Corrosion Resistance of a Sand Particle-Modified Enamel Coating Applied to Smooth Steel Bars
Abstract
:1. Introduction
2. Results and Discussion
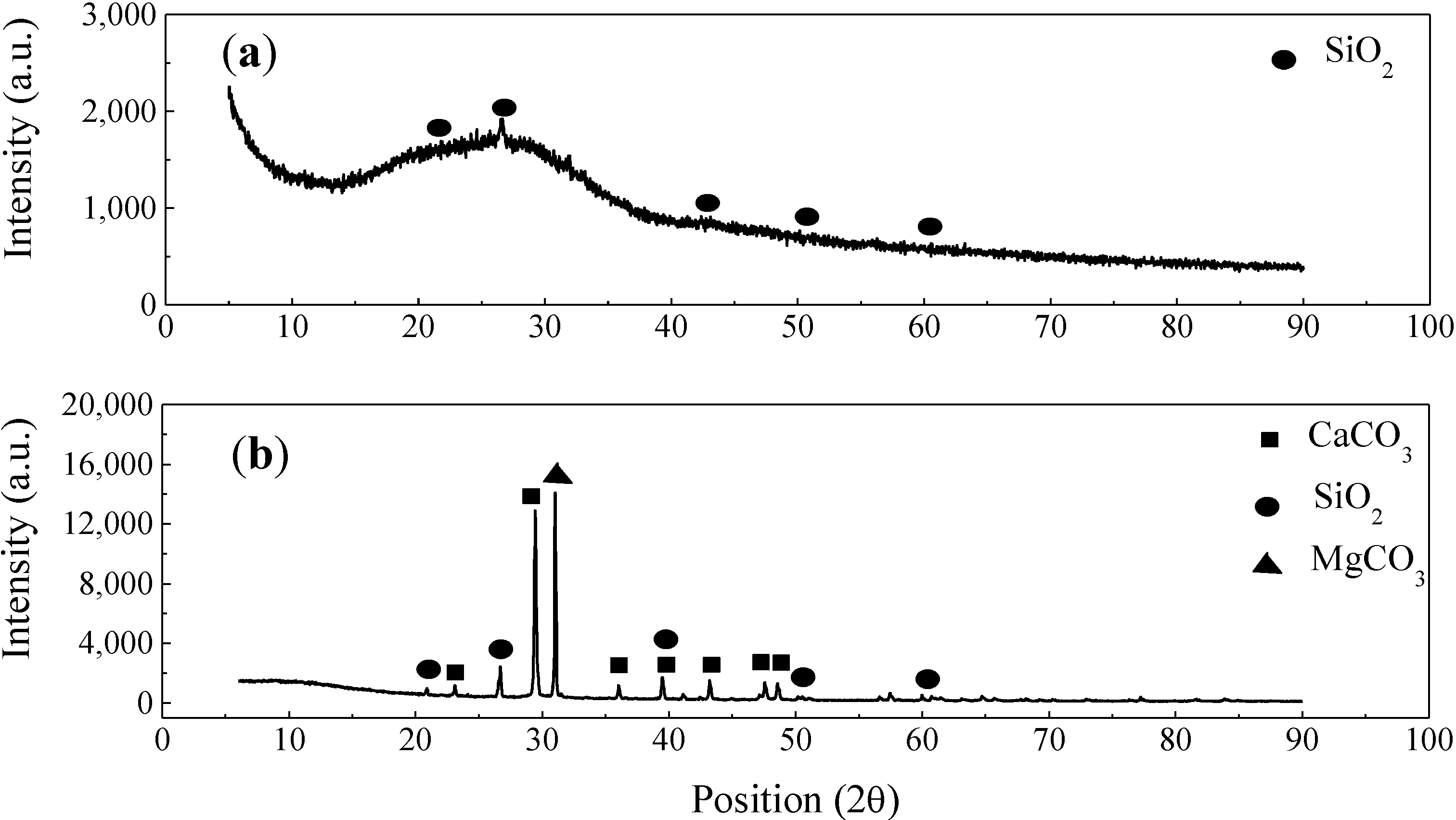
2.1. Characterization of Enamel Coating and Sand Particles

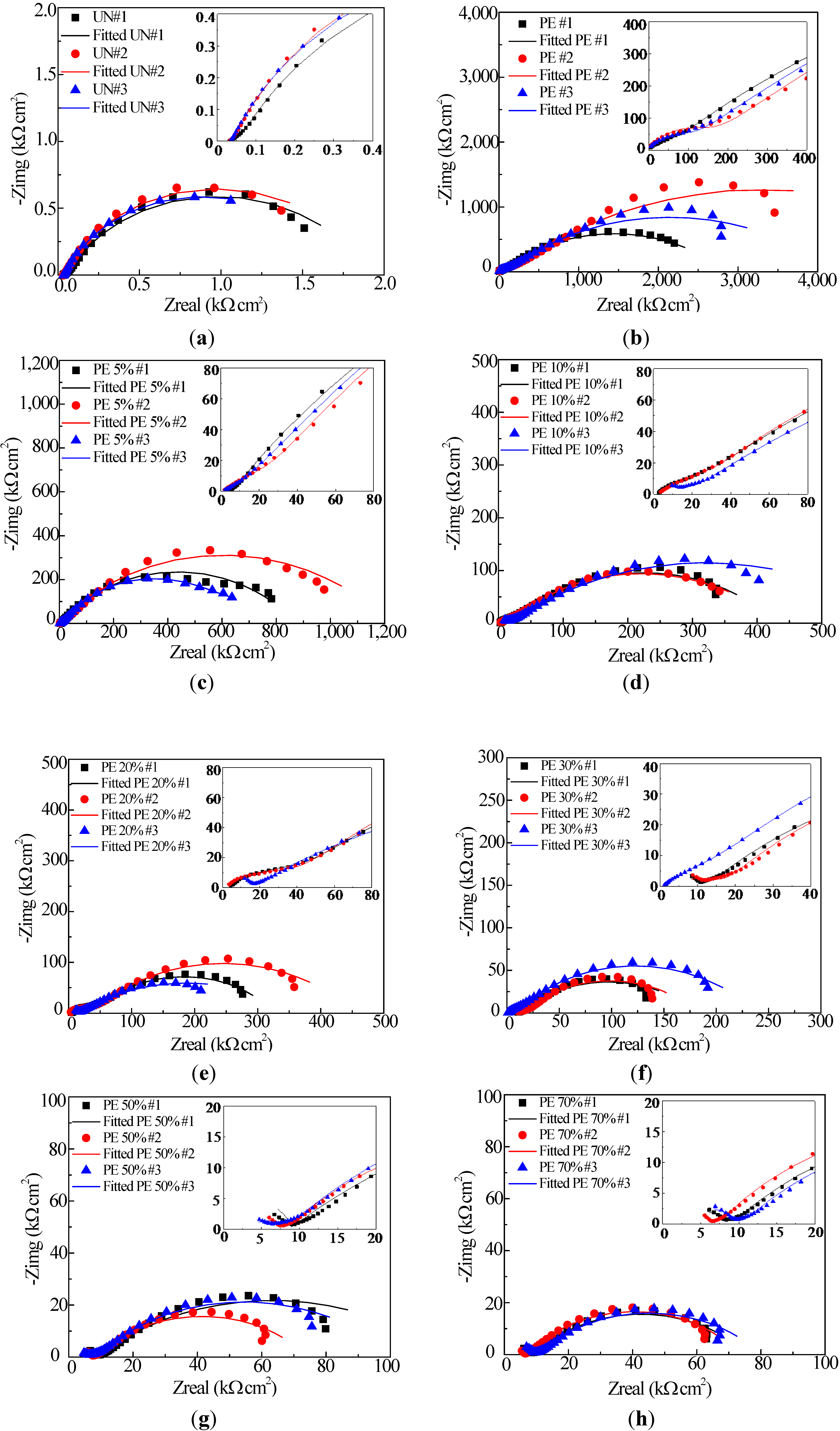
