Evaluation of the Adsorption Performance and Sustainability of Exfoliated Graphite Nanoplatelets (xGnP) for VOCs
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Samples | Specific Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) |
|---|---|---|
| xGnP | 20.4 | 0.082 |
| Perlite | 3.3 | 0.004 |
| Zeolite (Natural) | 23.4 | 0.031 |
| Zeolite (JST-MS100) | 755.6 | 0.352 |
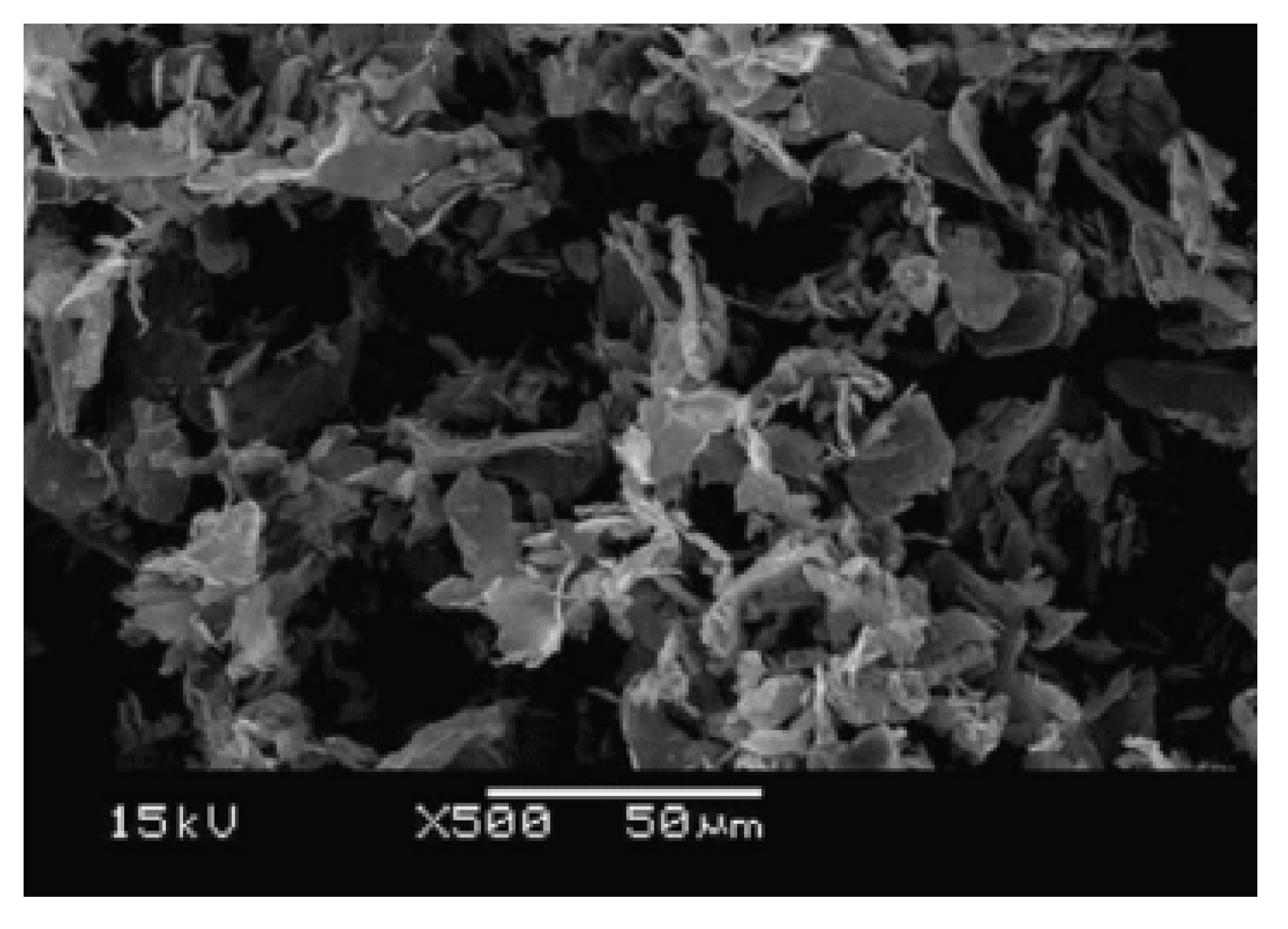
2.2. Methods
2.2.1. Thermal Extractor (TE)
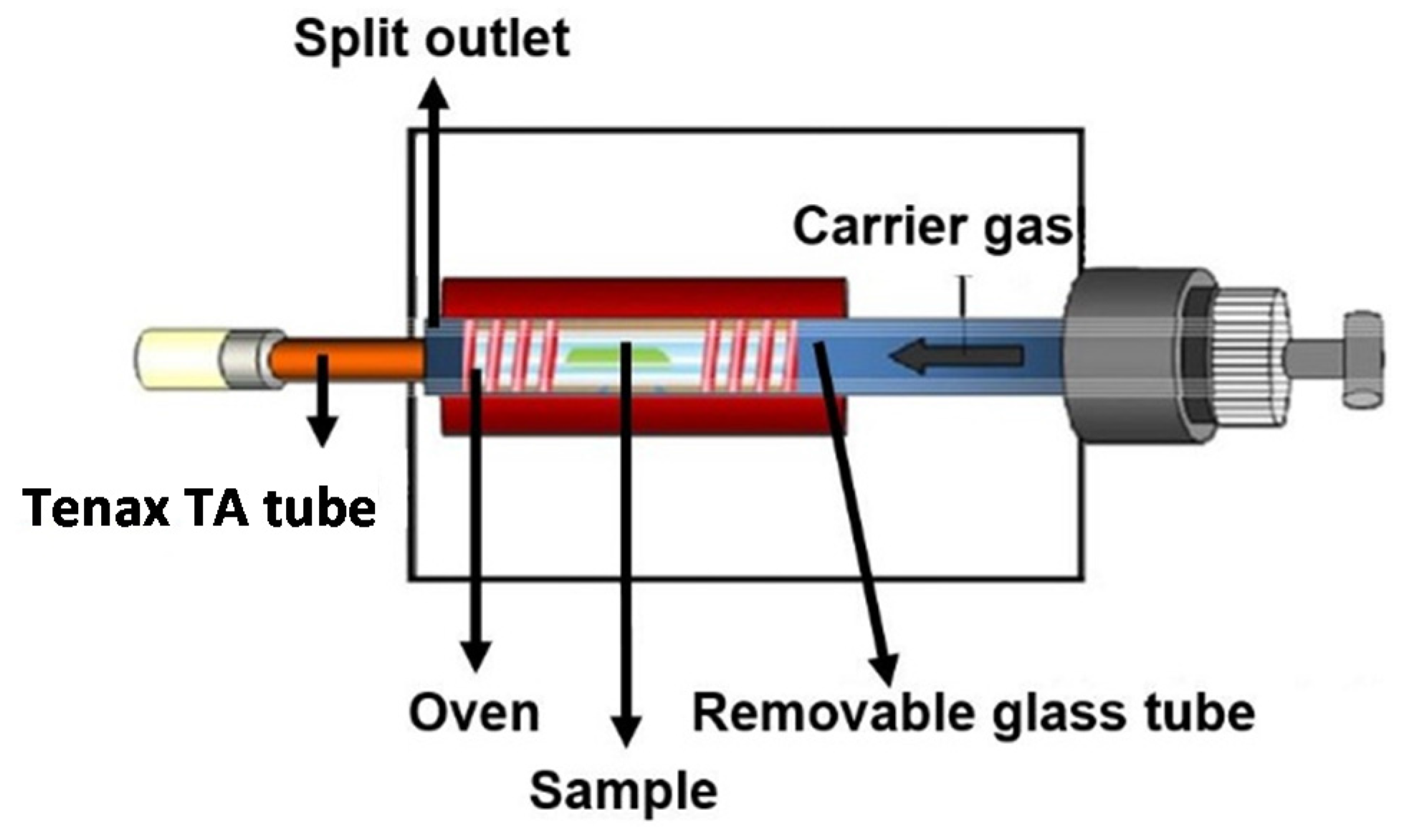
2.2.2. Evaluation of Adsorption Performance
3. Results and Discussion
3.1. Adsorption Performances of the Samples over Seven Days
| Samples | Zeolite (JST-MS100) | Zeolite (Natural) | Perlite | xGnP |
|---|---|---|---|---|
| TVOC (μg/m3) | 76.91 | 9.99 | 150.48 | 128.93 |
| Benzene (μg/m3) | 2.17 | 1.71 | 2.69 | 4.37 |
| Toluene (μg/m3) | 2.07 | 1.61 | 10.38 | 18.83 |
| Ethyl Benzene (μg/m3) | - | - | - | - |
| Xylene (μg/m3) | - | - | - | 1.89 |
| Styrene (μg/m3) | - | - | - | - |
| 5VOC (μg/m3) | 4.25 | 3.33 | 13.07 | 25.08 |
| 5VOC (%) | 5.52 | 33.13 | 8.69 | 19.45 |
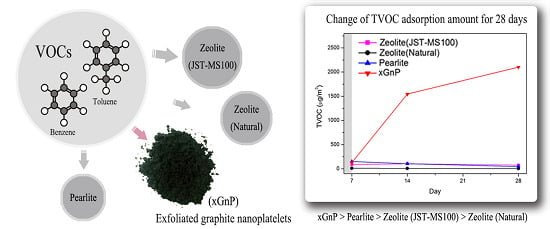
3.2. Sustainability Evaluation for 28 Days
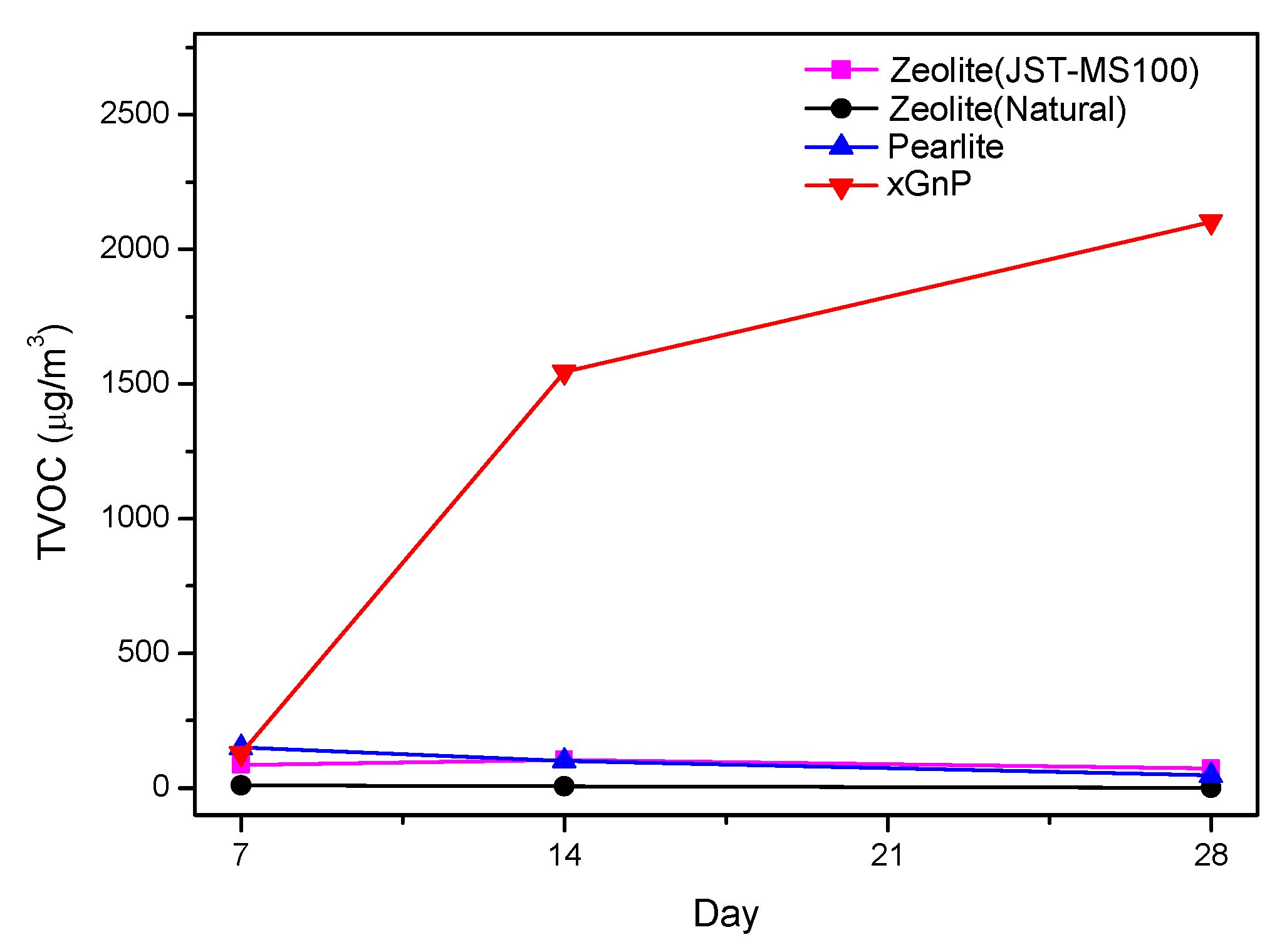

| TVOC | 7 days (μg/m3) | 14 days (μg/m3) | 28 days (μg/m3) |
|---|---|---|---|
| Zeolite (JST-MS100) | 86.96 | 105.50 | 72.49 |
| Zeolite (Natural) | 9.99 | 7.09 | 2.27 |
