Enhanced Erbium-Doped Ceria Nanostructure Coating to Improve Solar Cell Performance
Abstract
:1. Introduction
2. Results and Discussion
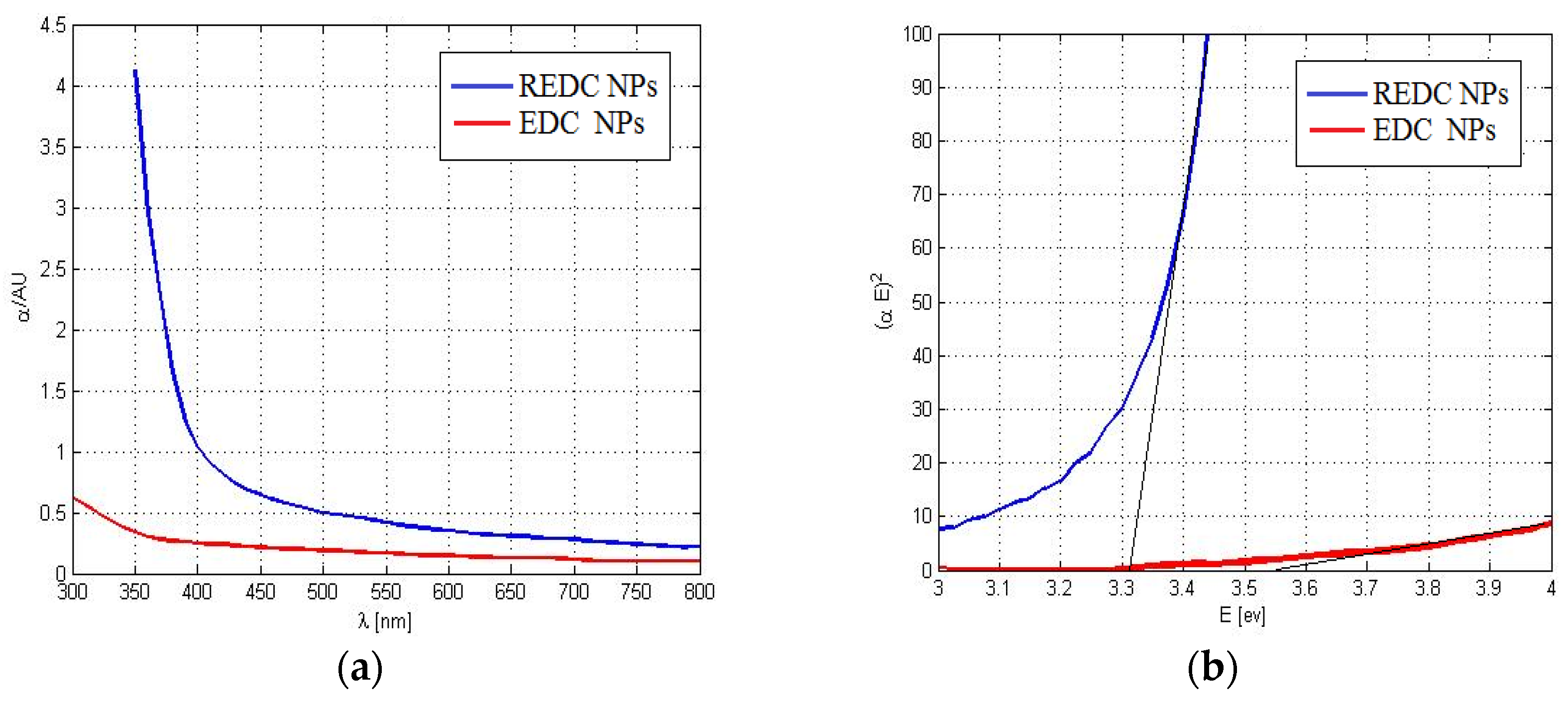
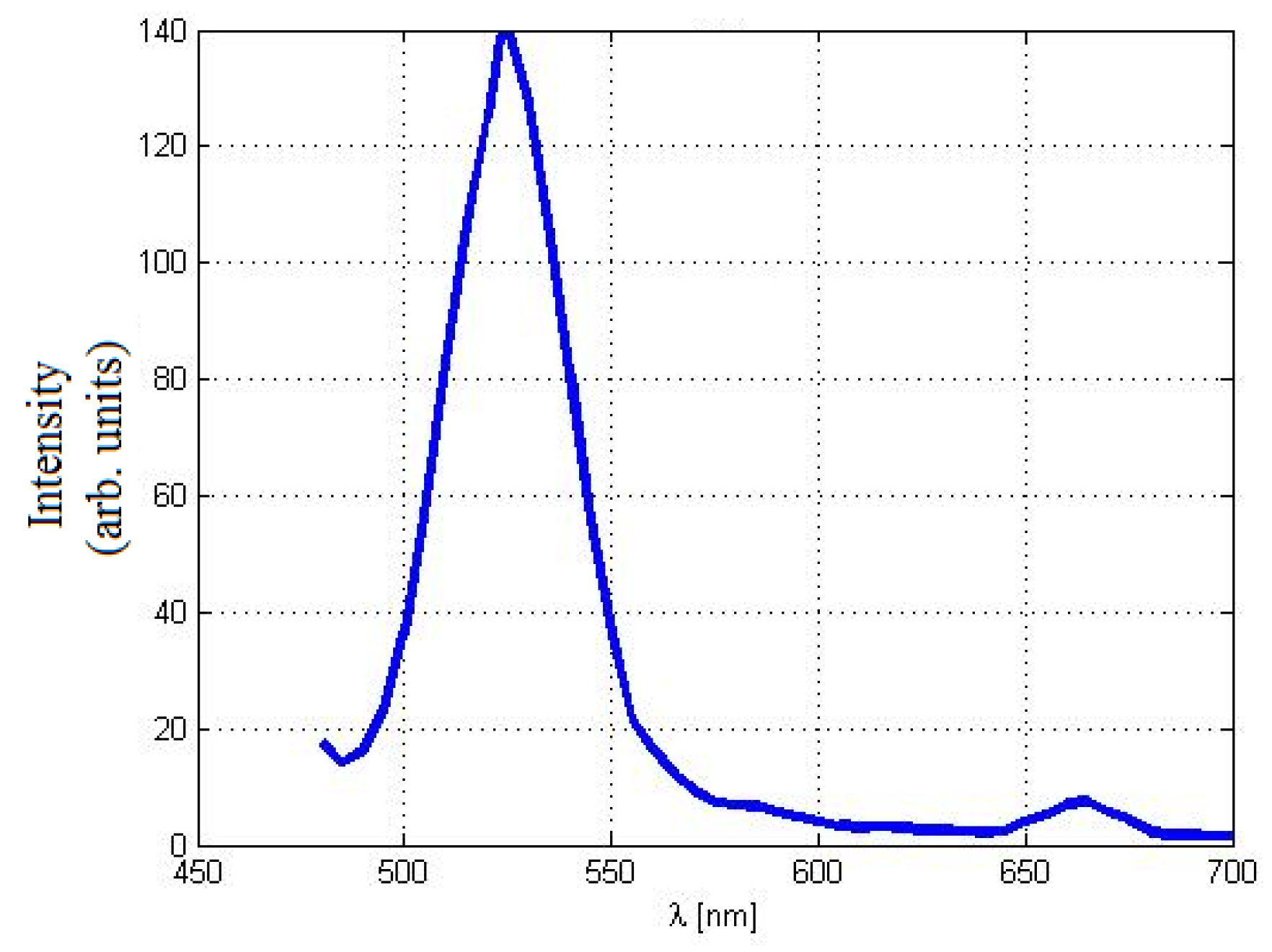
2.1. Nanoparticles Characterization

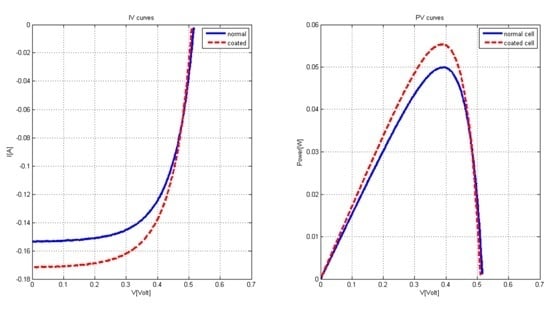
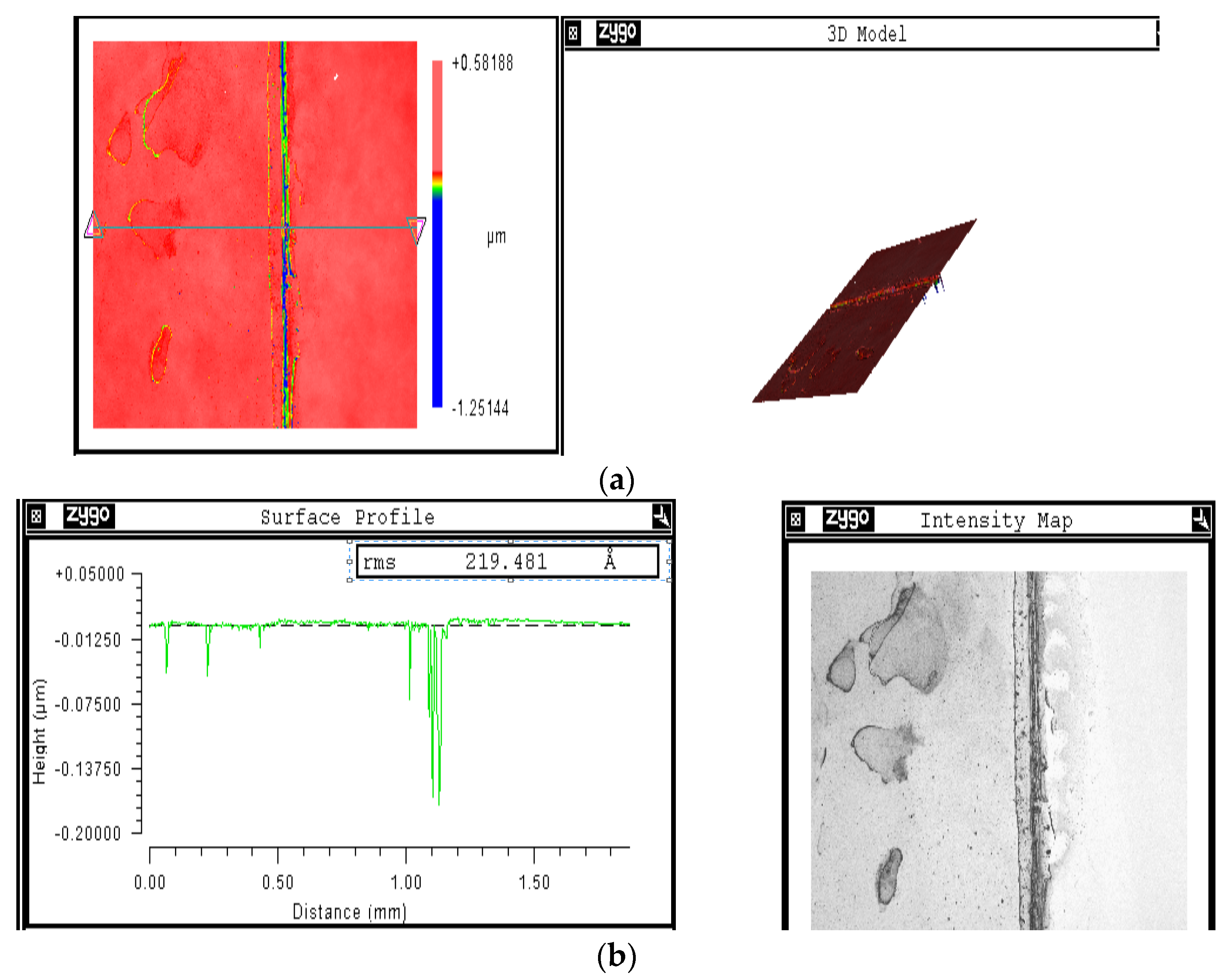
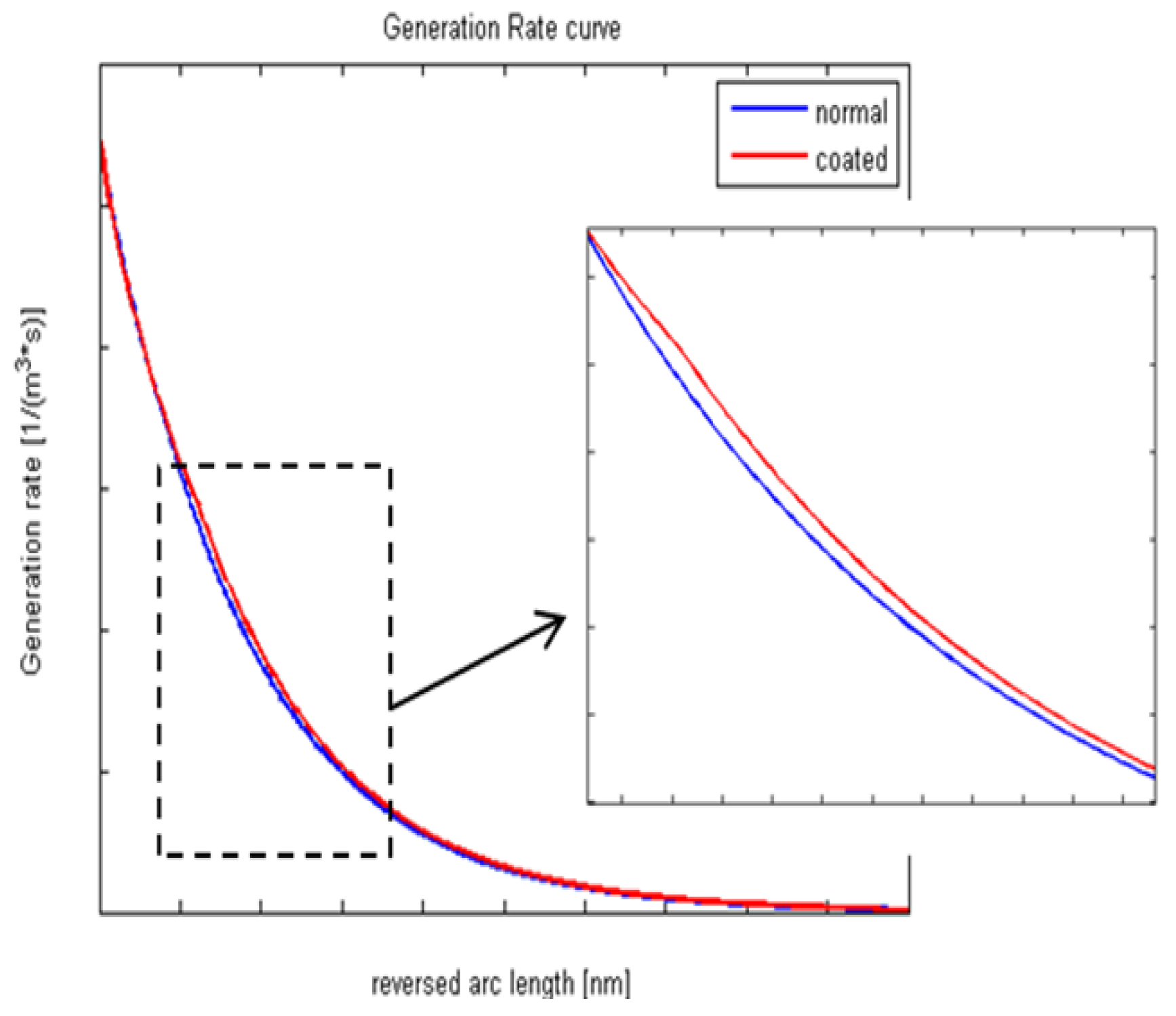
2.2. Coated Solar Cell Characterization
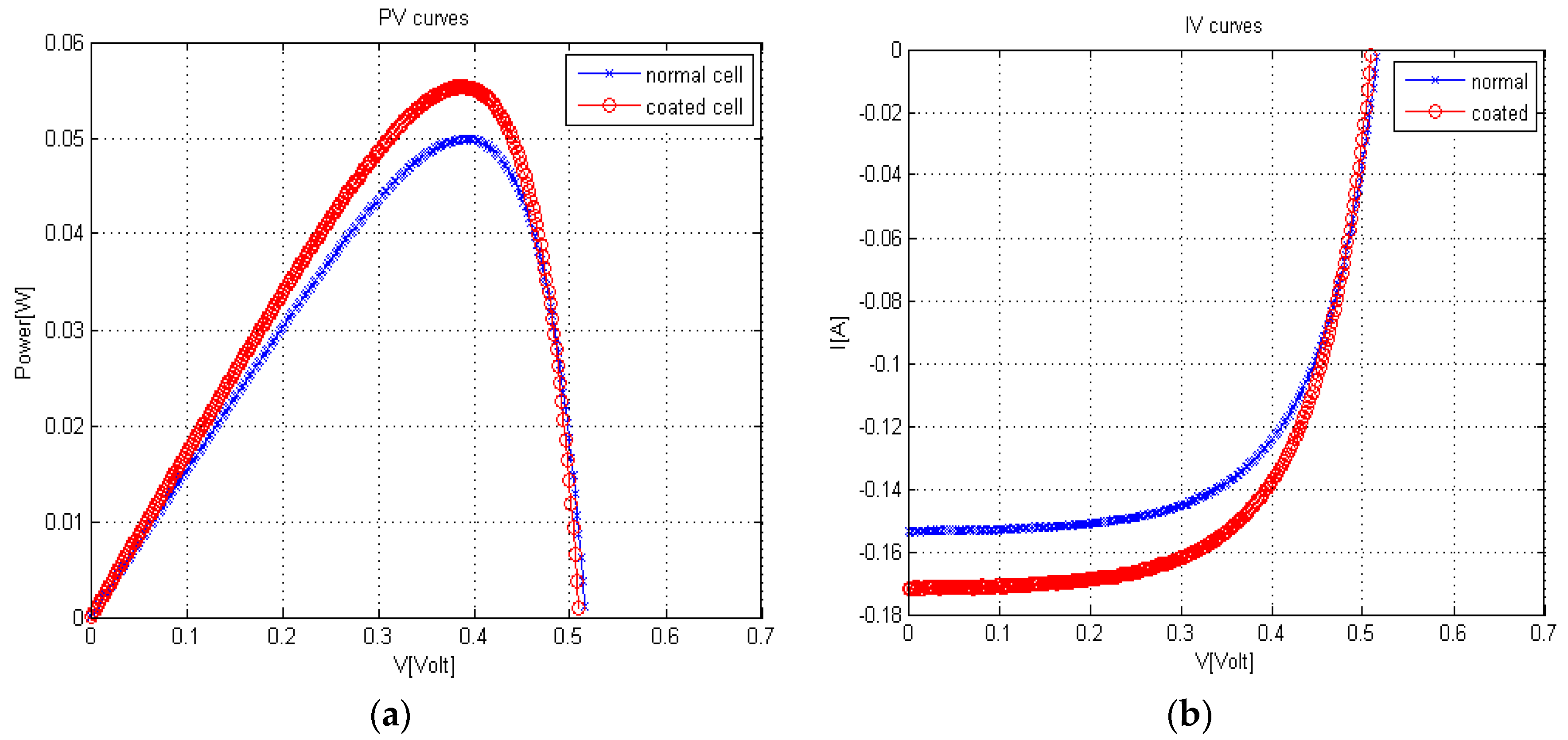
| Condition | F.F | % | ||
|---|---|---|---|---|
| Uncoated | 0.5155 | 0.1537 | 0.6301 | 15.1075 |
| coated cell | 0.5095 | 0.1718 | 0.6322 | 16.7452 |

3. Experimental Section
3.1. Nanoparticles Synthesis
3.2. Characterizations of Nanoparticles

3.3. Coating Procedure
3.4. Solar Cell Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maruyama, T.; Shinyashiki, Y.; Osako, S. Energy conversion efficiency of solar cells coated with fluorescent coloring agent. Sol. Energy Mater. Sol. Cells 1998, 56, 1–6. [Google Scholar] [CrossRef]
