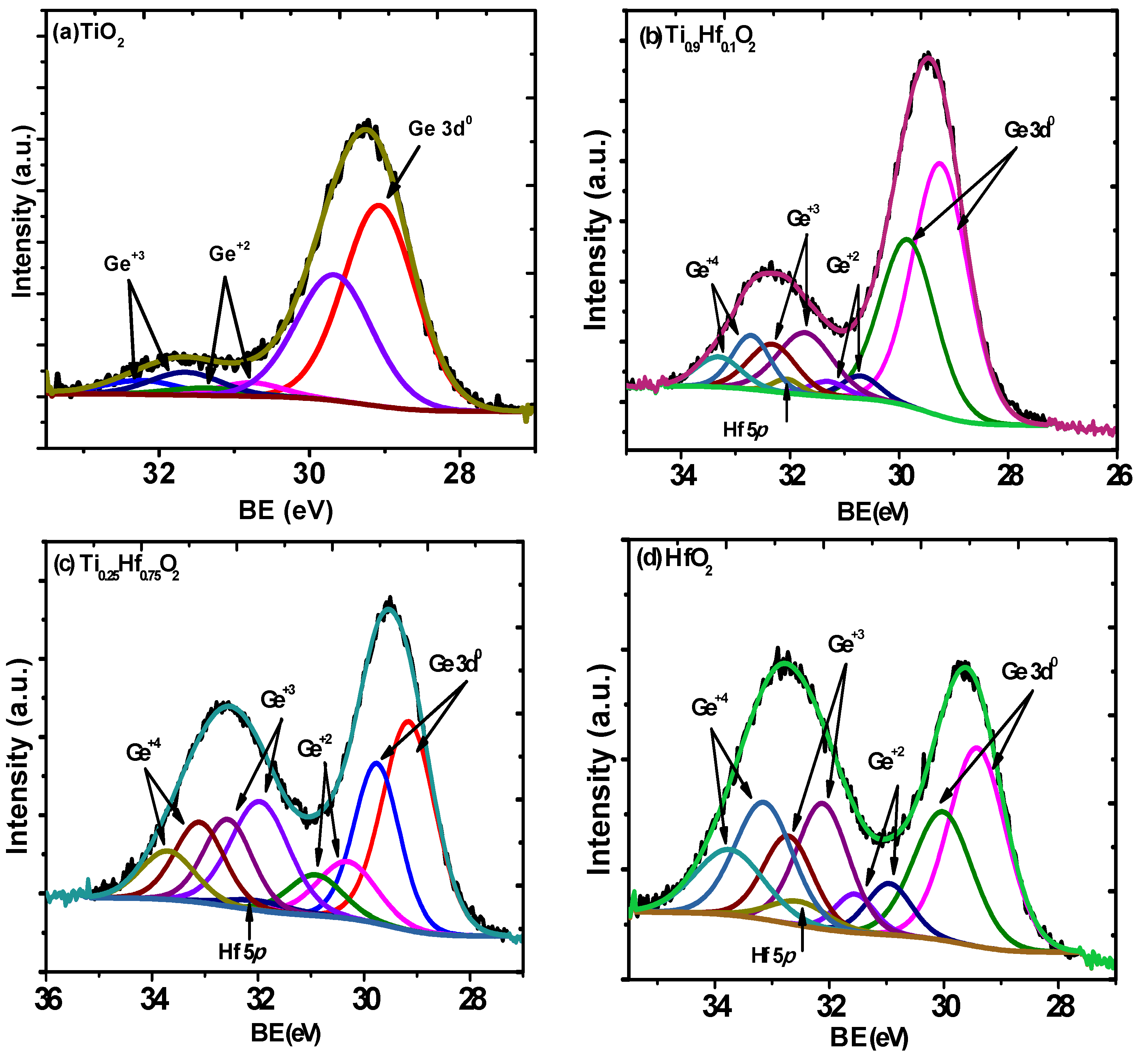

| Materials | Ge+2 | Ge+3 | Ge+4 |
|---|---|---|---|
| TiO2 | 0.06 | 0.12 | – |
| Ti0.9Hf 0.1O2 | 0.06 | 0.24 | 0.14 |
| Ti0.25Hf0.75O2 | 0.17 | 0.24 | 0.41 |
