Inexpensive Ipomoea aquatica Biomass-Modified Carbon Black as an Active Pt-Free Electrocatalyst for Oxygen Reduction Reaction in an Alkaline Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Catalyst Preparation
2.3. Characterization
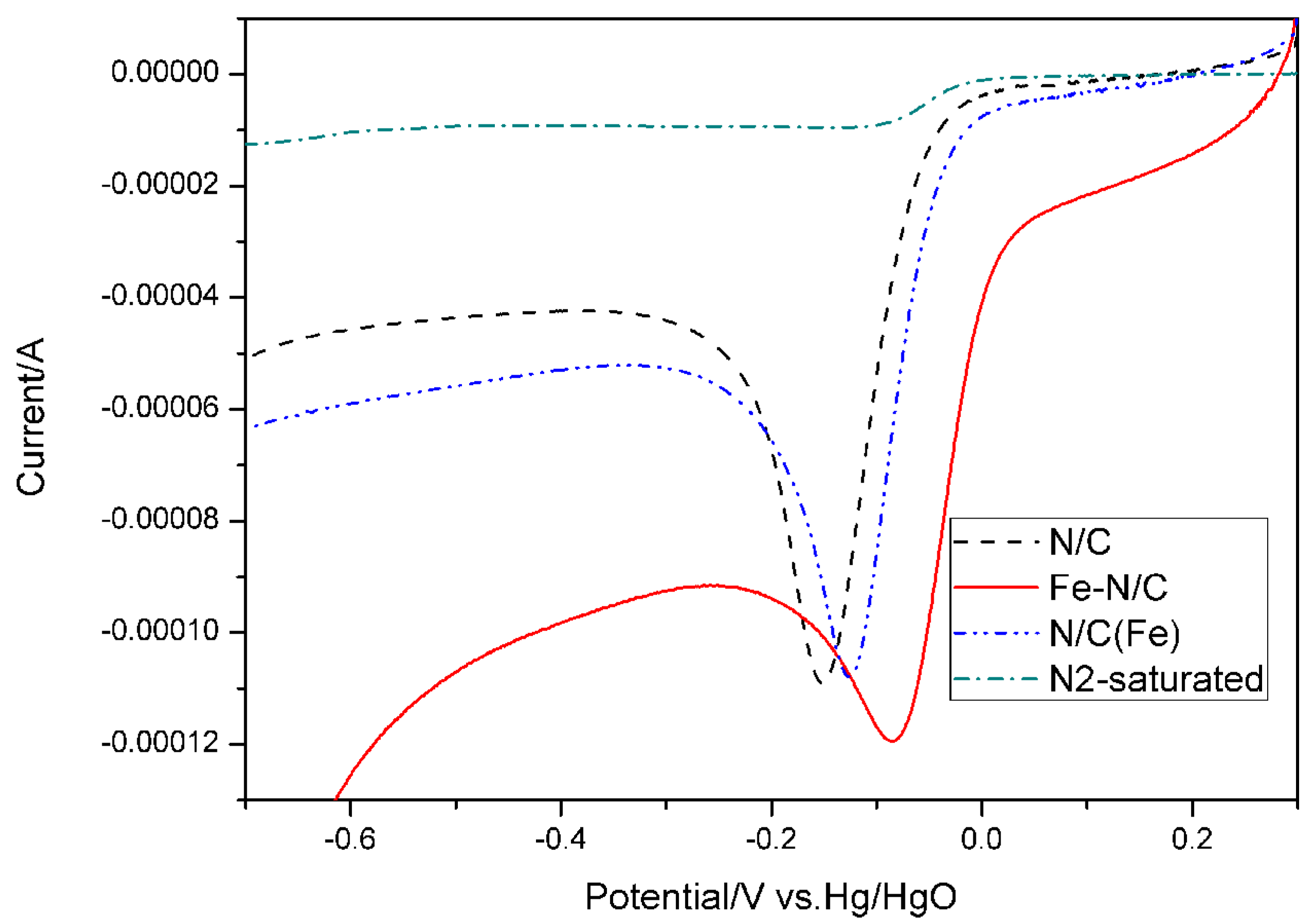
3. Results and Discussion





| Catalyst | Peak | Area | FWHM | Content |
|---|---|---|---|---|
| N/C | 398.497 eV | 803 | 2.600 eV | 35% |
| 400.936 eV | 909 | 2.300 eV | 40% | |
| 404.734 eV | 567 | 2.200 eV | 25% | |
| Fe–N/C | 399.436 eV | 2328 | 1.603 eV | 100% |
| N/C(Fe) | 398.803 eV | 599 | 2.167 eV | 25% |
| 400.743 eV | 12159 | 2.304 eV | 50% | |
| 404.173 eV | 612 | 2.050 eV | 25% |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Jégou, P.; Jousselme, B.; Palacin, S. Electrochemical performance of annealed cobalt–benzotriazole/CNTs catalysts towards the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2011, 13, 21600–21607. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Z.; Liao, W.L.; Sun, L.T.; Chen, C.G. Synthesis of non-noble nitrogen-containing catalysts for cathodic oxygen reduction reaction: A critical review. Int. J. Electrochem. Sci. 2015, 10, 2467–2477. [Google Scholar]
- Poh, C.K.; Lim, S.H.; Tian, Z.; Lai, L.; Feng, Y.P.; Shen, Z.; Lin, J. Pt-WxC nano-composites as an efficient electrochemical catalyst for oxygen reduction reaction. Nano Energy 2013, 2, 28–39. [Google Scholar]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Z.; Chen, C.G.; Luo, Z.L. A novel nitrogen-containing electrocatalyst for oxygen reduction reaction from blood protein pyrolysis. J. Power Sour. 2014, 245, 841–845. [Google Scholar] [CrossRef]
- Nabae, Y.; Kuang, Y.; Chokai, M.; Ichihara, T.; Isoda, A.; Hayakawa, T.; Aoki, T. High performance Pt-free cathode catalysts for polymer electrolyte membrane fuel cells prepared from widely available chemicals. J. Mater. Chem. A 2014, 2, 11561–11564. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nature Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Ji, S.; Feng, H.; Linkov, V.; Wang, R. Biomass-derived activated carbon as high-performance non-precious electrocatalyst for oxygen reduction. RSC Adv. 2013, 3, 12039–12042. [Google Scholar] [CrossRef]
- Si, Y.J.; Chen, C.G.; Yin, W.; Cai, H. Electrocatalytic activity of non-precious metal catalyst Co–N/C toward oxygen reduction reaction. Chin. Chem. Lett. 2010, 21, 983–986. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Li, Z.; Chen, C. Exploration of the catalytically active site structures of animal biomass-modified on cheap carbon nanospheres for oxygen reduction reaction with high activity, stability and methanol-tolerant performance in alkaline medium. Carbon 2015, 85, 279–288. [Google Scholar] [CrossRef]
