2.2.1. General Diffusion Considerations
In addition to thermally induced effects of dopant depletion, there also appear kinetic limitations due to dopant diffusion. Dopant diffusion has been evaluated during MCVD processing of silica preforms with various dopants [
5,
6,
8,
13]. The basic concepts developed there will be applied to the fiber drawing process. This will yield a rough estimate of dopant diffusion effects encountered during drawing process.
Diffusion effects of dopants during fiber drawing or cane stretching broaden the concentration profile. There is an interaction between the thermodynamic effects of gas emission reactions and kinetic effects of diffusion processes. The dopant transport from the inner of the preform or cane to its surface is determined by the evaporation rate and the volume of dopants, and it also impacts the above described (thermal) interface reaction. The diffusion process is modeled according to Fick’s second law in one-dimensional coordinates:
where
c is the local concentration of the dopant,
t is the time,
x is the coordinate and correlates to the radius, and
D is the diffusion coefficient.
The concentration profile is obtained from solving numerically the integral of Equation (3) where a possible solution is given by:
The characteristic diffusion length,
L is obtained from the solution of diffusion in Equation (4) setting erf(1), that is
t = τ
D, the effective time of diffusion:
It is known that the dopant diffusion coefficients are a function of temperature
T and of dopant concentration
c. The temperature and concentration correlation has been investigated by Kirchhof
et al. [
6] on the basis of MCVD processing. An empirical equation (Equation (6)) has been postulated describing the observed
D for binary SiO
2-GeO
2 glasses in the temperature range of 1200–2000 °C and at a concentration range from 0 mol% to 20 mol% GeO
2. A similar dependency was obtained for binary SiO
2-SiF
4 glasses over the temperature range of 1000–2000 °C and for concentrations of <1 mol% of SiF
4:
where
A0,
A1,
B1,
x are experimentally estimated parameters listed in
Table 1. This equation is similar to the common Arrhenius expression of the (concentration dependent) diffusion coefficient:
where
EA(
c) is the concentration dependent activation energy, R is the universal gas constant,
D0 is the pre-exponential factor, and
T the temperature.
The activation energies
EA can be deduced from comparison of Equations (6) and (7) using the parameters given in
Table 1 as:
Table 1.
Experimentally estimated parameters for temperature and concentration dependent diffusion equation (Equation (6)).
Table 1.
Experimentally estimated parameters for temperature and concentration dependent diffusion equation (Equation (6)).
| Dopant | A0 | A1 (K) | B1 (K) | x | EA (kJ/mol) | Reference |
|---|
| GeO2 | 3.63 | −30800 | 71 | 1.0 | (590-1.4c) | [6] |
| SiF4 | 0.24 | −20000 | 0 | 0 | 383 | [5] |
Note that the fluorine diffusion is assumed to be independent of concentration in a concentration regime up to 6 mol% of SiF4.
For simulation of germanium and fluorine concentration profiles due to dopant diffusion during the drawing process, the axial length of diffusion zone in the drawing furnace is estimated in analogy to the method described in reference [
13].
One important parameter for the simulation is the time the preform is exposed to such high temperatures in the furnace that noticeable diffusion of the dopants takes place. This time is governed by the speed of movement of the preform through the furnace as well as the heat zone length it is moving through. The heat zone length is the length where the temperature is sufficiently high enough for detectable diffusion. Thus, a temperature has to be estimated where the activation energy of diffusion induces a measurable dopant material flux. This temperature is called equivalent temperature
Teq and can be estimated as follows [
14]:
where
Tmax is the maximum temperature of the axial temperature profile, and
EA is the activation energy of dopant diffusion as can be calculated by Equation (8a), and R is the universal gas constant. Considering the intersection of equivalent temperature
Teq with axial position of the drawing temperature profile an effective axial heat zone length can be assessed which represents the effective length of diffusion,
zD, during drawing/stretching.