| HfO2 | 0.13 | 0.45 | 0.75 |


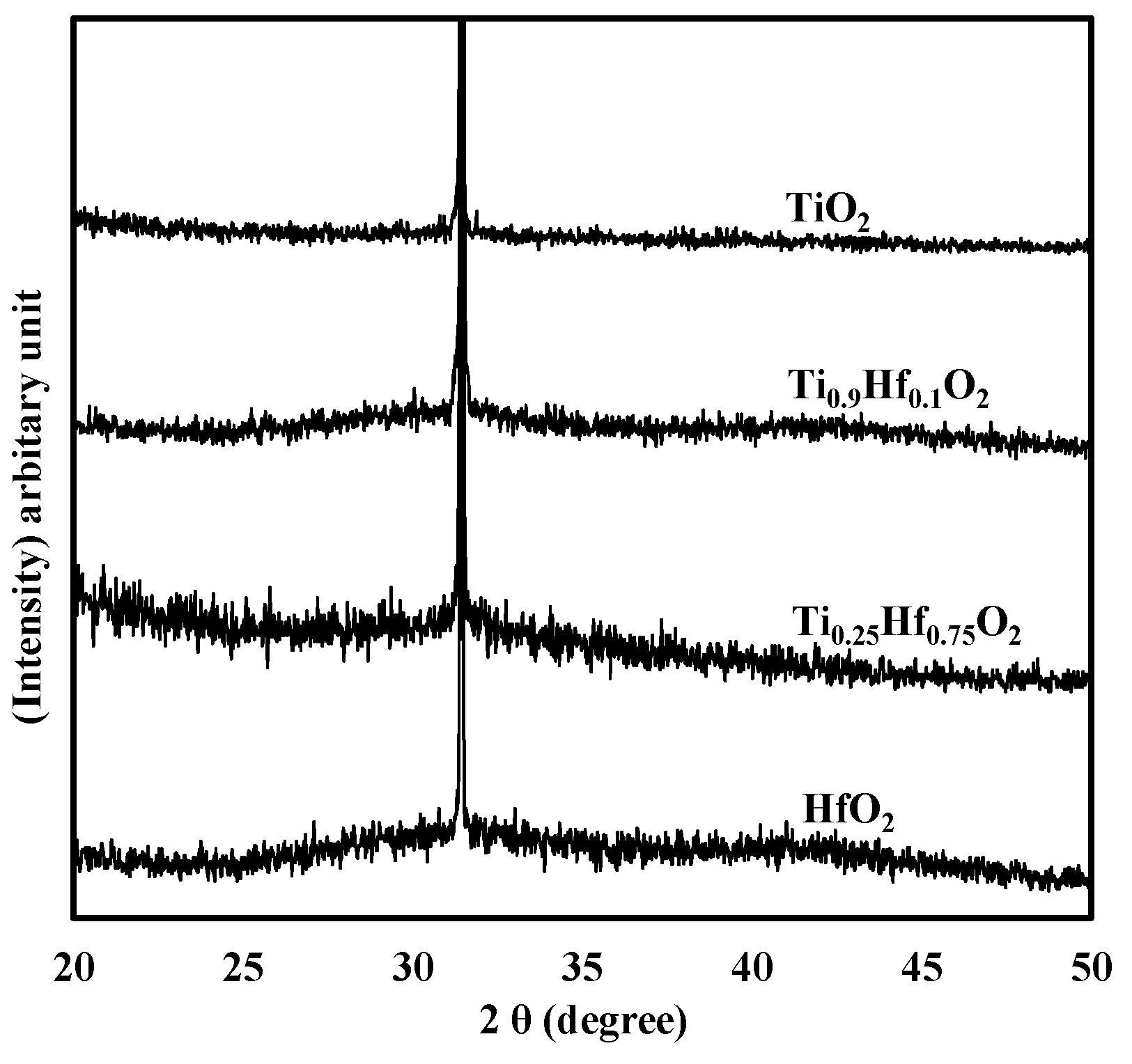



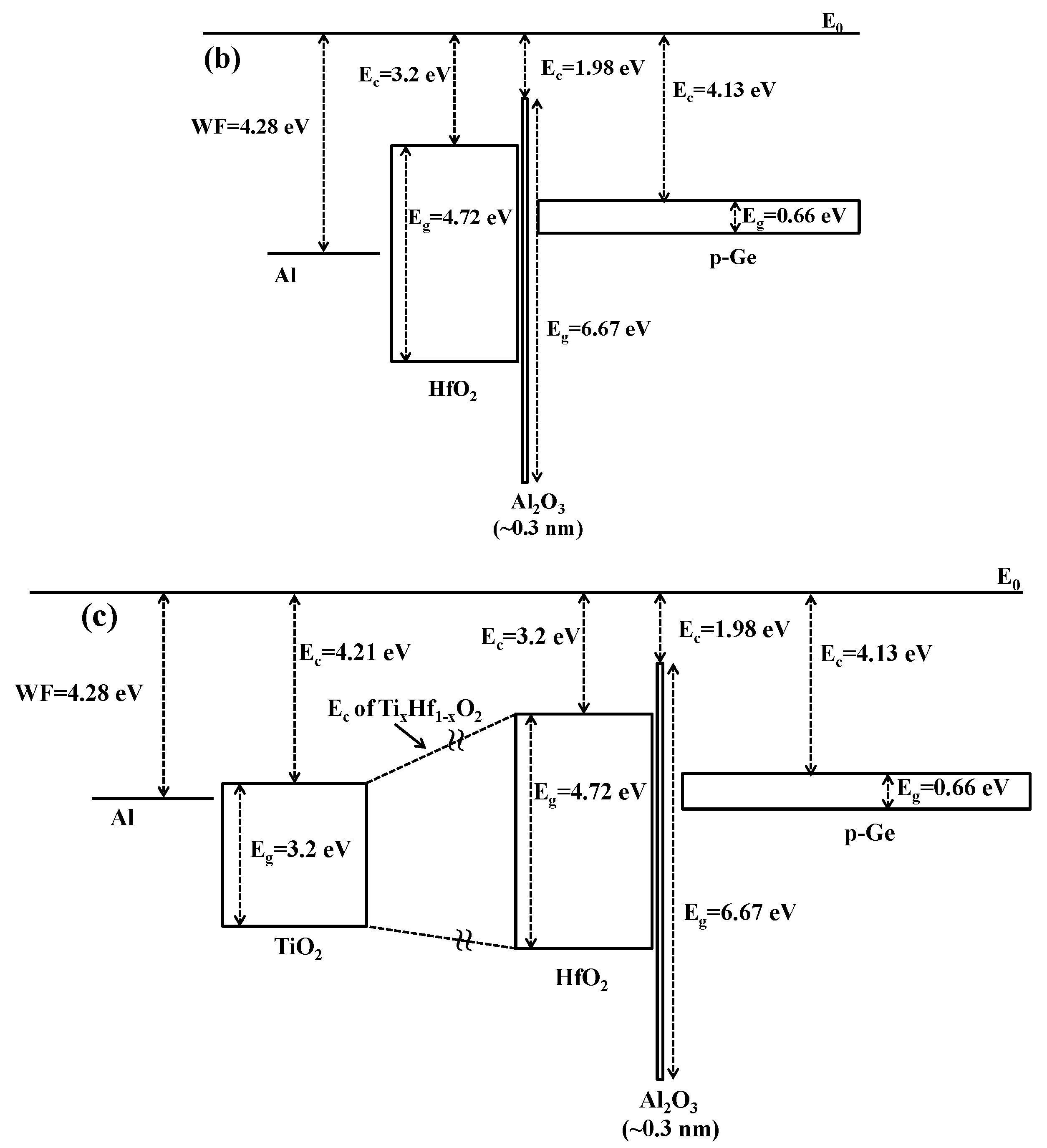
3. Experimental Section
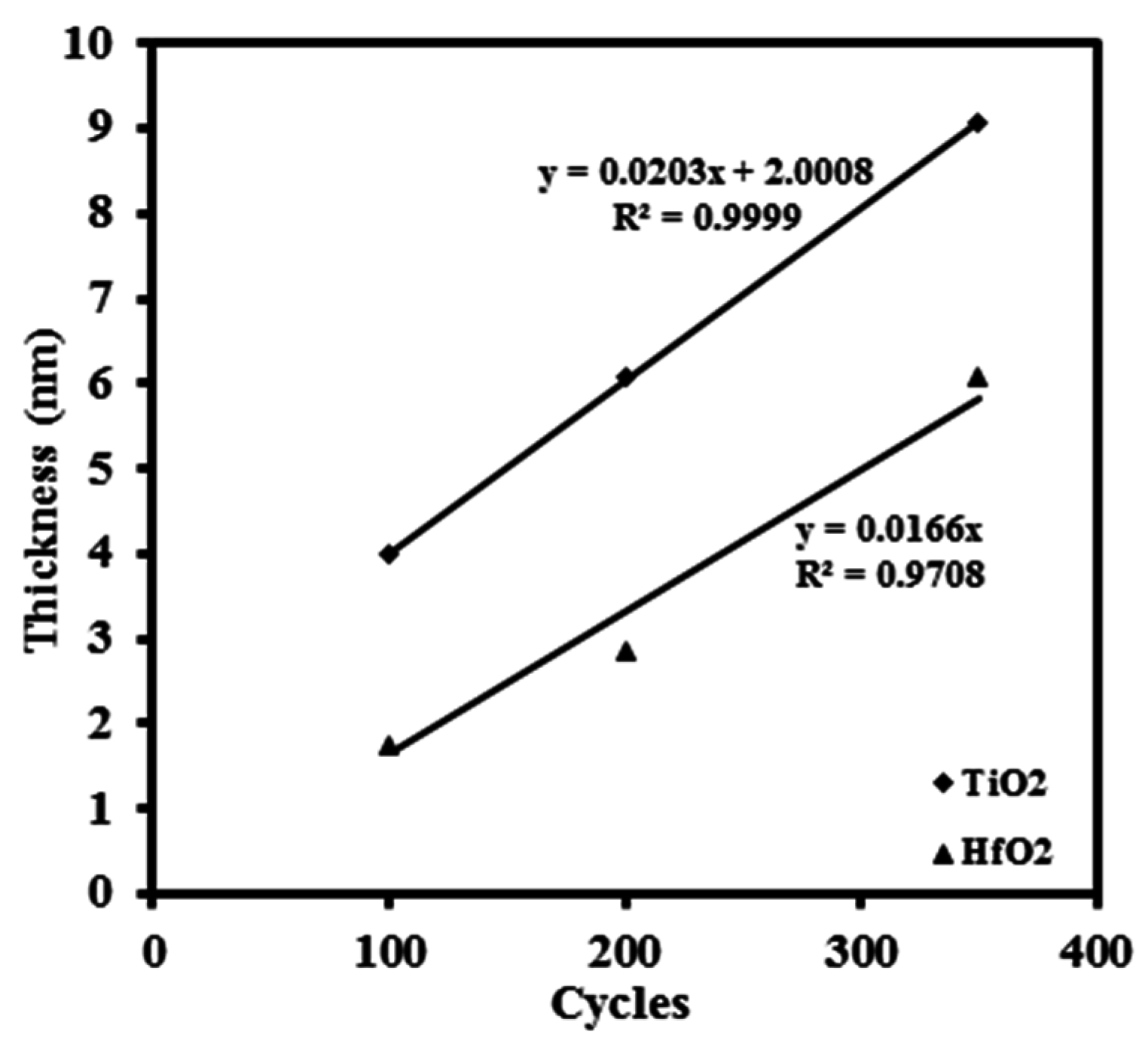
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caymax, M.; Eneman, G.; Bellenger, F.; Merckling, C.; Delabie, A.; Wang, G.; Loo, R.; Simoen, E.; Mitard, J.; de Jaeger, B. Germanium for advanced CMOS anno 2009: A SWOT analysis. In Proceedings of the International Electron Devices Meeting, Baltimore, MD, USA, 7–9 December 2009; pp. 428–431.
