Differently Shaped Au Nanoparticles: A Case Study on the Enhancement of the Photocatalytic Activity of Commercial TiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Proposed Research Plan
- (a)
- Spheres: 3D particles described by a single dimension: the radius of the sphere;
- (b)
- Triangles: 2D particles described by multiple geometric elements and for which one of the geometry defining element is significantly smaller than the other;
- (c)
- Wires: 1D particles where two of the geometry defining element is significantly smaller than the other.
2.2. Characterization of the Photocatalysts
2.2.1. X-ray Diffraction (XRD)

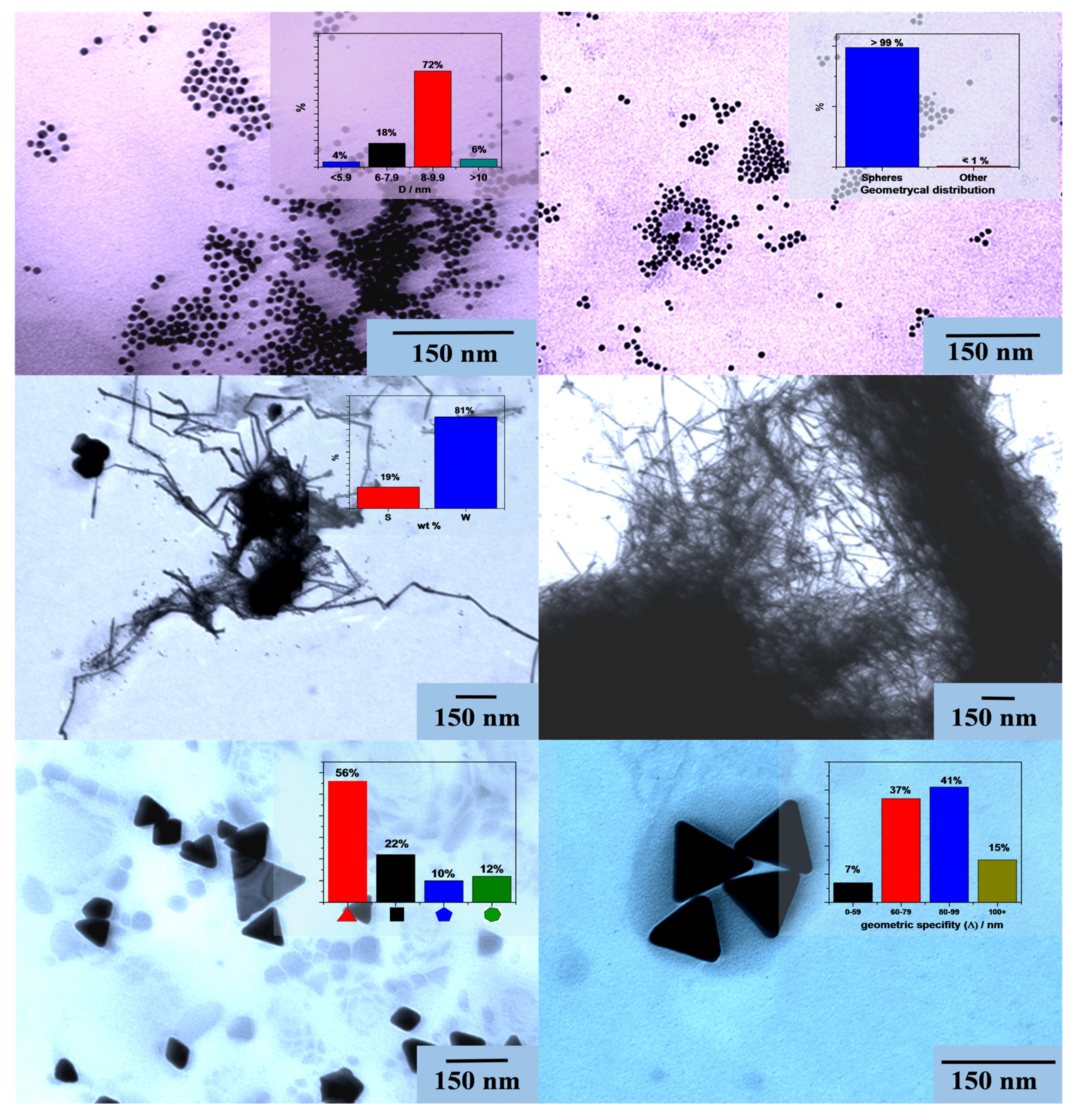
2.2.2. Transmission Electron Microscopy (TEM)

2.2.3. Diffuse Reflectance Spectroscopy (DRS)


2.3. Photocatalytic Performance of the Obtained Nanocomposites
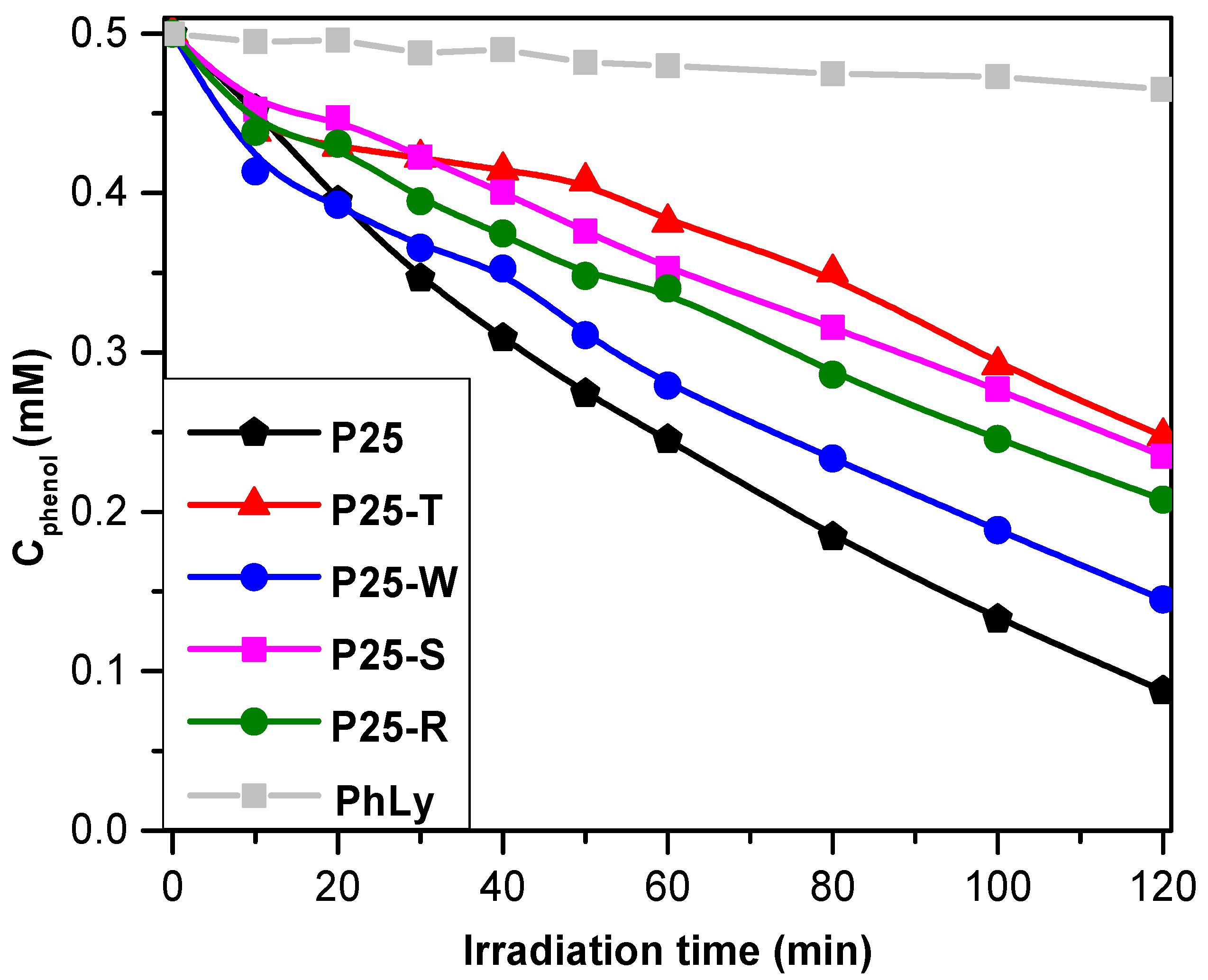
2.3.1. Photodegradation of Phenol
- (i)
- The particle’s size in the base catalyst—in the present study this was ruled out by using Aeroxide P25 in all the composite materials;
- (ii)
- Au NPs crystal size—all the Au NPs obtained are large Au NPs (except for P25-S).