Figure 2 shows the estimated germanium and fluorine diffusion zone lengths
zD for a maximum temperature of 1900 °C.
zD is about 38 mm for germanium diffusion and 48 mm for fluorine diffusion, respectively. The time the preform needs to pass that distance in the furnace at a given preform speed
vp is called the effective diffusion time τ
D. For fiber or cane drawing the preform feed rate determines the fiber drawing speed or cane drawing speed by fixed preform and fiber or cane diameters. The diffusion length
zD can be expressed either by preform or fiber material flow conditions:
where
vp is preform feed rate, τ
DP is the diffusion time on the preform scale,
vF is the fiber drawing speed, and τ
DF is an equivalent diffusion time in terms of fiber scale. Equation (10) results from mass conservation during the elongation of the preform to the fiber. The effective diffusion time is needed to estimate the diffusion on a radial scale of preform or fiber.
Figure 2.
Axial temperature profile of drawing furnace as determined from thermocouple measurements with a maximum temperature of 1900 °C. The effective lengths zD for radial diffusion of GeO2 and fluorine are marked in the curve in blue and red, respectively.
Figure 2.
Axial temperature profile of drawing furnace as determined from thermocouple measurements with a maximum temperature of 1900 °C. The effective lengths zD for radial diffusion of GeO2 and fluorine are marked in the curve in blue and red, respectively.
The simulation of changes in dopant concentration profiles due to diffusion was done with COMSOL
® Software (COMSOL AB, Stockholm, Sweden) [
15]. It is based on solving Equation (3) numerically taking into account:
The broadening of the dopant profiles can be roughly estimated from the characteristic diffusion length,
L, which is obtained from Equation (5) setting
t = τ
D, the effective time of diffusion. For the fiber scale, it follows:
Note that L can be calculated either for the preform scale using τD,P or for the fiber scale using τD,F as the effective time of diffusion and substitution according to Equation (10).
2.2.2. Effects of Germanium Diffusion
Germanium diffusions effects are considered for typical stacking elements that contain a GeO2 doped core and a SiO2 cladding. Thus evaporation effects according reaction in Equation (1) are negligible.