- Saraswat, K.; Chui, C.O.; Krishnamohan, T.; Kim, D.; Nayfeh, A.; Pethe, A. High performance germanium MOSFETs. Mater. Sci. Eng. B-Solid 2006, 135, 242–249. [Google Scholar] [CrossRef]
- Kamata, Y. High-κ /Ge MOSFETs for future nanoelectronics. Mater. Today 2008, 11, 1–2. [Google Scholar] [CrossRef]
- Shang, H.; Okorn-Schmidt, H.; Chan, K.K.; Copel, M.; Ott, J.A.; Kozlowski, P.M.; Steen, S.E.; Cordes, S.A.; Wong, H.-S.P.; Jones, E.C.; Haensch, W.E. High mobility p-channel germanium MOSFETs with a thin Ge oxynitride gate dielectric. In Proceedings of the International Electron Devices Meeting, San Francisco, CA, USA, 8–11 December 2002; pp. 441–444.
- Goley, P.S.; Hudait, M.K. Germanium based field-effect transistors: Challenges and opportunities. Materials 2014, 7, 2301–2339. [Google Scholar] [CrossRef]
- Wu, D.; Lindgren, A.-C.; Persson, S.; Sjoblom, G.; Haartman, M.V.; Seger, J.; Hellstrom, P.E.; Olsson, J.; Blom, H.O.; Zhang, S.L. A novel strained Si0.7Ge0.3 surface-channel pMOSFET with an ALD TiN/Al2O3/HfAlOx/Al2O3 Gate Stack. IEEE Electron Devices Lett. 2003, 24, 171–173. [Google Scholar] [CrossRef]
- Houssa, M.; Jaeger, B.D.; Delabie, A.; Elshocht, S.V.; Afanas’ev, V.V.; Autran, J.L.; Stesmans, A.; Meuris, M.; Heyns, M.M. Electrical characteristics of Ge/GeOx(N)/HfO2 gate stacks. J. Non-Cryst. Solids 2005, 351, 1902–1905. [Google Scholar] [CrossRef]
- Zhao, Y. Design of higher-k and more stable rare earth oxides as gate dielectrics for advanced CMOS devices. Materials 2012, 5, 1413–1438. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Q.C.; Zhu, C.X.; Chan, D.S.H.; Li, M.F.; Balasubramanian, N.; Chin, A.; Kwong, D.L. Alternative surface passivation on germanium for metal-oxide semiconductor applications with high-κ gate dielectric. Appl. Phys. Lett. 2004, 85, 4127–4129. [Google Scholar] [CrossRef]
- Chen, J.J.-H.; Bojarczuk, N.A.; Shang, H., Jr.; Copel, M.; Hannon, J.B.; Karasinski, J.; Preisler, E.; Banerjee, S.K.; Guha, S. Ultrathin Al2O3 and HfO2 gate dielectrics on surface-nitrided Ge. IEEE Trans. Electron Devices 2004, 51, 1441–1447. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, X.J.; Zhang, W.Q.; Fu, Y.; Li, A.D.; Li, H.; Wu, D. Comparison of the interfacial and electrical properties of HfAlO films on Ge with S and GeO2 passivation. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Taylor, S.; Werner, M.; Chalker, P.R.; Murray, R.T.; Gaskell, J.M.; Jones, A.C. Dielectric relaxation of lanthanum doped zirconium oxide. Appl. Phys. Lett. 2009, 105. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, C.; Mu, Y.; Zhao, C.Z.; Taylor, S.; Chalker, P.R. Hysteresis in lanthanide zirconium oxides observed using a pulse CV technique and including the effect of high temperature annealing. Materials 2015, 8, 4829–4842. [Google Scholar] [CrossRef]
- Huang, L.Y.; Li, A.D.; Zhang, W.Q.; Li, H.; Xia, Y.D.; Wu, D. Fabrication and characterization of La-doped HfO2 gate dielectrics by metal-organic chemical vapor deposition. Appl. Surf. Sci. 2010, 256, 2496–2499. [Google Scholar] [CrossRef]
- Wang, T.; Ekerdt, J.G. Atomic layer deposition of lanthanum stabilized amorphous hafnium oxide thin films. Chem. Mater. 2009, 21, 3096–3101. [Google Scholar] [CrossRef]
- Chen, F.; Bin, X.; Hella, C.; Shi, X.; Gladfelter, W.L.; Campbell, S.A. A Study of mixtures of HfO2 and TiO2 as high-κ gate dielectrics. Microelectron. Eng. 2004, 72, 263–266. [Google Scholar] [CrossRef]
