Preparation of Cotton-Wool-Like Poly(lactic acid)-Based Composites Consisting of Core-Shell-Type Fibers
Abstract
:1. Introduction
2. Results and Discussion
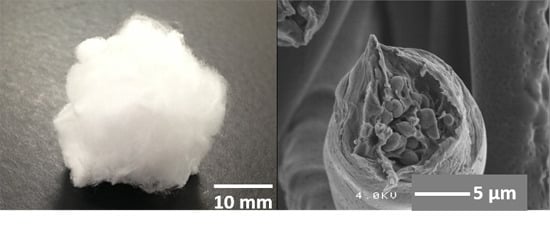
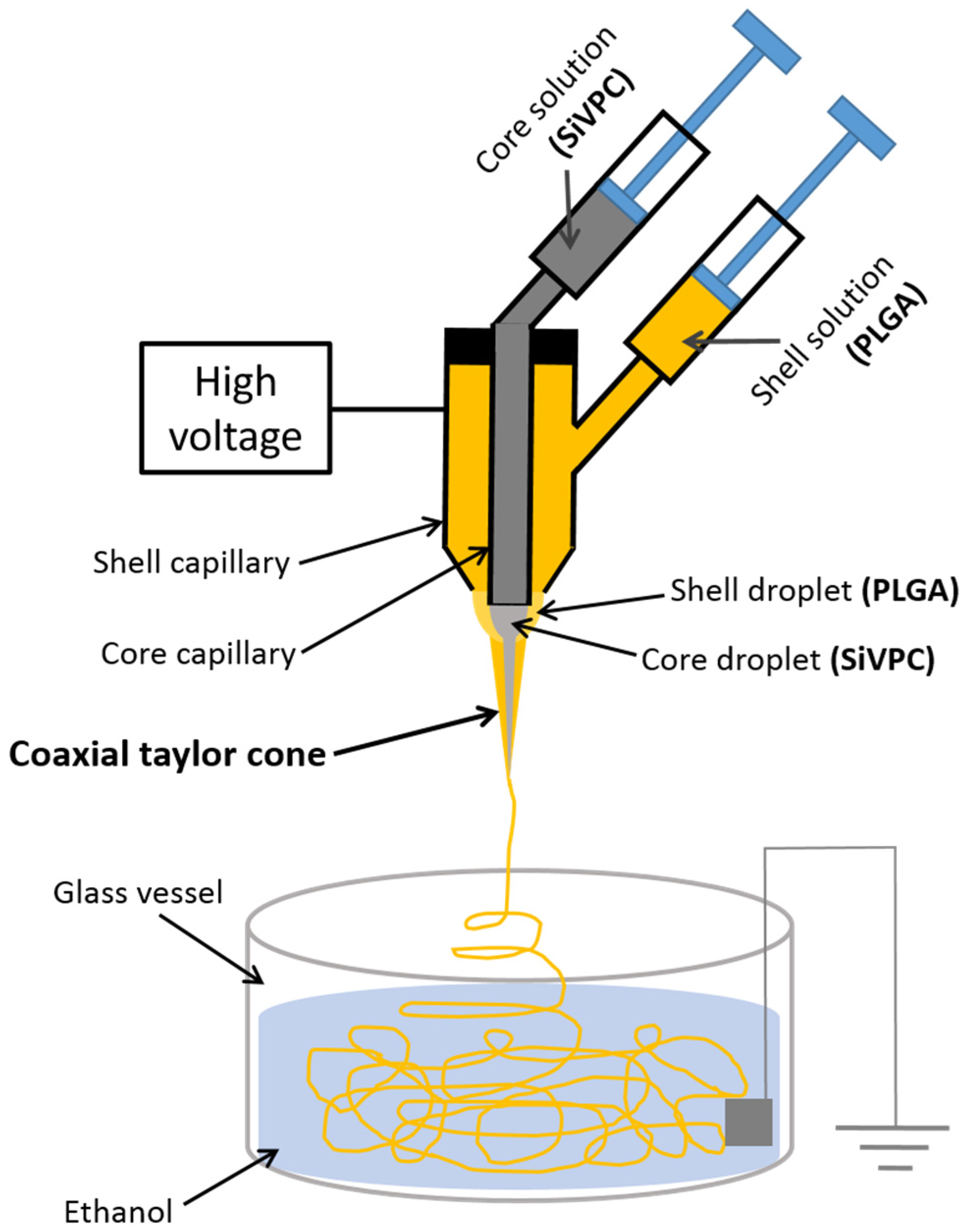
2.1. Preparation of Core-Shell-Type Fibers

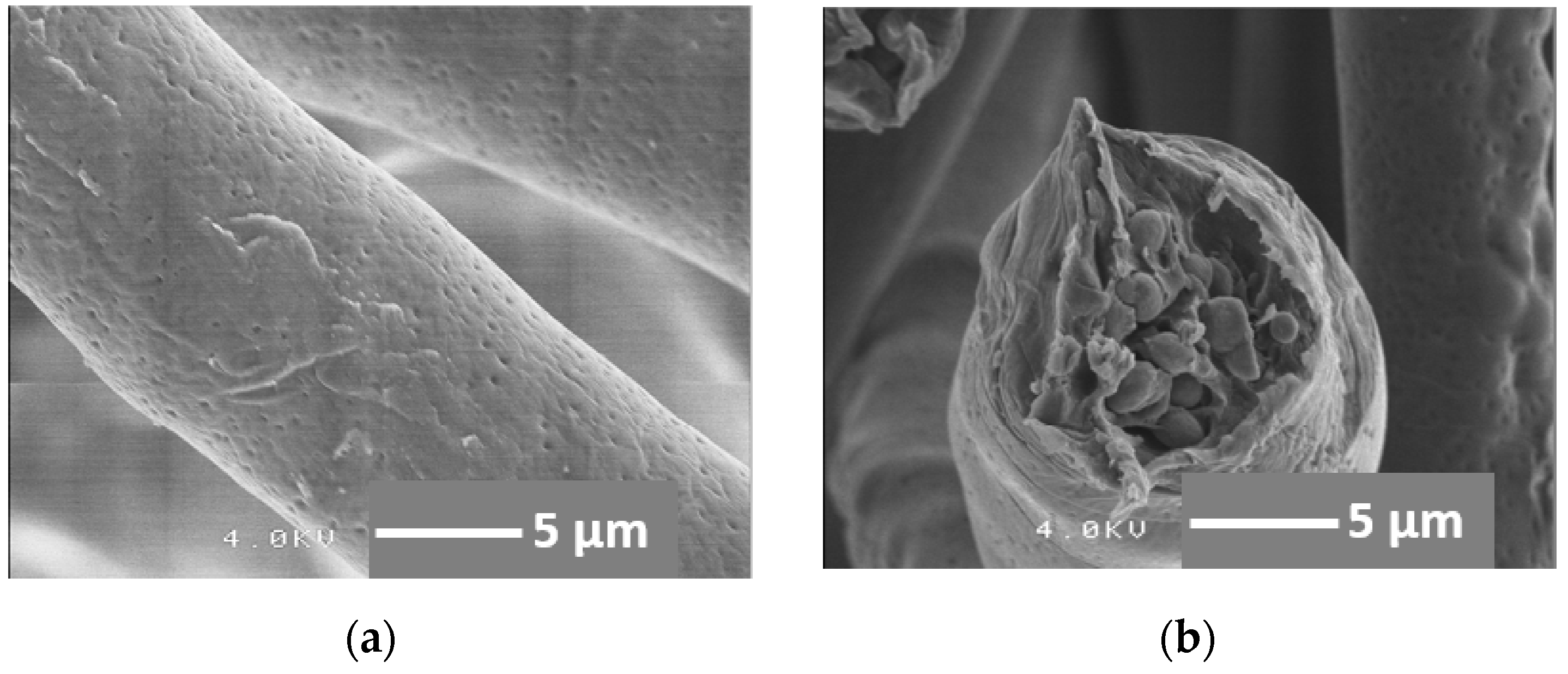
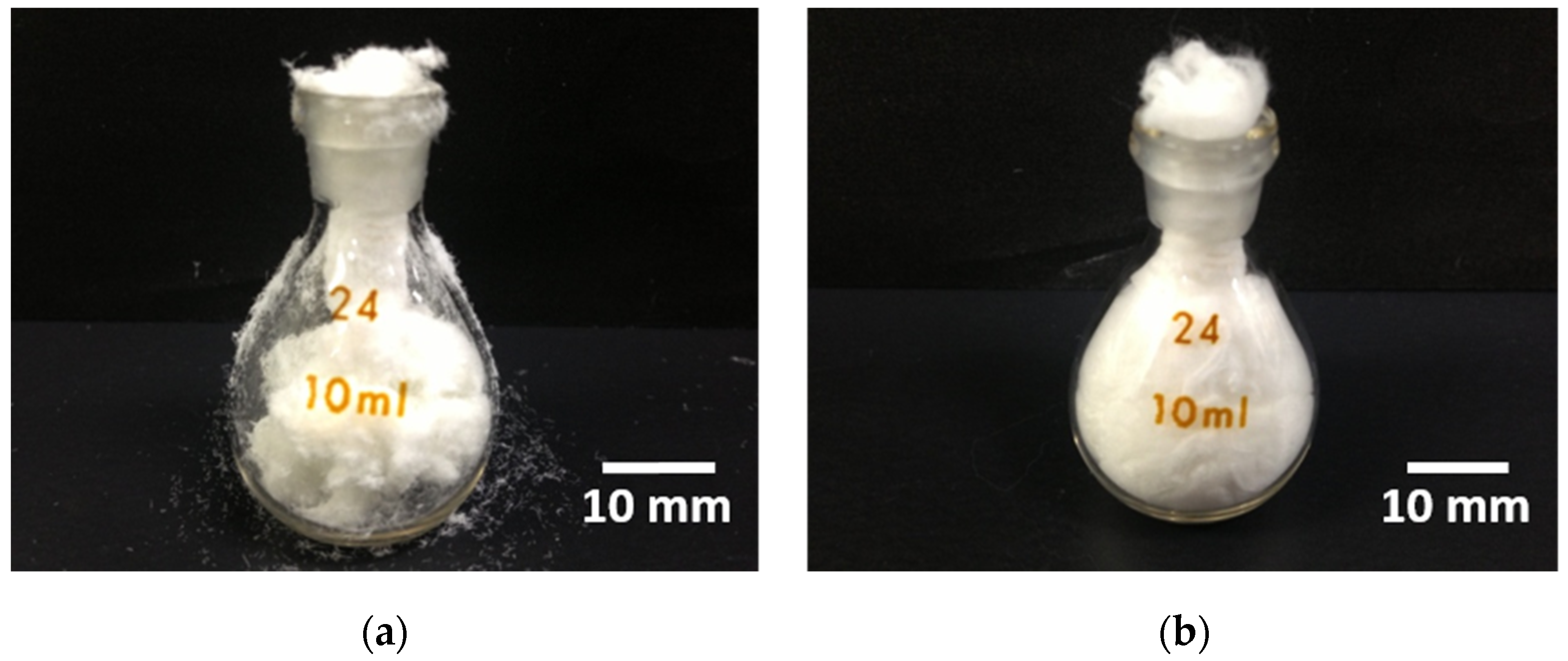
2.2. Mechanical Flexibility of Cotton-Wool-Like Materials

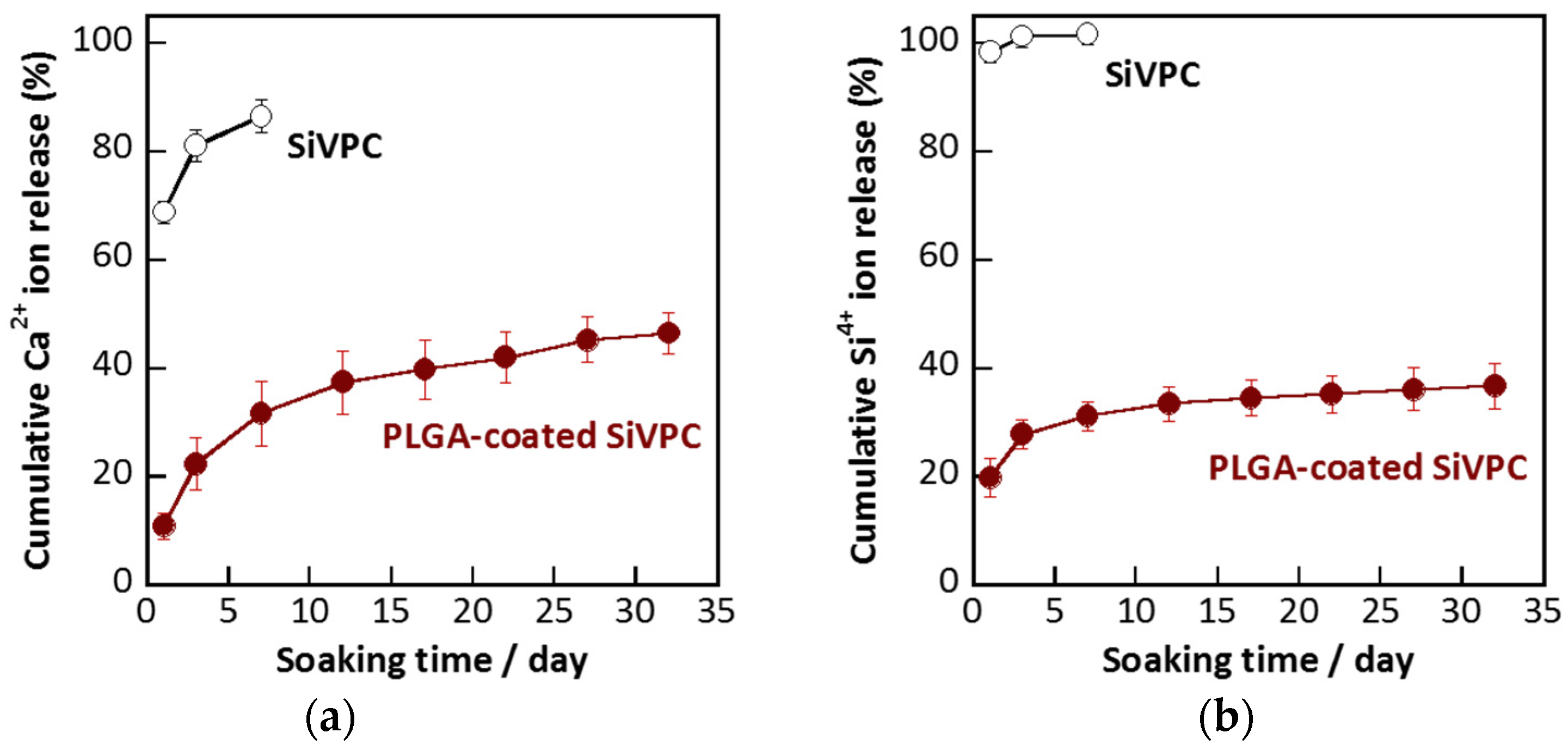
2.3. Dissolution of Calcium and Silicate Ions
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L.; Polak, J.M. Third generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Ronk, R. Calcium sulfate bone void filler: A review and a look ahead. J. Craniofacial Surg. 2000, 11, 327–333. [Google Scholar] [CrossRef]
- Kenny, S.M.; Buggy, M. Bone cements and fillers: A review. J. Mater. Sci. Mater. Med. 2003, 14, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Xynos, I.D.; Polak, J.M. Bioactive glasses for in situ tissue regeneration. J. Biomater. Sci. Polym. Ed. 