At a typical drawing temperature of 1900 °C the germanium diffusion coefficient increases by about a factor of 3 when increasing the GeO
2 concentration from 1 mol% to 20 mol%, that is an increase from 1 × 10
−10.5 cm
2·s
−1 to 1 × 10
−10 cm
2·s
−1, respectively [
6]. A slightly higher coefficient was estimated for fluorine diffusion. At 1900 °C, it was determined to be 1 × 10
−9.44 cm
2·s
−1 [
5].
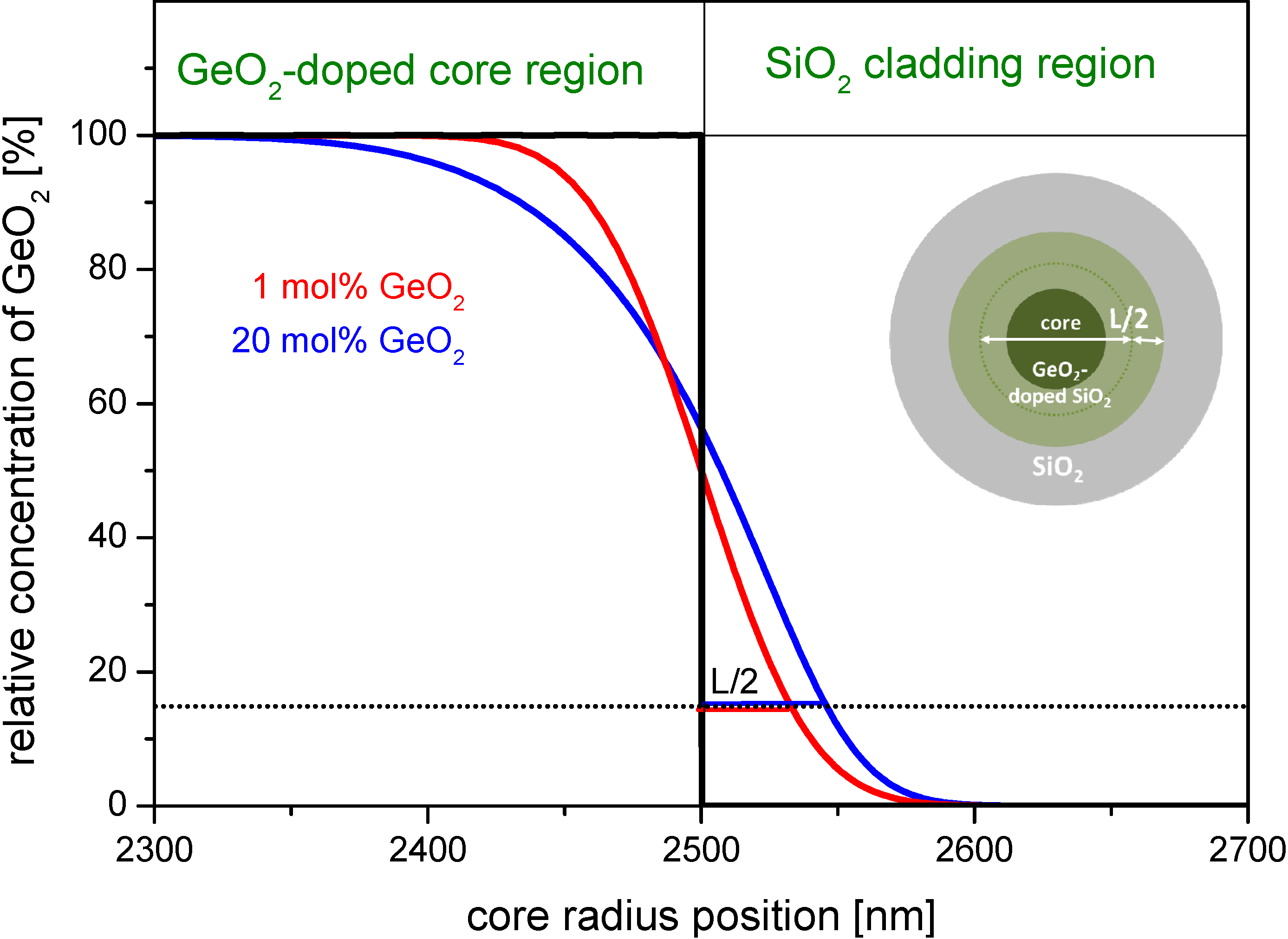
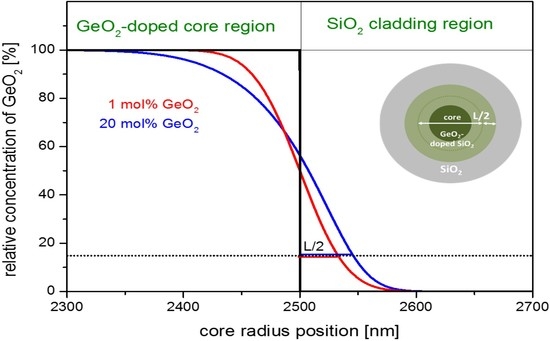
Figure 3 exemplarily shows a simulated GeO
2-concentration curve as a function of radial position. It simulates the diffusion of GeO
2 from the center of the core towards the cladding. The initial concentration profile is a step index profile of 5 µm diameter. The effective diffusion time τ
D used for the simulation is evaluated from Equation (10) using a drawing speed of
vF = 10 m·min
−1 and the afore determined
zD. The regions of SiO
2 cladding and GeO
2-doped core are marked in the upper part of the figure.
Figure 3.
Simulated dopant concentration profiles due to diffusion in the boundary region for germanium doped step index cores with a starting diameter of 5 µm at a drawing temperature of Tmax = 1900 °C and one drawing pass (simulated for fiber geometry conditions). Black curve: assumed initial profile; red curve: simulated profile starting with 1 mol% GeO2; and blue curve: simulated profile starting with 20 mol% GeO2. The inset figure shows a sketch of a GeO2-doped element, the dark green region represents the original concentration; and the light green represents the region with lower concentration.
Figure 3.
Simulated dopant concentration profiles due to diffusion in the boundary region for germanium doped step index cores with a starting diameter of 5 µm at a drawing temperature of Tmax = 1900 °C and one drawing pass (simulated for fiber geometry conditions). Black curve: assumed initial profile; red curve: simulated profile starting with 1 mol% GeO2; and blue curve: simulated profile starting with 20 mol% GeO2. The inset figure shows a sketch of a GeO2-doped element, the dark green region represents the original concentration; and the light green represents the region with lower concentration.