| Perlite | 150.48 | 101.95 | 48.20 |
| xGnP | 128.93 | 1545.31 | 2102.64 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.H.; Kim, S. The determination of the adsorption performance of graphite for VOCs and formaldehyde. Energy Build. 2012, 46, 56–61. [Google Scholar] [CrossRef]
- Yu, C.; Crump, D. A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ. 1998, 33, 357–374. [Google Scholar] [CrossRef]
- Choi, D.H.; Kang, D.H.; Kim, S.S.; Yeo, M.S.; Kim, K.W. The impact of a non-adhesive floating installation method on emissions and indoor concentrations of VOCs. Indoor Built. Environ. 2010, 19, 435–443. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, L.; Yang, K. Adsorbtion behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Lin, C.C. Binary VOCs absorption in a rotating packed bed with blade packings. J. Environ. Manag. 2012, 98, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, F.; Malhautier, L.; Roux, J.C.; Fanlo, J.L. Absorption of a mixture of volatile organic compounds (VOCs) in aqueous solutions of soluble cutting oil. Bioresour. Technol. 2008, 99, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Heymes, F.; Demoustier, P.M.; Charbit, F.; Fanlo, J.L.; Moulin, P. A new efficient absorption liquid to treat exhaust air loaded with toluene. Chem. Eng. J. 2006, 115, 225–231. [Google Scholar] [CrossRef]