| Sample | r0,oxalic acid (mM·min−1) ×(10−3) | r0, phenol (mM·min−1) ×(10−3) | Oxalic acidUV/Vis degradation rate (%) | PhenolUV/Vis degradation rate (%) | H2 production rate (mL·h−1) | Band gap (eV) |
|---|---|---|---|---|---|---|
| P25-W | 46.0 | 7.45 | 97.4/21.0 | 71.0/12.2 | 0.62 | 2.85 |
| P25-S | 71.7 | 3.19 | 97.6/20.8 | 52.9/8.6 | 0.78 | 2.70 |
| P25-R | 51.2 | 4.49 | 86.2/19.1 | 58.4/9.5 | 0.63 | 2.80 |
| P25-T | 69.0 | 5.01 | 75.5/16.8 | 50.5/7 | – | 2.85 |
| P25 | 41.9 | 5.71 | 54.3/12 | 82.4/13.8 | – | 3.11 |
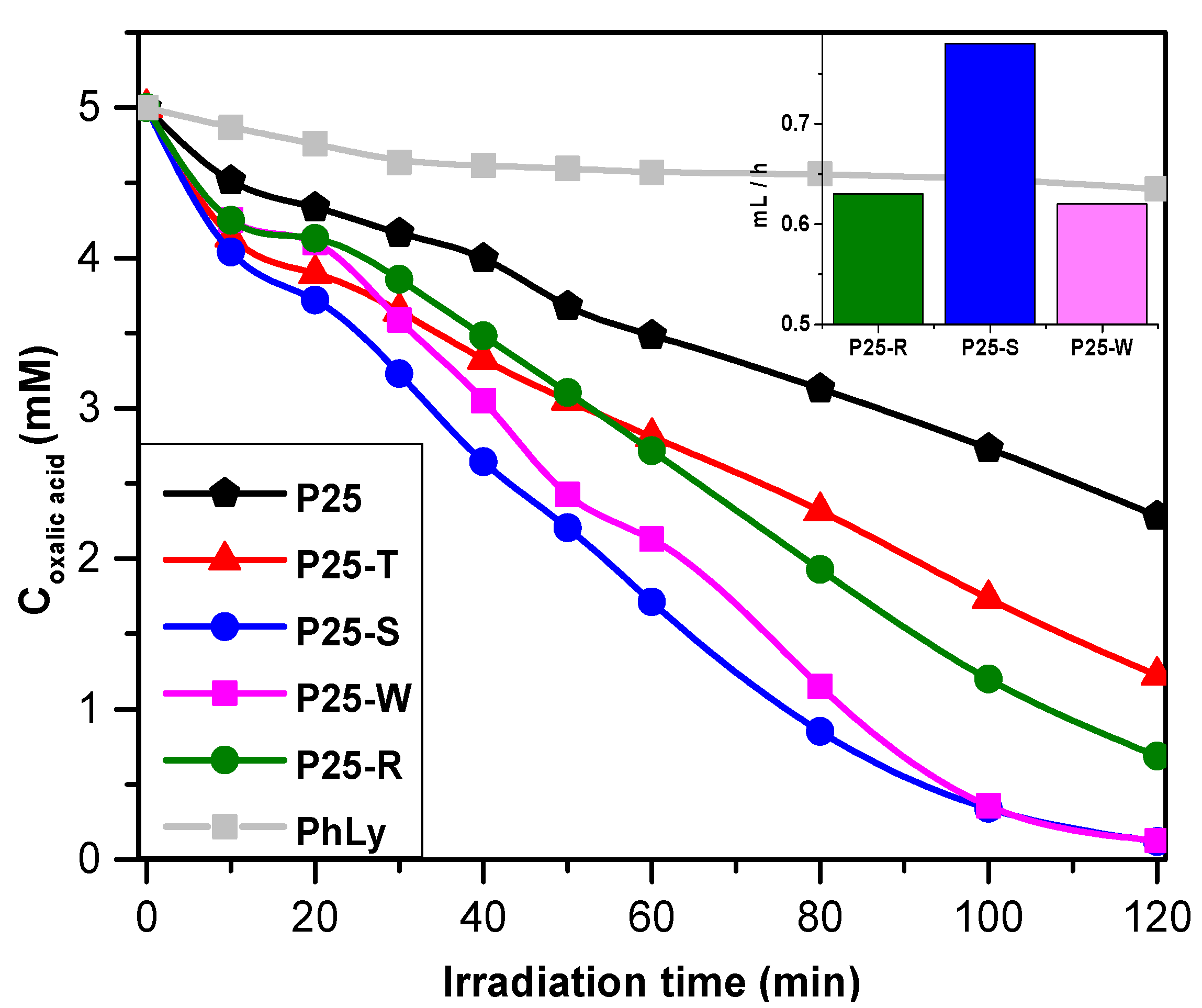
2.3.2. Photodegradation of Oxalic Acid and Photocatalytic Hydrogen Production
3. Experimental Section
3.1. Materials
3.2. The Synthesis of the Au-NPs
3.3. Synthesis of Au–TiO2 Composites
3.4. Characterization Methods and Instrumentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K.; Kikuchi, S. Photosensitized electrolytic oxidation on semiconducting n-type TiO2 electrode. J Soc. Chem. Ind. Jpn. 1969, 72, 108–113. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Rajbongshi, B.M.; Ramchiary, A.; Samdarshi, S.K. Influence of N-doping on photocatalytic activity of ZnO nanoparticles under visible light irradiation. Mater. Lett. 2014, 134, 111–114. [Google Scholar] [CrossRef]
- Siddiquey, I.; Furusawa, T.; Sato, M.; Suzuki, N. Microwave-assisted silica coating and photocatalytic activities of ZnO nanoparticles. Mater. Res. Bull. 2008, 43, 3416–3424. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhu, L.; Oh, W.-C. Preparation and high visible-light-induced photocatalytic activity of CdSe and CdSe–C60 nanoparticles. J. Ind. Eng. Chem. 2012, 18, 2004–2009. [Google Scholar] [CrossRef]
- Ghosh, T.; Cho, K.-Y.; Ullah, K.; Nikam, V.; Park, C.-Y.; Meng, Z.-D.; Oh, W.-C. High photonic effect of organic dye degradation by CdSe–graphene–TiO2 particles. J. Ind. Eng. Chem. 2013, 19, 797–805. [Google Scholar] [CrossRef]
- Talebian, N.; Jafarinezhad, F. Morphology-controlled synthesis of SnO2 nanostructures using hydrothermal method and their photocatalytic applications. Ceram. Int. 2013, 39, 8311–8317. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Farbod, M.; Meamar Ghaffari, N.; Kazeminezhad, I. Fabrication of single phase CuO nanowires and effect of electric field on their growth and investigation of their photocatalytic properties. Ceram. Int. 2014, 40, 517–521. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. Cuo nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Kim, J.; Bondarchuk, O.; Kay, B.D.; White, J.M.; Dohnálek, Z. Preparation and characterization of monodispersed WO3 nanoclusters on TiO2(110). Catal. Today 2007, 120, 186–195. [Google Scholar] [CrossRef]