- Shan, G.D.; Demopoulos, G.P. Near-infrared sunlight harvesting in dye-sensitized solar cells via the insertion of an upconverter-TiO2 nanocomposite layer. Adv. Mater. 2010, 22, 4373–4377. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, S.; Fukuda, T.; Kasuya, A. Blue shift in ultraviolet absorption spectra of monodisperse CeO2−x nanoparticles. J. Appl. Phys. 2000, 87, 1318–1321. [Google Scholar] [CrossRef]
- Oh, M.; Nho, J.; Cho, S.; Lee, J.; Sing, R. Polishing behaviors of ceria abrasives on silicon dioxide and silicon nitride CMP. Powder Technol. 2011, 206, 239–245. [Google Scholar] [CrossRef]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.; Riedel, L.; Seal, S.; Hickman, J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Steel, B.; Heinzel, B. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chang, W.; Huang, C.; Sun, K. Biomimetic nanostructured antireflection coating and its application on crystalline silicon solar cells. Opt. Express 2011, 18, 14411–14419. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Ray, M.; Ratan, N.; Pramanick, A.; Hossain, S. Performance enhancement of crystalline silicon solar cells by coating with luminescent silicon nanostructures. J. Electron. Mater. 2013, 42, 403–409. [Google Scholar] [CrossRef]
