Patchwork Coating of Fragmented Ultra-Thin Films and Their Biomedical Applications in Burn Therapy and Antithrombotic Coating
Abstract
:1. Introduction
2. Fabrication and Characterization of Centimeter-Sized Nanosheets
2.1. Biodegradable Nanosheets
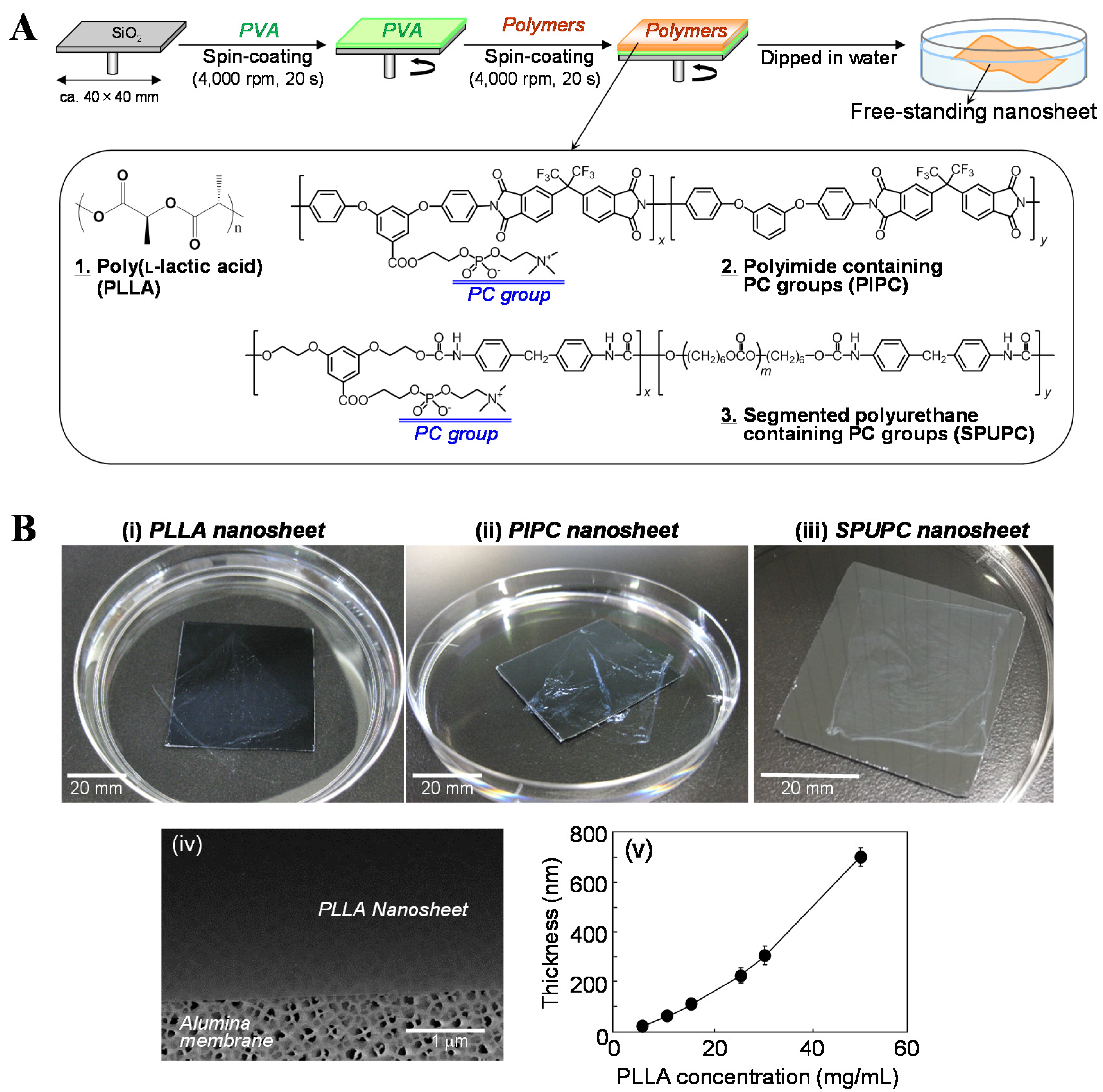
2.2. PC-Containing Polymer Nanosheets
2.3. Unique Property of the Nanosheets: High Adhesion
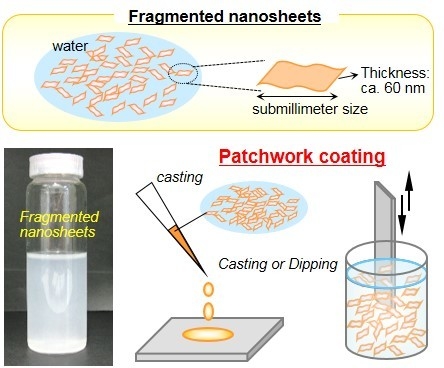
3. Fabrication and Characterization of Submillimeter-Sized Nanosheets (Fragmented Nanosheets)
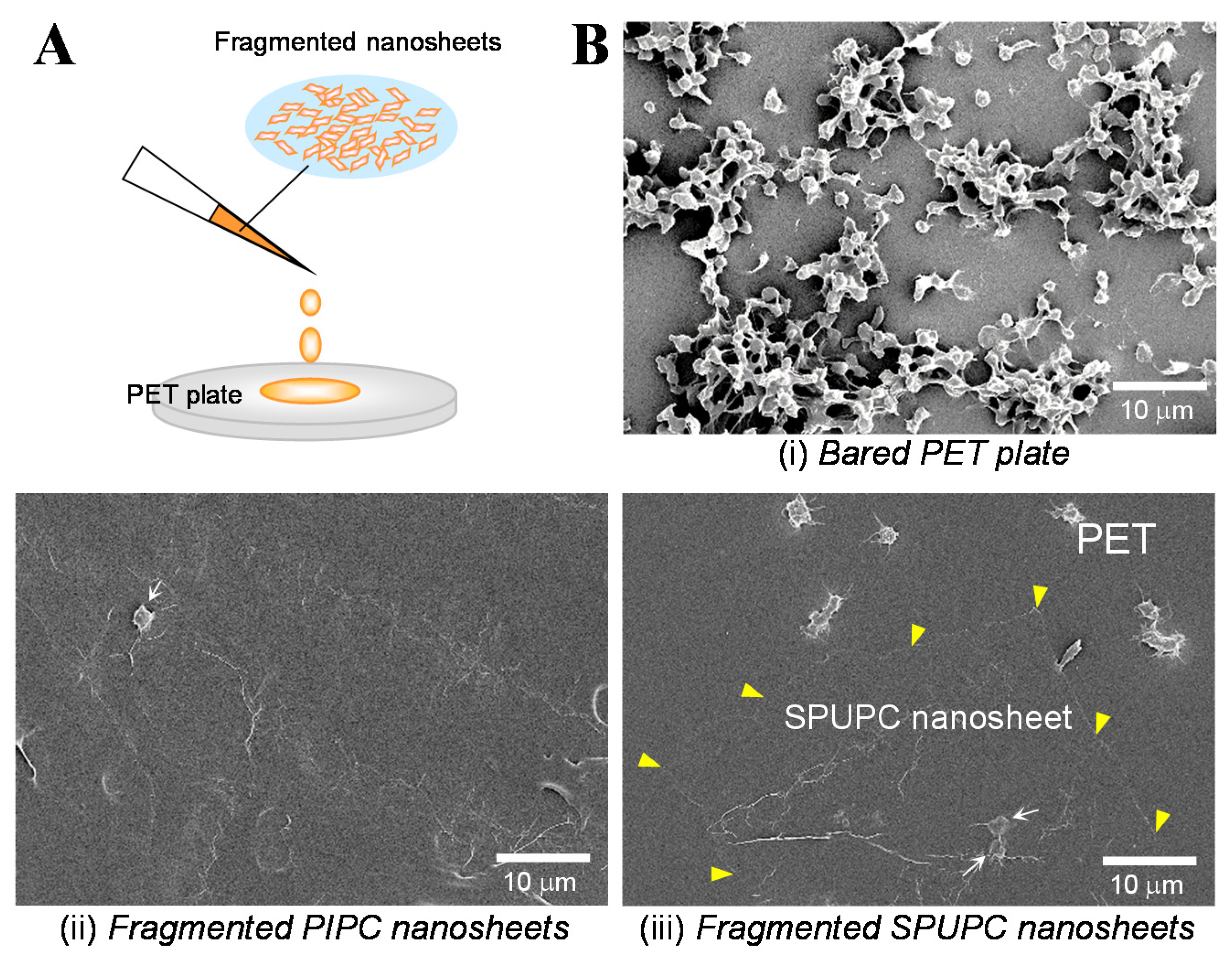
3.1. Fabrication of the Fragmented Nanosheets
3.2. Unique Adhesion Behavior of the Fragmented Nanosheets
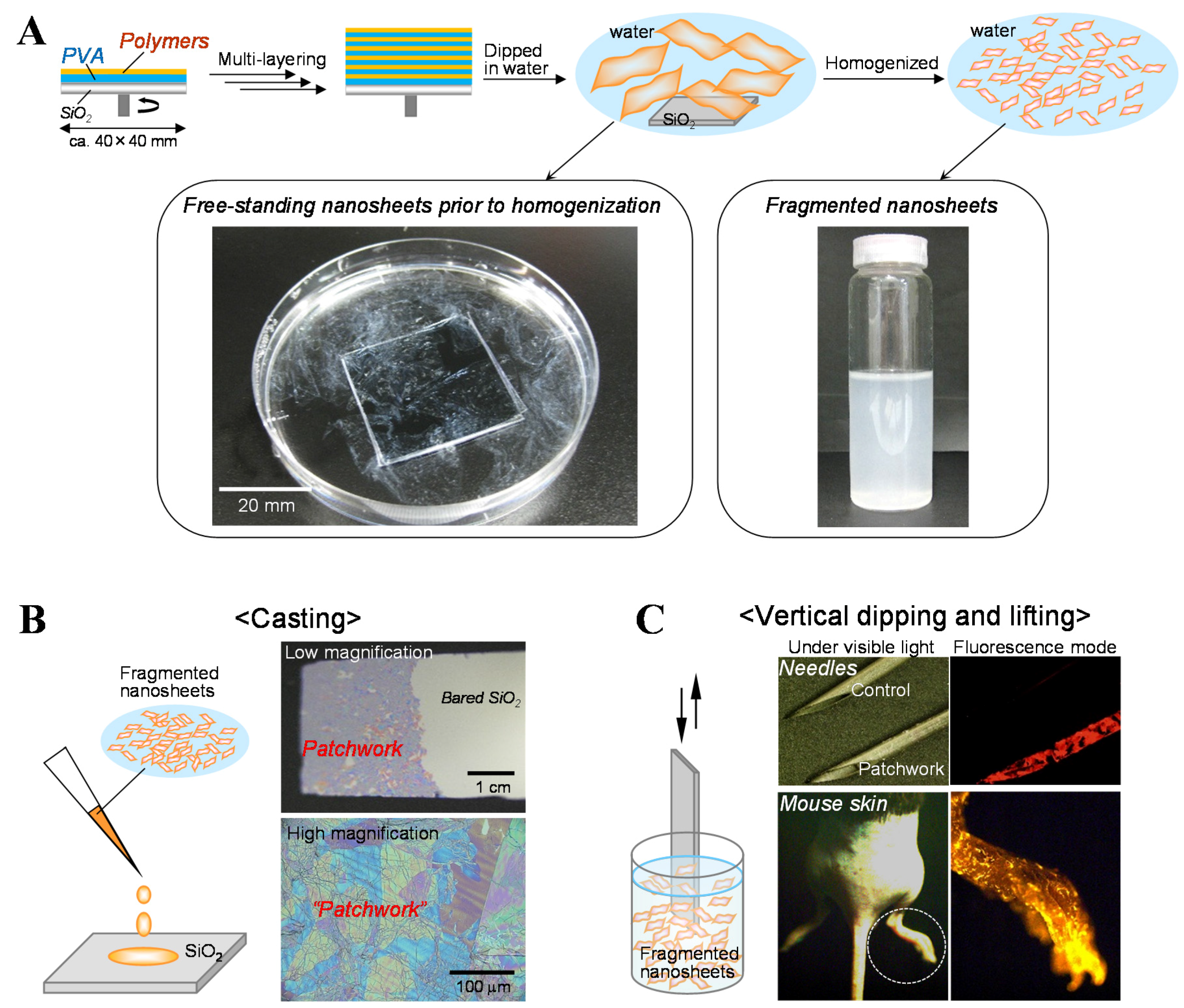
3.3. Biomedical Applications of the Fragmented Nanosheets as Aqueous Surface Modifiers
3.3.1. Burn Therapy
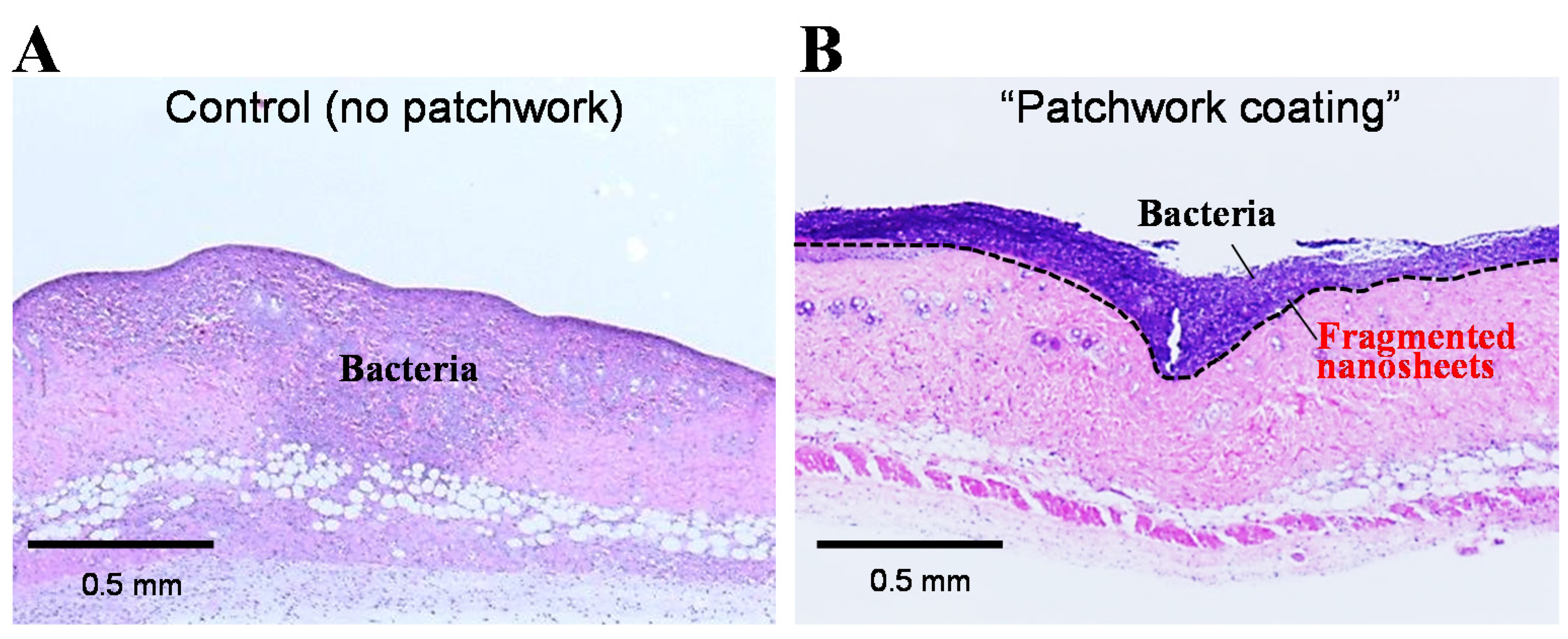
3.3.2. Antithrombotic Coating
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, W.; Campolongo, M.J.; Tan, S.J.; Luo, D. Freestanding ultrathin nano-membranes via self-assembly. Nano Today 2009, 4, 482–493. [Google Scholar] [CrossRef]
- Nikoobakht, B.; Li, X. Two-dimensional nanomembranes: Can they outperform lower dimensional nanocrystals? ACS Nano 2012, 6, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, S.; Okamura, Y.; Fujie, T.; Fukui, Y. Development of biodegradable nanosheets as nano-adhesive plaster. Pure Appl. Chem. 2008, 80, 2259–2271. [Google Scholar] [CrossRef]
- Mallwitz, F.; Laschewsky, A. Direct access to stable, freestanding polymer membranes by layer-by-layer assembly of polyelectrolytes. Adv. Mater. 2005, 17, 1296–1299. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Kotov, N.A.; Magonov, S.; Ozturk, B. Nanostructured artificial nacre. Nat. Mater. 2003, 2, 413–418. [Google Scholar] [CrossRef]