- Song, X.C.; Zheng, Y.F.; Yang, E.; Wang, Y. Large-scale hydrothermal synthesis of WO3 nanowires in the presence of K2SO4. Mater. Lett. 2007, 61, 3904–3908. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Vinu, R.; Madras, G. Renewable energy via photocatalysis. Curr. Org. Chem. 2013, 17, 2538–2558. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar]
- Huang, C.-J.; Wang, Y.-H.; Chiu, P.-H.; Shih, M.-C.; Meen, T.-H. Electrochemical synthesis of gold nanocubes. Mater. Lett. 2006, 60, 1896–1900. [Google Scholar] [CrossRef]
- Baik, H.J.; Hong, S.; Park, S. Surface plasmon modes of gold nanospheres, nanorods, and nanoplates in an organic solvent: Phase-transfer from aqueous to organic media. J. Colloid Interface Sci. 2011, 358, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Choudhury, S.; Tewari, R.; Tyagi, A.K.; Kale, S.P. Synthesis of uniform gold nanoparticles using non-pathogenic bio-control agent: Evolution of morphology from nano-spheres to triangular nanoprisms. J. Colloid Interface Sci. 2012, 367, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Gupta, R.; Gunupuru, R.; Srivastava, D.N.; Paul, P. Calix[4]arene functionalized gold nanoparticles: Application in colorimetric and electrochemical sensing of cobalt ion in organic and aqueous medium. Sens. Actuators Chem. 2014, 191, 757–764. [Google Scholar] [CrossRef]
- Benkovicova, M.; Vegso, K.; Siffalovic, P.; Jergel, M.; Luby, S.; Majkova, E. Preparation of gold nanoparticles for plasmonic applications. Thin Solid Films 2013, 543, 138–141. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Orza, A.; Olenic, L.; Pruneanu, S.; Pogacean, F.; Biris, A.S. Morphological and electrical characteristics of amino acid-AuNP nanostructured two-dimensional ensembles. Chem. Phys. 2010, 373, 295–299. [Google Scholar] [CrossRef]
- Wang, J.-D.; Liu, J.-K.; Lu, Y.; Hong, D.-J.; Yang, X.-H. Catalytic performance of gold nanoparticles using different crystallinity hap as carrier materials. Mater. Res. Bull. 2014, 55, 190–197. [Google Scholar] [CrossRef]
