Synthesis of Biocompatible Hydroxyapatite Using Chitosan Oligosaccharide as a Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
| Sample Name | COS (g/L) | Molecular Mass of COS | Ca(NO3)2 (mol/L) | (NH4)2HPO4 (mol/L) | T (°C) |
|---|---|---|---|---|---|
| CS1000-1 | 10 | 1000 | 0.167 | 0.1 | 85 |
| CS3000-1 | 10 | 3000 | 0.167 | 0.1 | 85 |
| CS5000-1 | 10 | 5000 | 0.167 | 0.1 | 85 |
| CS1000-2 | 2 | 1000 | 0.167 | 0.1 | 85 |
| CS3000-2 | 2 | 3000 | 0.167 | 0.1 | 85 |
| CS5000-2 | 2 | 5000 | 0.167 | 0.1 | 85 |
2.3. Physical Characterization Methods
2.3.1. X-ray Diffraction Measurement
2.3.2. Fourier Transform Infrared Spectrometry Measurement
2.3.3. X-ray Photoelectron Spectroscopy
2.3.4. Tecnai F30 Transmission Electron Microscope Measurement
2.4. Cell Viability Test, Cell Morphology and Alkaline Phosphatase Staining Assay
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
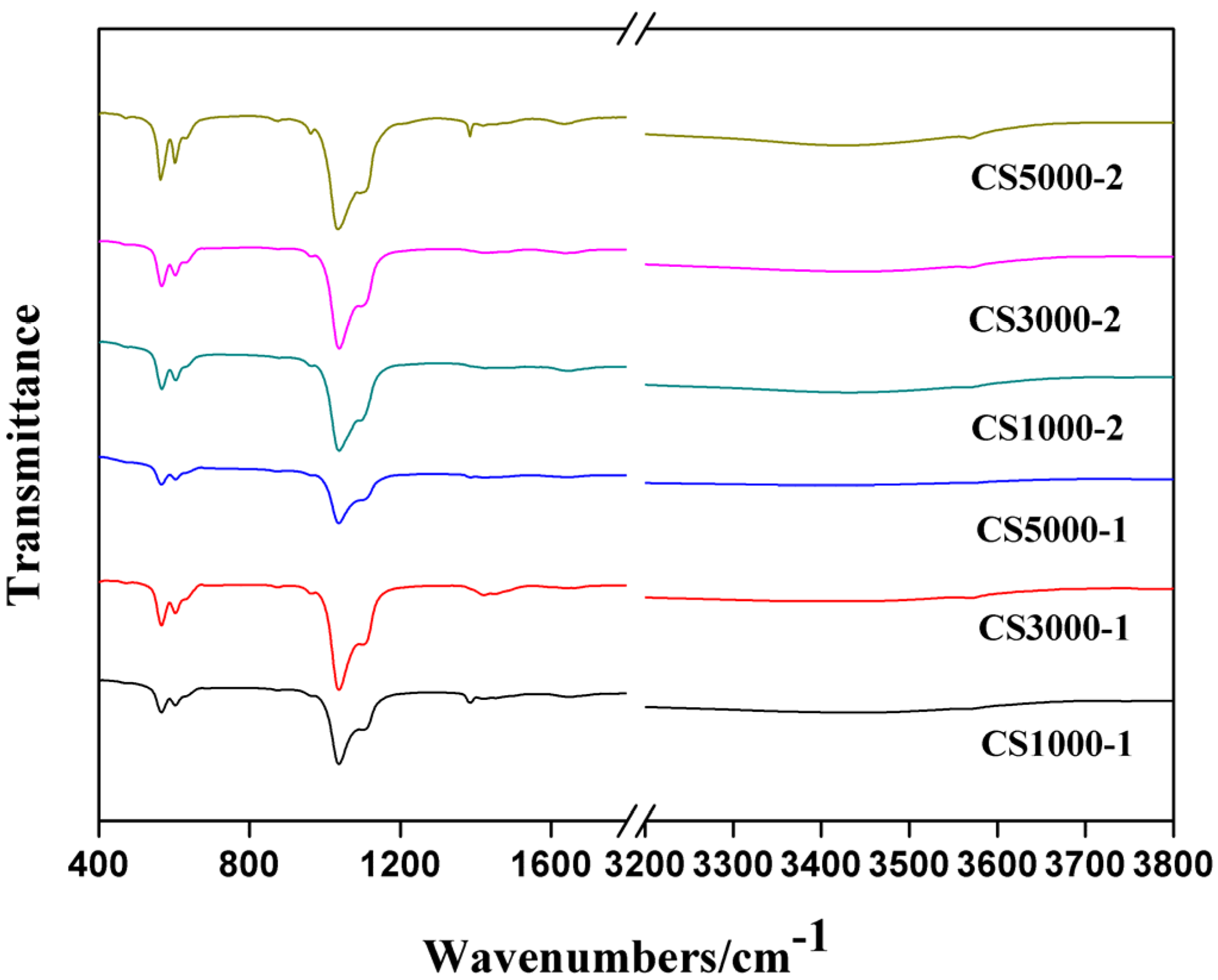
3.2. X-Ray Photoelectron Spectroscopy
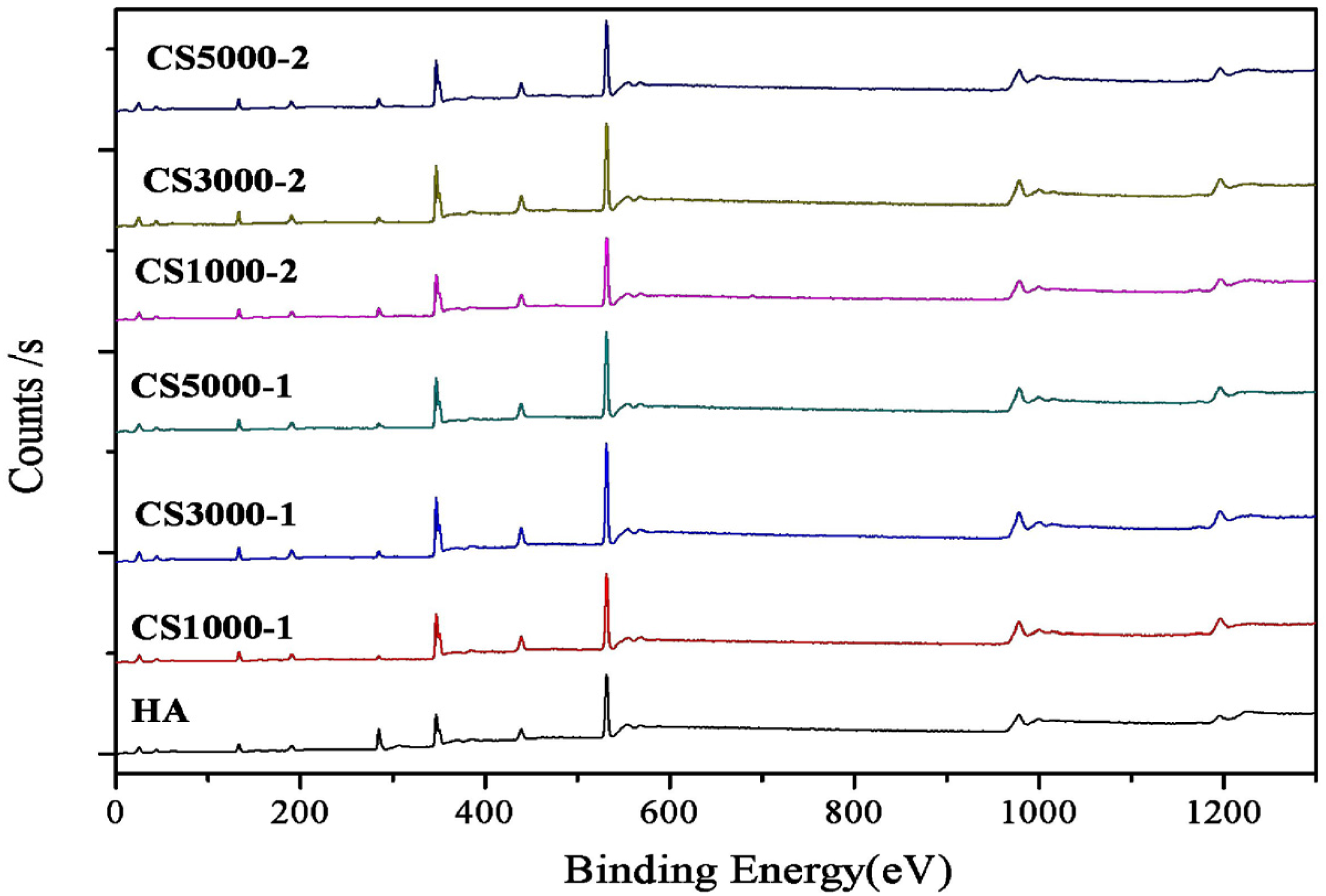
| wt % | CS1000-1 | CS3000-1 | CS5000-1 | CS1000-2 | CS3000-2 | CS5000-2 |
|---|---|---|---|---|---|---|
| Ca (atomic %) | 18.25 | 18.04 | 17.59 | 16.45 | 18.43 | 16.76 |
| P (atomic %) | 12.60 | 12.59 | 11.93 | 11.63 | 12.59 | 11.57 |