- Dwivedi, P.; Gaur, V.; Sharma, A.; Verma, N. Comparative study of removal of volatile organic compounds by cryogenic condensation and adsorption by activated carbon fiber. Sep. Purif. Technol. 2004, 39, 23–37. [Google Scholar] [CrossRef]
- Ji, W.; Sikdar, S.K.; Hwang, S.T. Modeling of multicomponent pervaporation for removal of volatile organic compounds from water. J. Membr. Sci. 1994, 93, 1–19. [Google Scholar] [CrossRef]
- Daubert, I.; Lafforgue, C.; Maranges, C.; Fonade, C. Feasibility study of a compact process for biological treatment of highly soluble VOCs polluted gaseous effluent. Biotechnol. Prog. 2001, 17, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Ahn, H.G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Rider, A.N.; An, Q.; Thostenson, E.T.; Brack, N. Ultrasonicated-ozone modification of exfoliated graphite for stable aqueous graphitic nanoplatelet dispersions. Nanotechnology 2014, 25, 495607. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Do, I.; Drzal, L.T. Multifunctional exfoliated graphite nanoplatelets-LLDPE nanocomposites fabricated by solution compounding method and various screw rotating systems. Macromol. Mater. Eng. 2009, 294, 196–205. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Mechanical properties and morphological characterization of exfoliated graphite-polypropylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1675–1682. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Yoon, D.W. Evaluation studies on the adsorption VOCs performance of the natural porous materials for the development of absorptive building materials. J. KIAEBS 2011, 5, 115–121. (In Korean) [Google Scholar]
- International Organization for Standardization. Indoor Air—Part 23: Performance Test for Evaluating the Reduction of Formaldehyde Concentrations by Sorptive Building Materials; ISO/DIS 16000-23; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization. Indoor Air—Part 24: Performance Test for Evaluating the Reduction of Volatile Organic Compounds and Carbonyl Compounds without Formaldehyde Concentrations by Sorptive Building Materials; ISO/DIS 16000-24; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- Japan Industrial Standard. Performance Test of Sorptive Building Materials of Reducing Indoor Air Pollution with Small Chamber—Part 1: Measurement of Adsorption Flux with Supplying Constant Concentration of Formaldehyde; JIS A 1905-1; Japanese Standard Association: Tokyo, Japan, 2007. [Google Scholar]
- Japan Industrial Standard. Performance Test for Sorptive Building Materials of Reducing Indoor Air Pollution with Small Chamber—Part 2: Measurment of Capability for Suppressing Formaldehyde Emission; JIS A 1905-2; Japanese Standards Association: Tokyo, Japan, 2007. [Google Scholar]