The dopant profiles of the drawn fiber broaden by L/2, that is about 32 nm for 1 mol% GeO2 and by 45 nm in case of 20 mol% GeO2 doping. The relative error of this broadening at the very high doping concentration of 20 mol% GeO2 amounts to maximum 6% for a typical core size of 5 µm, and is significantly less for lower dopant concentrations.
Practically spoken, the penetration of GeO2 into the surface area of the undoped silica cladding can be neglected for most drawing scenarios.
2.2.3. Effects of Fluorine Diffusion
A different situation is faced during drawing of active doped filament canes and fibers which are overcladded with a highly fluorine doped silica jacketing tube. Here, the SiF4-doped SiO2 region is directly adjoined by air. Thus evaporation reactions according to reaction in Equation (2) have to be taken into account.
Fluorine doped silica tubes and rods (e.g., Heraeus Fluosil® from Heraeus Quarzglas GmbH & Co. KG, Hanau, Germany) are used for low index overcladding of laser fiber pump cores and/or the introduction of non-guiding/symmetry-breaking elements to increase the pump efficiency. The typical fabrication approach is the arrangement of rods in a fluorine doped overcladding tube.
The expected change in SiF
4 concentration profile was again simulated with COMSOL
® Software [
15]. Due to the high vapor pressure at the SiO
2-SiF
4 equilibrium (see
Figure 1) under drawing conditions, the fluorine dopants directly leak into the space between preform stack and cladding. Thus, a zero concentration of fluorine at the surface during diffusion is assumed for the simulation.
Figure 4 shows the simulated progress of the fluorine dopant profiles starting with the initial step index profile (Line a) and for the cases of cane drawing and fiber drawing (Lines b and c, respectively).
Figure 4.
Simulated profiles of fluorine diffusion as a function of time starting with a step index profile (simulated for preform geometry conditions): (a) assumed initial profile; (b) simulated profile after 394 s; and (c) simulated profile after 3941 s. The inset figure shows a sketch of an F-doped tube, the dark green region represents the original F-concentration, the light green represents the region with lower concentration.
Figure 4.
Simulated profiles of fluorine diffusion as a function of time starting with a step index profile (simulated for preform geometry conditions): (a) assumed initial profile; (b) simulated profile after 394 s; and (c) simulated profile after 3941 s. The inset figure shows a sketch of an F-doped tube, the dark green region represents the original F-concentration, the light green represents the region with lower concentration.
Exemplary values of diffusion lengths are given for a fluorinated overcladding tube with a mole fraction of xSiF4 = 5.4 mol%, an outer diameter of 25 mm and an inner diameter of 19 mm, thus d0 = 3 mm; at Tmax = 1900 °C, a diffusion length of L = 9 µm for cane drawing (vP = 8 mm·min−1) can be estimated, whereas for fiber drawing the equivalent diffusion length is enlarged to L = 35 µm due to the longer process time with vP = 0.75 m·min−1.
The surface transfer of fluorine dopants into the space between preform stack and cladding creates SiF4-rich bubbles during drawing process. In the following, we approximate the expected volume of SiF4 bubbles surrounding a silica rod stack with a fluorinated silica tube.
The assumption is that all of the diffused SiF
4 is completely evaporated into the cavities. The corresponding molar amount of vaporized SiF
4 is converted into an equivalent bubble volume using the ideal gas equation. The ratio of SiF
4 bubble volume to the SiO
2 solid volume can then be expressed in the simplified form:
where
VSiF4(g) is the volume of evaporated gas SiF
4,
VSiO2(s) is the volume of the overcladding tube,
L is the characteristic diffusion length of fluorine,
d0 is the wall thickness of the fluorinated cladding tube,
xSiF4 is the mole fraction, and
VM(SiO2) is the molar volume of the matrix material SiO
2. For simplification, standard conditions for the gas phase (
T = 273 K, and
p = 101325 Pa) are assumed.
Using Equation (12), and inserting xSiF4 = 5.4 mol% and d0 = 3 mm, a bubble volume of 11% and 35% relative to the overcladding material volume for the drawn cane and fiber can be estimated, respectively. Note that the difference in those values results from the different times the preforms stays in the heat zone of the oven: at high fiber drawing speed the preform motion is slow, whereas in case of cane drawing the preform motion is higher.