- Omidfar, K.; Khorsand, F.; Darziani Azizi, M. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Bannat, I.; Wessels, K.; Oekermann, T.; Rathousky, J.; Bahnemann, D.; Wark, M. Improving the photocatalytic performance of mesoporous titania films by modification with gold nanostructures. Chem. Mater. 2009, 21, 1645–1653. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; Garcia, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.C.; Maicu, M.; Navío, J.A.; Colón, G. Effect of sulfate pretreatment on gold-modified TiO2 for photocatalytic applications. J. Phys. Chem. C 2009, 113, 12840–12847. [Google Scholar] [CrossRef]
- Veréb, G.; Ambrus, Z.; Pap, Z.; Kmetykó, Á.; Dombi, A.; Danciu, V.; Cheesman, A.; Mogyorósi, K. Comparative study on UV and visible light sensitive bare and doped titanium dioxide photocatalysts for the decomposition of environmental pollutants in water. Appl. Catal. A Gen. 2012, 417–418, 26–36. [Google Scholar] [CrossRef]
- Iliev, V.; Tomova, D.; Bilyarska, L.; Tyuliev, G. Influence of the size of gold nanoparticles deposited on TiO2 upon the photocatalytic destruction of oxalic acid. J. Mol. Catal. A Chem. 2007, 263, 32–38. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Paulsi, S. Gold nanoparticles in early detection and treatment of cancer: Biodistribution and toxicities. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 80–88. [Google Scholar]
- Kumar, D.; Saini, N.; Jain, N.; Sareen, R.; Pandit, V. Gold nanoparticles: An era in bionanotechnology. Expert Opin. Drug Deliv. 2013, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Integrated nanoparticle–biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.; Zhang, T.; Mou, C.-Y. Catalysis by gold: New insights into the support effect. Nano Today 2013, 8, 403–416. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Petkov, V.; Peng, Y.; Williams, G.; Huang, B.; Tomalia, D.; Ren, Y. Structure of gold nanoparticles suspended in water studied by X-ray diffraction and computer simulations. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Kumar, D.V.R.; Kulkarni, A.A.; Prasad, B.L.V. Synthesis of triangular gold nanoplates: Role of bromide ion and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 181–190. [Google Scholar] [CrossRef]
- Bernardi, M.; Raja, S.N.; Lim, S.K. Nanotwinned gold nanowires obtained by chemical synthesis. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Karácsonyi, É.; Baia, L.; Dombi, A.; Danciu, V.; Mogyorósi, K.; Pop, L.C.; Kovács, G.; Coşoveanu, V.; Vulpoi, A.; Simon, S.; et al. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitectures obtained by selective photodeposition. Catal. Today 2013, 208, 19–27. [Google Scholar] [CrossRef]