| Ca/P ratio | 1.45 | 1.43 | 1.47 | 1.41 | 1.46 | 1.45 |
3.3. X-Ray Diffraction Studies
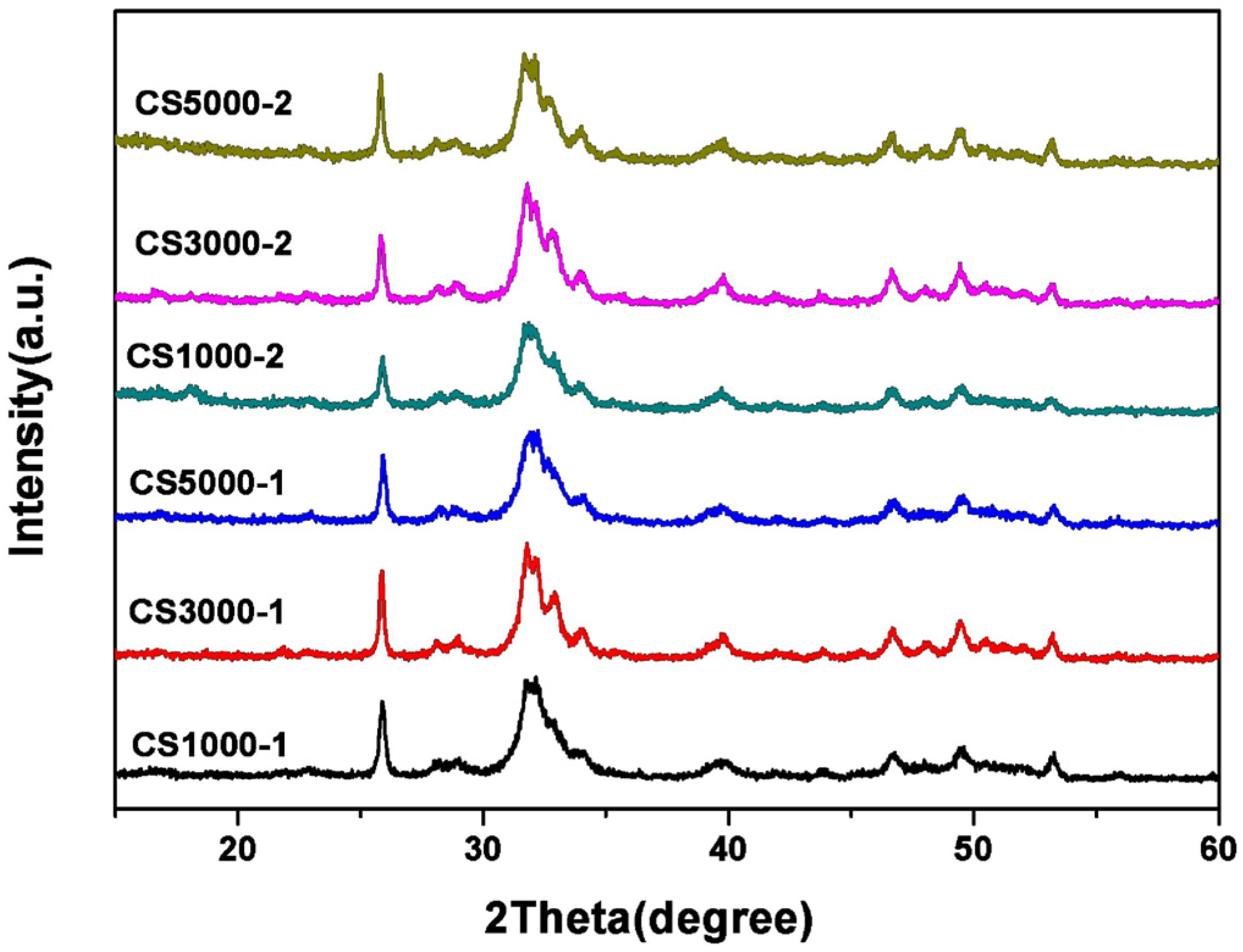
3.4. Morphology Analysis Using TEM
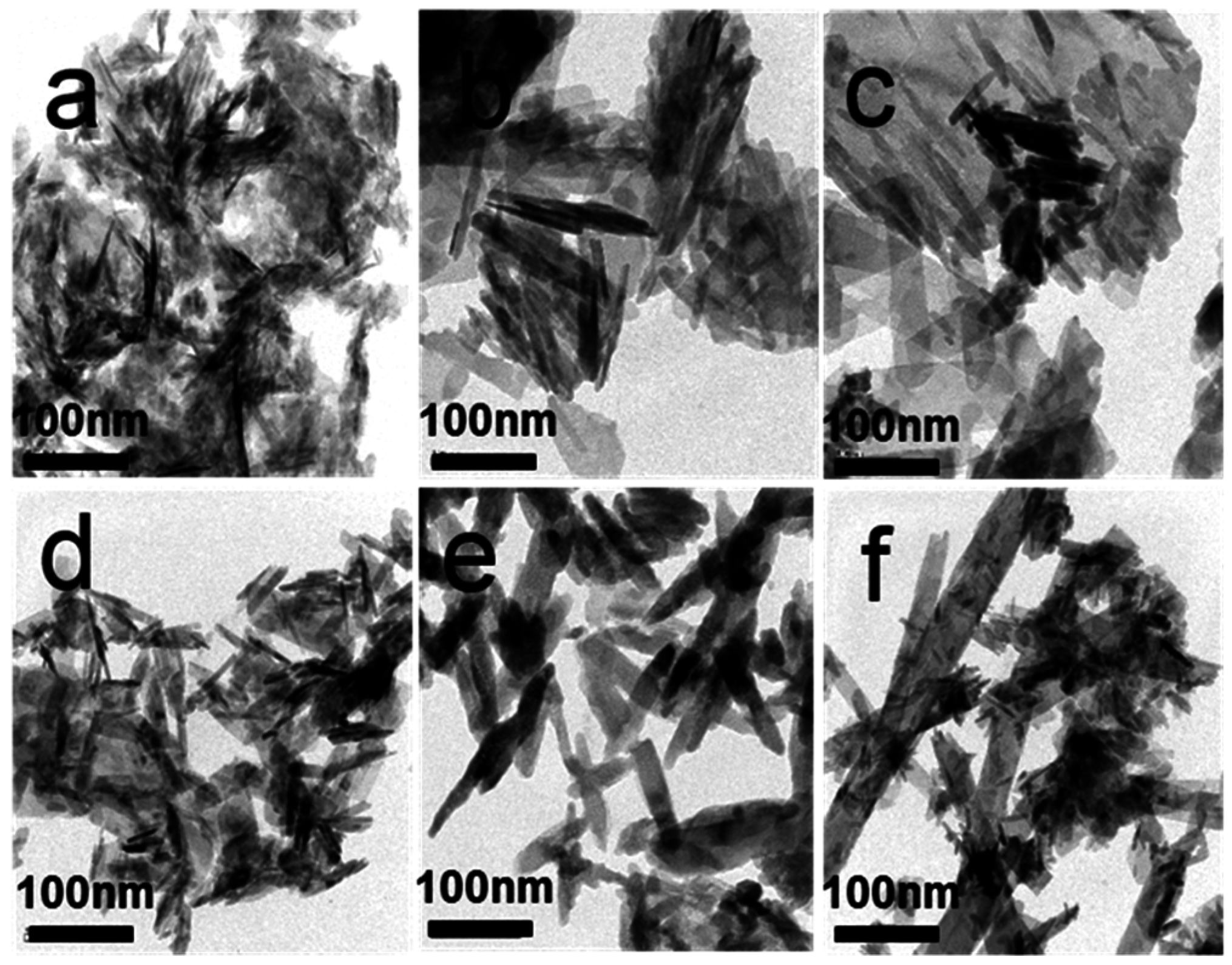
3.5. Cell Viability Test, Cell Morphology and Alkaline Phosphatase Staining Assay



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Iwasashi, M.; Funayama, T.; Watanabe, A.; Noguchi, H.; Tsukanishi, T.; Suetsugu, Y.; Makihara, T.; Ochiai, N.; Yamazaki, M.; Sakane, M. Bone regeneration and remodeling within a unidirectional porous hydroxyapatite bone substitute at a cortical bone defect site: Histological analysis at one and two years after implantation. Materials 2015, 8, 4884–4894. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Tayebi, L. Surface modification of biodegradable porous Mg bone scaffold using polycaprolactone/bioactive glass composite. Mater. Sci. Eng. C 2015, 49, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Y.; Cui, Y.; Tao, C.S.; Zhang, Z.Q.; Tang, P.F.; Mao, K.Y.; Wang, X.M.; Cui, F.Z. Mineralized collagen: Rationale, current status, and clinical applications. Materials 2015, 8, 4733–4750. [Google Scholar] [CrossRef]