- Guo, C.Z.; Chen, C.G.; Luo, Z.L. The structural changes of blood pyropolymers and their beneficial electrocatalytic activity toward oxygen reduction. Chin. Sci. Bull. 2013, 58, 3698–3703. [Google Scholar] [CrossRef]
- Maruyama, J.; Fukui, N.; Kawaguchi, M.; Abe, I. Application of nitrogen-rich amino acids to active site generation in oxygen reduction catalyst. J. Power Sour. 2008, 182, 489–495. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.Y.; Chen, D.J.; Lin, J.L.; Ke, F.S.; Xu, G.L.; Sun, S.G. Study of pyrolyzed hemin/C as non-platinum cathodic catalyst for direct methanol fuel cells. Sci. China Chem. 2010, 53, 2057–2062. [Google Scholar] [CrossRef]
- Jiang, R.; Tran, D.T.; McClure, J.; Chu, D. Heat-treated hemin supported on graphene nanoplatelets for the oxygen reduction reaction. Electrochem. Commun. 2012, 19, 73–76. [Google Scholar] [CrossRef]
- Xi, P.B.; Liang, Z.X.; Liao, S.J. Stability of hemin/C electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2012, 37, 4606–4611. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.K.; Wang, G.; Gao, M.R.; Ge, J.; Yuan, W.J.; Shen, Y.H.; Xie, A.J.; Yu, S.H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, D.; Li, Y.; Liu, X.; Liu, P.; Yang, H.; An, T.; Tang, Z.; Zhao, H. Hydrothermal transformation of dried grass into graphitic carbon-based high performance electrocatalyst for oxygen reduction reaction. Small 2014, 10, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, Z.; Wang, C.; Zhang, W.; Liu, G. An absolutely green approach to fabricate carbon nanodots from soya bean grounds. RSC Adv. 2013, 3, 20662–20665. [Google Scholar] [CrossRef]
- Guo, C.Z.; Liao, W.L.; Chen, C.G. Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures. J. Power Sour. 2014, 269, 841–847. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; García, H. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, C.; Yin, Y.; Zhang, Y.; Wu, H.; Chen, C. The use of cheap polyaniline and melamine co-modified carbon nanotubes as active and stable catalysts for oxygen reduction reaction in alkaline medium. Electrochim. Acta 2015, 160, 357–362. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Antonietti, M. A zero-emission fuel cell that uses carbonaceous colloids from biomass waste as fuel source. Chem. Sus. Chem. 2010, 3, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Cui, L.; Wu, Y.; Liu, Y.; Pu, T.; He, X. A novel cobalt tetranitrophthalocyanine/graphene composite assembled by an in situ solvothermal synthesis method as a highly efficient electrocatalyst for the oxygen reduction reaction in alkaline medium. Phys. Chem. Chem. Phys. 2013, 15, 13093–13100. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sour. 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Feng, H.; Ji, S.; Mao, X.; Wang, R. Three-dimensional iron, nitrogen-doped carbon foams as efficient electrocatalysts for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 142, 317–323. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Andersen, N.I.; Stariha, S.; Atanassov, P. Original mechanochemical synthesis of non-platinum group metals oxygen reduction reaction catalysts assisted by sacrificial support method. Electrochim. Acta 2015, in press. [Google Scholar] [CrossRef]
- Moon, J.S.; Lee, Y.W.; Han, S.B.; Kwak, D.H.; Lee, K.H.; Park, A.R.; Sohn, J.I.; Cha, S.N.; Park, K.W. Iron–nitrogen-doped mesoporous tungsten carbide nanostructures as oxygen reduction electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 14644–14650. [Google Scholar] [CrossRef] [PubMed]
- Serov, A.; Tylus, U.; Artyushkova, K.; Mukerjee, S.; Atanassov, P. Mechanistic studies of oxygen reduction on Fe-PEI derived non-PGM electrocatalysts. Appl. Catal. B Environ. 2014, 150, 179–186. [Google Scholar] [CrossRef]
- Singh, D.; Soykal, I.I.; Tian, J.; Deak, D.; King, J.; Miller, J.T.; Ozkan, U.S. In situ characterization of the growth of CNx carbon nano-structures as oxygen reduction reaction catalysts. J. Catal. 2013, 304, 100–111. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chang, K.H.; Huang, Y.L.; Lee, Y.F.; Tien, H.W.; Li, S.M.; Lee, Y.H.; Liu, C.H.; Ma, C.C.M.; Hu, C.C. A powerful approach to fabricate nitrogen-doped graphene sheets with high specific surface area. Electrochem. Commun. 2012, 14, 39–42. [Google Scholar] [CrossRef]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, C.; Ma, Z.; Wu, H.; Chen, C. Inexpensive Ipomoea aquatica Biomass-Modified Carbon Black as an Active Pt-Free Electrocatalyst for Oxygen Reduction Reaction in an Alkaline Medium. Materials 2015, 8, 6658-6667. https://doi.org/10.3390/ma8105331
Zhang Y, Guo C, Ma Z, Wu H, Chen C. Inexpensive Ipomoea aquatica Biomass-Modified Carbon Black as an Active Pt-Free Electrocatalyst for Oxygen Reduction Reaction in an Alkaline Medium. Materials. 2015; 8(10):6658-6667. https://doi.org/10.3390/ma8105331
Chicago/Turabian StyleZhang, Yaqiong, Chaozhong Guo, Zili Ma, Huijuan Wu, and Changguo Chen. 2015. "Inexpensive Ipomoea aquatica Biomass-Modified Carbon Black as an Active Pt-Free Electrocatalyst for Oxygen Reduction Reaction in an Alkaline Medium" Materials 8, no. 10: 6658-6667. https://doi.org/10.3390/ma8105331