- Werner, M.; Peter, P.J.; Hindley, S.; Romani, S.; Mather, S.; Chalker, P.R.; Williams, P.A.; Van, D.B.; Jakob, A. Atomic layer deposition of Ti-HfO2 dielectrics. Appl. Phys. Lett. 2013, 31. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Van Elshocht, S.; Brijs, B.; Caymax, M.; Conard, T.; Chiarella, T.; de Gendt, S.; de Jaeger, B.; Kubicek, S.; Meuris, M.; Onsia, B.; et al. Deposition Of HfO2 on germanium and the impact of surface pretreatments. Appl. Phys. Lett. 2004, 85. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, C. Effects of sulfur passivation on germanium MOS capacitors with HfON gate dielectric. IEEE Electron Devices Lett. 2007, 28, 976–979. [Google Scholar] [CrossRef]
- Kato, R.; Kyogoku, S.; Sakashita, M.; Kondo, H.; Zaima, S. Effects of atomic layer deposition-Al2O3 interface layers on interfacial properties of ge metal oxide semiconductor capacitors. Jpn. J. Appl. Phys. 2009, 48. [Google Scholar] [CrossRef]
- Sahin, D.; Yildiz, I.; Gencer, A.I.; Aygun, G.; Turan, R. Evolution of SiO2/Ge/HfO2(Ge) multilayer structure during high temperature annealing. Thin Solid Films 2010, 518, 2365–2369. [Google Scholar] [CrossRef]
- Cho, M.H.; Roh, Y.S.; Whang, C.N.; Jeong, K.; Nahm, S.W.; Ko, D.H.; Lee, J.H.; Lee, N.I.; Fujihara, K. Thermal stability and structural characteristics of HfO2 films on Si(100) grown by atomic-layer deposition. Appl. Phys. Lett. 2002, 81, 472–474. [Google Scholar] [CrossRef]
- Opila, R.L.; Wilk, G.D.; Alam, M.A.; van Dover, R.B.; Busch, B.W. Photoemission study of Zr- and Hf-silicates for use as high-κ oxides: Role of second nearest neighbors and interface charge. Appl. Phys. Lett. 2002, 81, 1788–1790. [Google Scholar] [CrossRef]
- Van Elshocht, S.; Caymax, M.; Conard, T.; de Gendt, S.; Hoflijk, I.; Houssa, M.; de Jaeger, B.; van Steenbergen, J.; Heyns, M.; Meuris, M. Effect of hafnium germanate formation on the interface of HfO2/germanium metal oxide semiconductor devices. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, S.; Schaekers, M.; Lin, D.; Caymax, M.; Delabie, A.; Qu, X.; Jiang, Y.; Deduytsche, D.; Detavernier, C. Germanium surface passivation and atomic layer deposition of high-κ dielectrics—A tutorial review on Ge-based MOS capacitors. Semicond. Sci. Technol. 2012, 27. [Google Scholar] [CrossRef]
- Curreem, K.K.S.; Lee, P.F.; Wong, K.S.; Dai, J.Y.; Zhou, M.J.; Wang, J.; Li, Q. Comparison of interfacial and electrical characteristics of HfO2 and HfAlO high-κ dielectrics on compressively strained Si1−xGex. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Maeda, F.; Watanabe, Y.; Ogino, T. Distinctly different thermal decomposition pathways of ultrathin oxide layer on Ge and Si surfaces. Appl. Phys. Lett. 2000, 76, 2244–2246. [Google Scholar] [CrossRef]
- Schmeisser, D.; Schnell, R.D.; Bogen, A.; Himpsel, F.J.; Rieger, D.; Landgren, G.; Morar, J.F. Surface oxidation states of germanium. Surf. Sci. 1986, 172, 455–465. [Google Scholar] [CrossRef]
- Caymax, M.; van Elshocht, S.; Houssa, M.; Delabie, A.; Conard, T.; Meuris, M.; Heyns, M.M.; Dimoulas, A.; Spiga, S.; Fanciulli, M.; et al. HfO2 as gate dielectric on Ge: Interfaces and deposition techniques. Mat. Sci. Eng. B-Solid 2006, 135, 256–260. [Google Scholar] [CrossRef]