- Japan Industrial Standard. Performance Test of Sorptive Building Materials of Reducing Indoor Air Pollution with Small Chamber—Measurement of Adsorption Flux with Supplying Constant Concentration of Contaminant Air of VOC and Aldehydes without Formaldehyde; JIS A 1906; Japanese Standards Association: Tokyo, Japan, 2008. [Google Scholar]
- Wolkoff, P. Photocopiers and indoor air pollution. Atmos. Environ. 2011, 33, 2029–2030. [Google Scholar] [CrossRef]
- Kim, S.; Drzal, L.T. High latent storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, H.J. The effect of temperature on VOCs and carbonyl compounds emission from wooden flooring by thermal extractor test method. Build. Environ. 2012, 53, 95–99. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.J.; Wi, S.; Jeong, S.-G.; Kim, S. Evaluation of the Adsorption Performance and Sustainability of Exfoliated Graphite Nanoplatelets (xGnP) for VOCs. Materials 2015, 8, 7615-7621. https://doi.org/10.3390/ma8115412
Chang SJ, Wi S, Jeong S-G, Kim S. Evaluation of the Adsorption Performance and Sustainability of Exfoliated Graphite Nanoplatelets (xGnP) for VOCs. Materials. 2015; 8(11):7615-7621. https://doi.org/10.3390/ma8115412
Chicago/Turabian StyleChang, Seong Jin, Seunghwan Wi, Su-Gwang Jeong, and Sumin Kim. 2015. "Evaluation of the Adsorption Performance and Sustainability of Exfoliated Graphite Nanoplatelets (xGnP) for VOCs" Materials 8, no. 11: 7615-7621. https://doi.org/10.3390/ma8115412