- Pinna, A.; Figus, C.; Lasio, B.; Piccinini, M.; Malfatti, L.; Innocenzi, P. Release of ceria nanoparticles grafted on hybrid organic–inorganic films for biomedical application. ACS Appl. Mater. Interfaces 2012, 4, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Lasio, B.; Piccinini, M.; Marmiroli, B.; Amenitsch, H.; Falcaro, P.; Tokudome, Y.; Malfatti, L.; Innocenzi, P. Combining top-down and bottom-up routes for fabrication of mesoporous titania films containing ceria nanoparticles for free radical scavenging. ACS Appl. Mater. Interfaces 2013, 5, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Barbara, B.; Lasio, B.; Malfatti, L. Engineering the surface of hybrid organic-inorganic films with orthogonal grafting of oxide nanoparticles. J. Nanopart. Res. 2014, 16, 2463–2466. [Google Scholar] [CrossRef]
- Pankove, P. Optical Processes in Semiconductors; Dover Publications Inc.: New York, NY, USA, 1971. [Google Scholar]
- Shehata, N.; Meehan, K.; Leber, D. Fluorescence quenching in ceria nanoparticles: A dissolved oxygen molecular probe with a relatively temperature insensitive Stern-Volmer constant up to 50 °C. J. Nanophotonics 2012, 6. [Google Scholar] [CrossRef]
- Dhannia, T.; Jayalekshmi, S.; Kumar, M.; Rao, T.; Bose, A. Effect of aluminium doping and annealing on structural and optical properties of cerium oxide nanocrystals. J. Phys. Chem. Solids 2009, 70, 1443–1447. [Google Scholar] [CrossRef]
- Lawrence, N.; Jiang, K.; Cheung, C.L. Formation of a porous cerium oxide membrane by anodization. Chem. Commun. 2011, 47, 2703–2705. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Meehan, K.; Hassounah, I.; Hudait, M.; Jain, N.; Clavel, M.; Elhelw, S.; Madi, N. Reduced erbium-doped ceria nanoparticles: One nano-host applicable for simultaneous optical down- and up-conversions. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Devi, S.; Maiti, S. Synthesis and properties of nanocrystalline ceria powders. J. Mater. Res. 2004, 19, 3162–3171. [Google Scholar] [CrossRef]
- Guo, H. Green and red upconversion luminescence in CeO2:Er3+ powders produced by 785 nm laser. J. Solid State Chem. 2007, 180, 127–131. [Google Scholar] [CrossRef]