- Endo, H.; Mitsuishi, M.; Miyashita, T. Free-standing ultrathin films with universal thickness from nanometer to micrometer by polymer nanosheet assembly. J. Mater. Chem. 2008, 18, 1302–1308. [Google Scholar] [CrossRef]
- Vendamme, R.; Onoue, S.Y.; Nakao, A.; Kunitake, T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat. Mater. 2006, 5, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Mitrokotsa, M. Stabilized lipid film based biosensor for atenolol. Biosens. Bioelectron. 2002, 17, 565–572. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Theoharis, G. Rapid detection of vanillin in alcoholic beverages using stabilized polymerized lipid film based biosensors. Electroanalysis 2002, 14, 1661–1667. [Google Scholar] [CrossRef]
- Fujie, T.; Okamura, Y.; Takeoka, S. Ubiquitous transference of a free-standing polysaccharide nanosheet with the development of a nano-adhesive plaster. Adv. Mater. 2007, 19, 3549–3553. [Google Scholar] [CrossRef]
- Fujie, T.; Matsutani, N.; Kinoshita, M.; Okamura, Y.; Saito, A.; Takeoka, S. Adhesive, flexible, and robust polysaccharide nanosheets integrated for tissue-defect repair. Adv. Funct. Mater. 2009, 19, 2560–2568. [Google Scholar] [CrossRef]
- Fujie, T.; Okamura, Y.; Takeoka, S. Fabrication, Properties and Biomedical Applications of Nanosheets. In Functional Polymer Films, 2nd ed.; Advincula, R.C., Knoll, W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 2, pp. 907–931. [Google Scholar]