- Gentile, P.; Wilcock, C.J.; Miller, C.A.; Moorehead, R.; Hatton, P.V. Process optimisation to control the physico-chemical characteristics of biomimetic nanoscale hydroxyapatites prepared using wet chemical precipitation. Materials 2015, 8, 2297–2310. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, Y.H.; Chen, X.F.; Zhu, P.Z.; Wei, S.C. Biomimetic synthesis and biocompatibility evaluation of carbonated apatites template-mediated by heparin. Mater. Sci. Eng. C 2013, 33, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Y.; Cao, W.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J. Biomed. Mater. Res. A 2005, 75, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Kellermeier, M. Beyond biomineralization. Science 2009, 323, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chu, C.C. Biomimetic mineralization of acid polysaccharide-based hydrogels: Towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 2012, 22, 6080–6087. [Google Scholar] [CrossRef]
- Li, Q.H.; Li, M.; Zhu, P.Z.; Wei, S.C. In vitro synthesis of bioactive hydroxyapatite using sodium hyaluronate as a template. J. Mater. Chem. 2012, 22, 20257–20265. [Google Scholar] [CrossRef]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Roveri, N.; Falini, G.; Sidoti, M.C.; Tampieri, A.; Landi, E.; Sandri, M.; Parma, B. Biologically inspired growth of hydroxyapatite nanocrystals inside self-assembled collagen fibers. Mater. Sci. Eng. C 2003, 23, 441–446. [Google Scholar] [CrossRef]