- Liu, T.; Hon, M.; Teoh, L.G. Structure and optical properties of CeO2 nanoparticles synthesized by precipitation. J. Electron. Mater. 2013, 42, 2536–2541. [Google Scholar] [CrossRef]
- Lappalainen, J.; Tuller, H.; Lantto, V. Electronic conductivity and dielectric properties of nanocrystalline CeO2 films. J. Electroceram. 2004, 13, 129–133. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, F.; Zhao, L.; Ma, Y.; Ya, J. Comparative XPS study of surface reduction for nanocrystalline and microcrystalline ceria powder. Appl. Surf. Sci. 2006, 25, 4931–4935. [Google Scholar] [CrossRef]
- Chen, H.; Chang, H. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf. A 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Shehata, N.; Meehan, K.; Hudait, M.; Jain, N. Control of oxygen vacancies and Ce+3 concentrations in doped ceria nanoparticles via the selection of lanthanide element. J. Nanopart. Res. 2012, 14, 1173–1183. [Google Scholar] [CrossRef]
- Chui, C.O.; Kim, H.; McIntyre, P.C.; Saraswat, K.C. Atomic layer deposition of high-κ dielectric for germanium MOS applications—Substrate surface preparation. IEEE Electron. Device Lett. 2004, 25, 274–276. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2005. [Google Scholar]
- Shehata, N.; Meehan, K.; Leber, D. Study of fluorescence quenching in aluminum-doped ceria nanoparticles: Potential molecular probe for dissolved oxygen. J. Fluoresc. 2013, 23, 527–532. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, N.; Clavel, M.; Meehan, K.; Samir, E.; Gaballah, S.; Salah, M. Enhanced Erbium-Doped Ceria Nanostructure Coating to Improve Solar Cell Performance. Materials 2015, 8, 7663-7672. https://doi.org/10.3390/ma8115399
Shehata N, Clavel M, Meehan K, Samir E, Gaballah S, Salah M. Enhanced Erbium-Doped Ceria Nanostructure Coating to Improve Solar Cell Performance. Materials. 2015; 8(11):7663-7672. https://doi.org/10.3390/ma8115399
Chicago/Turabian StyleShehata, Nader, Michael Clavel, Kathleen Meehan, Effat Samir, Soha Gaballah, and Mohammed Salah. 2015. "Enhanced Erbium-Doped Ceria Nanostructure Coating to Improve Solar Cell Performance" Materials 8, no. 11: 7663-7672. https://doi.org/10.3390/ma8115399