- Okamura, Y.; Kabata, K.; Kinoshita, M.; Saitoh, D.; Takeoka, S. Free-standing biodegradable poly(lactic acid) nanosheet for sealing operations in surgery. Adv. Mater. 2009, 21, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Kabata, K.; Kinoshita, M.; Miyazaki, H.; Saito, A.; Fujie, T.; Ohtsubo, S.; Saitoh, D.; Takeoka, S. Fragmentation of poly(lactic acid) nanosheets and patchwork treatment for burn wounds. Adv. Mater. 2013, 25, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y. Fabrication of ultra-thin nanosheets with unique properties for biomedical applications. Kobunshi Ronbunshu 2013, 70, 351–359. [Google Scholar] [CrossRef]
- Okamura, Y.; Nagase, Y. Fabrication of bio-friendly polymer nanosheets for biomedical applications. Trans. Mat. Res. Soc. Jpn. 2014, 39, 379–384. [Google Scholar] [CrossRef]
- Nagase, Y.; Okamura, Y. Synthesis of New Biocompatible Polymers and Fabrication of Nanosheets. In Advances in Bioengineering, 2nd ed.; Andrea Serra, P.A., Ed.; InTech: Rijeka, Croatia, 2015; Volume 1, pp. 3–20. [Google Scholar]
- Conn, J., Jr.; Oyasu, R.; Welsh, M.; Beal, J.M. Vicryl (polyglactin 910) synthetic absorbable sutures. Am. J. Surg. 1974, 128, 19–23. [Google Scholar] [CrossRef]
- McGuire, D.A.; Barber, F.A.; Elrod, B.F.; Paulos, L.E. Bioabsorbable interference screws for graft fixation in anterior cruciate ligament reconstruction. Arthroscopy 1999, 15, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Wan, C.; Tamm, L.M. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta 2009, 1788, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and thier properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ueda, T.; Oshida, H.; Kurita, K.; Ishihara, K.; Nakabayashi, N. Preparation of 2-methacryoyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J. 1992, 24, 1259–1269. [Google Scholar] [CrossRef]