- Kita, K.; Takahashi, T.; Nomura, H.; Suzuki, S.; Nishimura, T.; Toriumi, A. Control of high-κ /germanium interface properties through selection of high-κ materials and suppression of GeO volatilization. Appl. Surf. Sci. 2008, 254, 6100–6105. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Campbell, S.A.; Li, H.J.; Peterson, J.J. Hafnium titanate as a high permittivity gate insulator: Electrical and physical characteristics and thermodynamic stability. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Delabie, A.; Puurunen, R.L.; Brijs, B.; Caymax, M.; Conard, T.; Onsia, B.; Richard, O.; Vandervorst, W.; Zhao, C.; Heyns, M.M.; et al. Atomic layer deposition of hafnium oxide on germanium substrates. J. Appl. Phys. 2005, 97, 64104. [Google Scholar] [CrossRef]
- Ardalan, P.; Pickett, E.R.; Harris, J.S.; Marshall, A.F.; Bent, S.F. Formation of an oxide-free Ge/TiO2 interface by atomic layer deposition on brominated Ge. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, C.Z.; Zhao, C.; Taechakumput, P.; Werner, M.; Taylor, S.; Chalker, P.R. Extrinsic and intrinsic frequency dispersion of high-κ materials in capacitance-voltage measurements. Materials 2012, 5, 1005–1032. [Google Scholar] [CrossRef]
- Lee, B.; Moon, T.; Kim, T.; Choi, D.; Park, B. Dielectric relaxation of atomic-layer-deposited HfO2 thin films from 1 kHz to 5 GHz. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, C.Z.; Werner, M.; Taylor, S.; Chalker, P. Dielectric relaxation of high-κ oxides. Nanoscale Res. Lett. 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.S.; Tan, T.S.; Babu, P.; Sandler, N.P. Mechanism of leakage current reduction of tantalum oxide capacitors by titanium doping. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Chen, Y.Y. Electrical characteristics of the uniaxial-strained nMOSFET with a fluorinated HfO2/SiON gate stack. Materials 2014, 7, 2370–2381. [Google Scholar] [CrossRef]
- Ji, M.; Wang, L.; Du, J. Preparation and characterization of Gd2O3-doped HfO2 high-κ gate dielectric thin films by RF sputtering. J. Phys. Conf. Ser. 2009, 152. [Google Scholar] [CrossRef]
- Guha, S.; Narayanan, V. Oxygen vacancies in high dielectric constant oxide-semiconductor films. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N. Study of direct-contact HfO2/Si interfaces. Materials 2012, 5, 512–527. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Mu, Y.; Roberts, J.W.; Althobaiti, M.; Dhanak, V.R.; Wu, J.; Zhao, C.; Zhao, C.Z.; Zhang, Q.; Yang, L.; et al. Electrical Properties and Interfacial Studies of HfxTi1–xO2 High Permittivity Gate Insulators Deposited on Germanium Substrates. Materials 2015, 8, 8169-8182. https://doi.org/10.3390/ma8125454
Lu Q, Mu Y, Roberts JW, Althobaiti M, Dhanak VR, Wu J, Zhao C, Zhao CZ, Zhang Q, Yang L, et al. Electrical Properties and Interfacial Studies of HfxTi1–xO2 High Permittivity Gate Insulators Deposited on Germanium Substrates. Materials. 2015; 8(12):8169-8182. https://doi.org/10.3390/ma8125454
Chicago/Turabian StyleLu, Qifeng, Yifei Mu, Joseph W. Roberts, Mohammed Althobaiti, Vinod R. Dhanak, Jingjin Wu, Chun Zhao, Ce Zhou Zhao, Qian Zhang, Li Yang, and et al. 2015. "Electrical Properties and Interfacial Studies of HfxTi1–xO2 High Permittivity Gate Insulators Deposited on Germanium Substrates" Materials 8, no. 12: 8169-8182. https://doi.org/10.3390/ma8125454