- Rosseeva, E.V.; Buder, J.; Simon, P.; Schwarz, U.; Frank-Kamenetskaya, O.V.; Kniep, R. Synthesis, characterization, and morphogenesis of carbonated fluorapatite-gelatine nanocomposites: A complex biomimetic approach toward the mineralization of hard tissues. Chem. Mater. 2008, 20, 6003–6013. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Gungormus, M.; Branco, M.; Fong, H.; Schneider, J.P.; Tamerler, C.; Sarikaya, M. Self assembled bi-functional peptide hydrogels with biomineralization-directing peptides. Biomaterials 2010, 31, 7266–7274. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-Assembly and Mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Geng, Y.; Henrikson, K.J.; Aparicio, C.; Stock, S.R.; Satcher, R.L.; Stupp, S.I. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 2010, 31, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.R.; Maltsev, S.; Davies, M.E.; Duer, M.J.; Jaeger, C.; Loveridge, N.; Murray, R.C.; Reid, D.G. The organic-mineral interface in bone is predominantly polysaccharide. Chem. Mater. 2007, 19, 5055–5057. [Google Scholar] [CrossRef]
- Zhu, P.Z.; Xu, J.D.; Sahar, N.; Morris, M.D.; Kohn, D.H.; Ramamoorthy, A. Time-resolved dehydration-induced structural changes in an intact bovine cortical bone revealed by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2009, 131, 17064–17065. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2015, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.; Michaud, P.; Mati, F.; Harrat, Z.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N. Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. Int. J. Biol. Macromol. 2013, 52, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kikuchi, M.; Koyama, Y.; Takakuda, K. Osteogenic activity of MG63 cells on bone-like hydroxyapatite/collagen nanocomposite sponges. J. Mater. Sci. 2010, 21, 1263–1272. [Google Scholar]
- Kalita, S.J.; Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C 2010, 30, 295–303. [Google Scholar] [CrossRef]
- He, Q.J.; Huang, Z.L.; Liu, Y.; Chen, W.; Xu, T. Template-directed one-step synthesis of flowerlike porous carbonated hydroxyapatite spheres. Mater. Lett. 2007, 61, 141–143. [Google Scholar] [CrossRef]
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, C.; Dalconi, M.C.; Nuzzo, S.; Mobilio, S.; Wenk, R.H. Rietveld refinement on X-ray diffraction patterns of bioapatite in human fetal bones. Biophys. J. 2003, 84, 2021–2029. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, G.; Liu, H.; Niu, X.; Han, J.; Zheng, L.; Fan, Y. Nano-hydroxyapatite particles induce apoptosis on MC3T3-E1 cells and tissue cells in SD rats. Nanoscale 2012, 4, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Narasaraju, T.; Phebe, D. Some physico-chemical aspects of hydroxylapatite. J. Mater. Sci. 1996, 31, 1–21. [Google Scholar] [CrossRef]
- Hattar, S.; Berdal, A.; Asselin, A.; Loty, S.; Greenspan, D.; Sautier, J. In vitro interactions of glasses with osteoblast-like cells. Eur. Cell Mater. 2002, 4, 61–69. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, G.; Chen, J.; Zhao, B.; Zhu, P. Synthesis of Biocompatible Hydroxyapatite Using Chitosan Oligosaccharide as a Template. Materials 2015, 8, 8097-8105. https://doi.org/10.3390/ma8125440
Wang J, Liu G, Chen J, Zhao B, Zhu P. Synthesis of Biocompatible Hydroxyapatite Using Chitosan Oligosaccharide as a Template. Materials. 2015; 8(12):8097-8105. https://doi.org/10.3390/ma8125440
Chicago/Turabian StyleWang, Jinyu, Guanxiong Liu, Jinshuai Chen, Bo Zhao, and Peizhi Zhu. 2015. "Synthesis of Biocompatible Hydroxyapatite Using Chitosan Oligosaccharide as a Template" Materials 8, no. 12: 8097-8105. https://doi.org/10.3390/ma8125440