- Nagase, Y.; Oku, M.; Iwasaki, Y.; Ishihara, K. Preparations of aromatic diamine monomers and copolyamides containing phosphorylcholine moiety and the biocompatibility of copolyamides. Polymer J. 2007, 39, 712–721. [Google Scholar] [CrossRef]
- Nagase, Y.; Nakajima, S.; Oku, M.; Iwasaki, Y.; Ishihara, K. Synthesis and properties of segmented poly(urethane-urea)s containing phosphorylcholine moiety in the side-chain. Polymer J. 2008, 40, 1149–1156. [Google Scholar] [CrossRef]
- Sakagami, Y.; Horiguchi, K.; Narita, Y.; Sirithep, W.; Morita, K.; Nagase, Y. Syntheses of a novel diol monomer and polyurethane elastomers containing phospholipid moieties. Polymer J. 2013, 45, 1159–1166. [Google Scholar] [CrossRef]
- Sirithep, W.; Morita, K.; Iwano, A.; Komachi, T.; Okamura, Y.; Nagase, Y. Syntheses and properties of elastic copoly(ester-urethane)s containing a phospholipid moiety and the fabrication of nanosheets. J. Biomater. Sci. Polymer Ed. 2014, 25, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- Iwano, A.; Morita, K.; Sirithep, W.; Okamura, Y.; Nagase, Y. Synthesis of biocompatible elastic polyurethane containing phospholipid moiety. Trans. Mat. Res. Soc. Jpn. 2014, 39, 411–414. [Google Scholar] [CrossRef]
- Baba, S.; Midorikawa, T.; Nakano, T. Unambiguous detection of the adhesive failure of metal films in the microscratch test by waveform analysis of the friction signal. Appl. Surf. Sci. 1999, 145–145, 344–349. [Google Scholar] [CrossRef]