2004, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Day, D.E.; Höland, W.; Rheinberger, V.M. Glass and medicine. Int. J. Appl. Glass Sci. 2010, 1, 104–117. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ji, K.; Kirkham, J.; Yan, Y.; Boccaccini, A.R.; Kellett, M.; Jin, Y.; Yang, X. Bone tissue engineering by using a combination of polymer/Bioglass composites with human adipose-derived stem cells. Cell Tissue Res. 2014, 356, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Obata, A.; Tokuda, S.; Kasuga, T. Enhanced in vitro cell activity on silicon-doped vaterite/poly(lactic acid) composites. Acta Biomater. 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Hotta, T.; Wakita, T.; Ota, Y.; Kasuga, T. Electrospun microfiber meshes of silicon-doped vaterite/poly(lactic acid) hybrid for guided bone regeneration. Acta Biomater. 2010, 6, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Obata, A.; Lin, S.; Jones, J.R.; Law, R.V.; Kasuga, T. Preparation of electrospun poly(lactic acid)-based hybrids containing siloxane-doped vaterite particles for bone regeneration. J. Biomater. Sci. Ploym. Ed. 2012, 23, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Poologasundarampillai, G.; Jones, J.R.; Kasuga, T. Tracking the formation of vaterite particles containing aminopropyl-functionalized silsesquioxane and their structure for bone regenerative medicine. J. Mater. Chem. B 2013, 1, 4446–4454. [Google Scholar] [CrossRef]
- Kasuga, T.; Obata, A.; Maeda, H.; Ota, Y.; Yao, X.F.; Oribe, K. Siloxane-poly(lactic acid)-vaterite composites with 3D cotton-like structure. J. Mater. Sci. Mater. Med. 2012, 23, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.P.; Fan, M.; Chen, Z.; Jansen, J. Bioactive Electrospun Scaffolds Delivering Growth Factors and Genes for Tissue Engineering Applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, X.Q.; Wang, H.S.; He, C.L.; Mo, X.M. Fabrication and characterization of biodegradable nanofibrous mats by mix and coaxial electrospinning. J. Mater. Sci. Mater. Med. 