- Kuwauchi, Y.; Yoshida, H.; Akita, T.; Haruta, M.; Takeda, S. Intrinsic catalytic structure of gold nanoparticles supported on TiO2. Angew. Chem. Int. Ed. 2012, 51, 7729–7733. [Google Scholar] [CrossRef]
- Pap, Z.; Radu, A.; Hidi, I.J.; Melinte, G.; Diamandescu, L.; Popescu, T.; Baia, L.; Danciu, V.; Baia, M. Behavior of gold nanoparticles in a titania aerogel matrix: Photocatalytic activity assessment and structure investigations. Chin. J. Catal. 2013, 34, 734–740. [Google Scholar] [CrossRef]
- Maicu, M.; Hidalgo, M.C.; Colon, G.; Navio, J.A. Comparative study of the photodeposition of Pt, Au and Pd on pre-sulphated TiO2 for the photocatalytic decomposition of phenol. J. Photochem. Photobiol. A Chem. 2011, 217, 275–283. [Google Scholar] [CrossRef]
- Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Norén, K.; Canton, S.E.; et al. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity “from degradation intermediates to catalysts’ structural peculiarities”, part II: Aerogel based composites—fine details by spectroscopic means. Appl. Catal. B Environ. 2014, 148–149, 589–600. [Google Scholar] [CrossRef]
- Kovács, G.; Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Pap, Z. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, part I: Aeroxide P25 based composites. Appl. Catal. B Environ. 2014, 147, 508–517. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Bannat, I.; Wark, M. Gold nanoparticles on mesoporous interparticle networks of titanium dioxide nanocrystals for enhanced photonic efficiencies. J. Phys. Chem. C 2009, 113, 7429–7435. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pap, Z.; Tóth, Z.R.; Danciu, V.; Baia, L.; Kovács, G. Differently Shaped Au Nanoparticles: A Case Study on the Enhancement of the Photocatalytic Activity of Commercial TiO2. Materials 2015, 8, 162-180. https://doi.org/10.3390/ma8010162
Pap Z, Tóth ZR, Danciu V, Baia L, Kovács G. Differently Shaped Au Nanoparticles: A Case Study on the Enhancement of the Photocatalytic Activity of Commercial TiO2. Materials. 2015; 8(1):162-180. https://doi.org/10.3390/ma8010162
Chicago/Turabian StylePap, Zsolt, Zsejke Réka Tóth, Virginia Danciu, Lucian Baia, and Gábor Kovács. 2015. "Differently Shaped Au Nanoparticles: A Case Study on the Enhancement of the Photocatalytic Activity of Commercial TiO2" Materials 8, no. 1: 162-180. https://doi.org/10.3390/ma8010162