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Two-dimensional dielectric nanosheets: Novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 2012, 24, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, X.; Liang, J.; Bando, Y.; Sasaki, T. Molecular-scale heteroassembly of redoxable hydroxide nanosheets and conductive graphene into superlattice composites for high-performance supercapacitors. Adv. Mater. 2014, 26, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, T.; Okubo, M. Preparation and thermodynamic stability of micron-sized, monodisperse composite polymer particles of disc-like shapes by seeded dispersion polymerization. Langmuir 2007, 23, 7958–7962. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nakatsuru, R.; Kagari, Y.; Okubo, M. Formation of “snowmanlike” polystyrene/poly(methyl methacrylate)/toluene droplets dispersed in an aqueous solution of a nonionic surfactant at thermodynamic equilibrium. Langmuir 2007, 23, 11506–11512. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, J.; Cleland, H.; Campbell, F. Dressings for superficial and partial thickness burns. Cochrane Database Syst. Rev. 2008, 4. [Google Scholar] [CrossRef]
- Satoh, Y.; Saitoh, D.; Takeuchi, A.; Ojima, K.; Kouzu, K.; Kawakami, S.; Ito, M.; Ishihara, M.; Sato, S.; Takishima, K. ERK2 dependent signaling contributes to wound healing after a partial-thickness burn. Biochem. Biophys. Res. Commun. 2009, 381, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Haith, L.R.; Stair-Buchmann, M.E.; Ackerman, B.H.; Herder, D.; Reigart, C.L.; Stoering, M.; Guilday, R.E.; Patton, M.L.; Ross, K.M. Evaluation of Aquacel Ag for Autogenous Skin Donor Sites. J. Burn Care. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.B.; Sparnon, A.L.; Byard, R.W. Unusual donor site reactions to calcium alginate dressings. Burns 2000, 26, 393–398. [Google Scholar] [CrossRef]
- Fuster, V.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N. Engl. J. Med. 1992, 326, 242–250. [Google Scholar] [PubMed]
- Iwasaki, Y.; Mikami, A.; Kurita, K.; Yui, N.; Ishihara, K.; Nakabayashi, N. Reduction of surface-induced platelet activation on phospholipid polymer. J. Biomed. Mater. Res. 1997, 36, 508–515. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamasaki, A.; Ishihara, K. Platelet compatible blood filtration fabrics using a phosphorylcholine polymer having high surface mobility. Biomaterials 2003, 24, 3599–3604. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamura, Y.; Nagase, Y.; Takeoka, S. Patchwork Coating of Fragmented Ultra-Thin Films and Their Biomedical Applications in Burn Therapy and Antithrombotic Coating. Materials 2015, 8, 7604-7614. https://doi.org/10.3390/ma8115404
Okamura Y, Nagase Y, Takeoka S. Patchwork Coating of Fragmented Ultra-Thin Films and Their Biomedical Applications in Burn Therapy and Antithrombotic Coating. Materials. 2015; 8(11):7604-7614. https://doi.org/10.3390/ma8115404
Chicago/Turabian StyleOkamura, Yosuke, Yu Nagase, and Shinji Takeoka. 2015. "Patchwork Coating of Fragmented Ultra-Thin Films and Their Biomedical Applications in Burn Therapy and Antithrombotic Coating" Materials 8, no. 11: 7604-7614. https://doi.org/10.3390/ma8115404