2009, 20, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotakic, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Fujikura, K.; Obata, A.; Kasuga, T. Cellular migration to electrospun poly(lactic acid) fibermats. J. Biomater. Sci. Polym. Ed. 2012, 23, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Ran, S.; Kim, K.-S.; Fang, D.; Hsiao, B.S.; Chu, B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules 2003, 4, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wang, X.; Feng, Y.; Li, J.; Lim, C.T.; Ramakrishna, S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(ε-caprolactone) Nanofibers for Sustained Release. Biomacromolecules 2006, 7, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core-Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Marquez, M.; Spretz, R.; Velarde-Ortiz, R.; Larsen, G. Electrically forced coaxial nanojets for one-step hollow nanofiber design. J. Am. Chem. Soc. 2004, 126, 5376–5377. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y.N. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 5, 933–938. [Google Scholar] [CrossRef]
- Díaz, J.E.; Barrero, A.; Márquez, M.; Loscertales, I.G. Controlled encapsulation of hydrophobic liquids in hydrophilic polymer nanofibers by co-electrospinning. Adv. Funct. Mater. 2006, 16, 2110–2116. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, P.; Obata, A.; Jones, J.R.; Kasuga, T. Preparation of Cotton-Wool-Like Poly(lactic acid)-Based Composites Consisting of Core-Shell-Type Fibers. Materials 2015, 8, 7979-7987. https://doi.org/10.3390/ma8115434
Wang J, Zhou P, Obata A, Jones JR, Kasuga T. Preparation of Cotton-Wool-Like Poly(lactic acid)-Based Composites Consisting of Core-Shell-Type Fibers. Materials. 2015; 8(11):7979-7987. https://doi.org/10.3390/ma8115434
Chicago/Turabian StyleWang, Jian, Pin Zhou, Akiko Obata, Julian R. Jones, and Toshihiro Kasuga. 2015. "Preparation of Cotton-Wool-Like Poly(lactic acid)-Based Composites Consisting of Core-Shell-Type Fibers" Materials 8, no. 11: 7979-7987. https://doi.org/10.3390/ma8115434