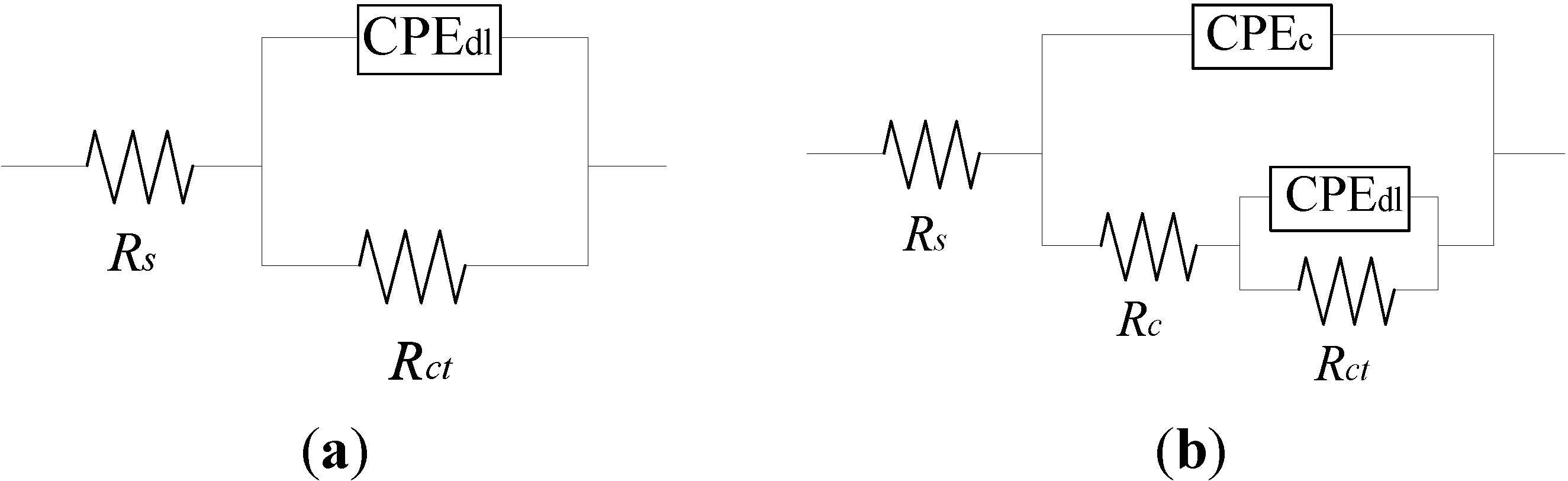
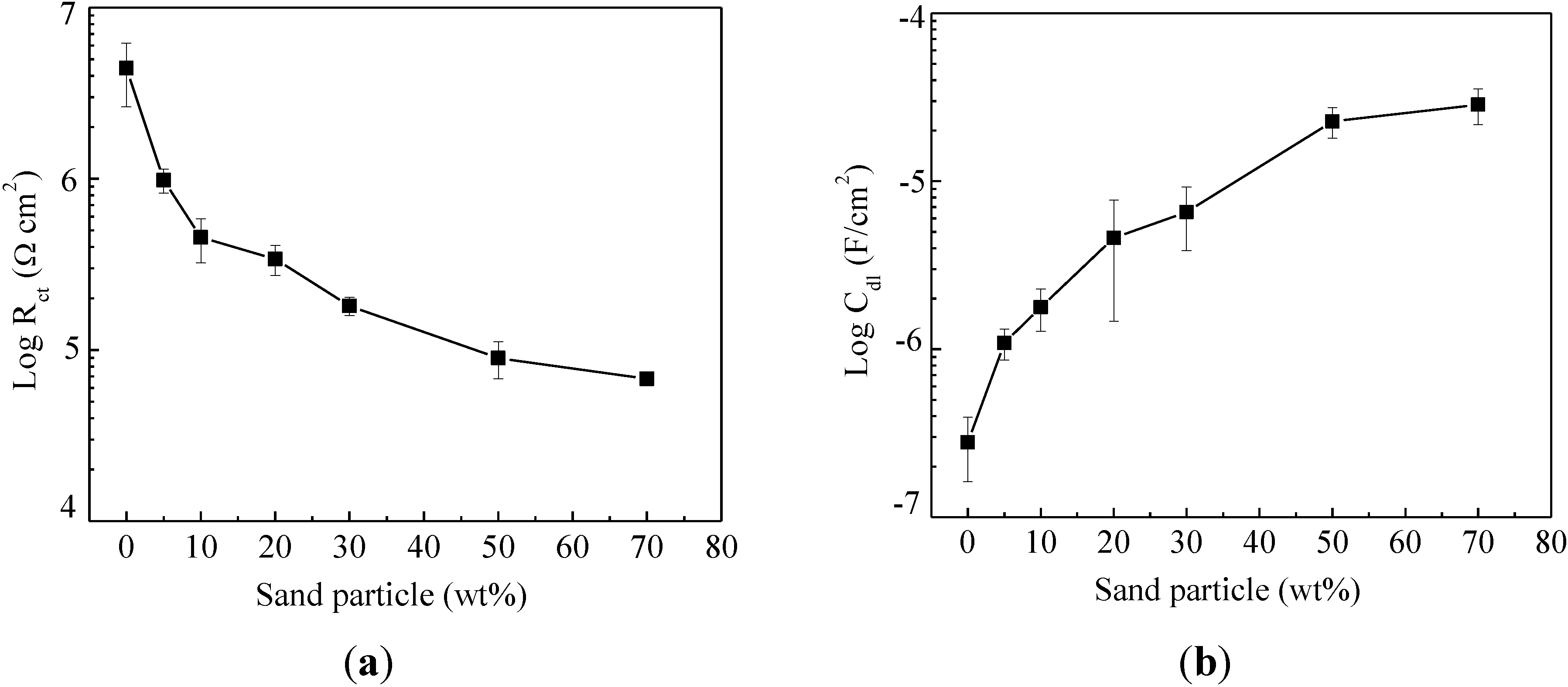
2.2. Electrochemical Impedance Spectroscopy


2.3. Visual Observation and Analysis
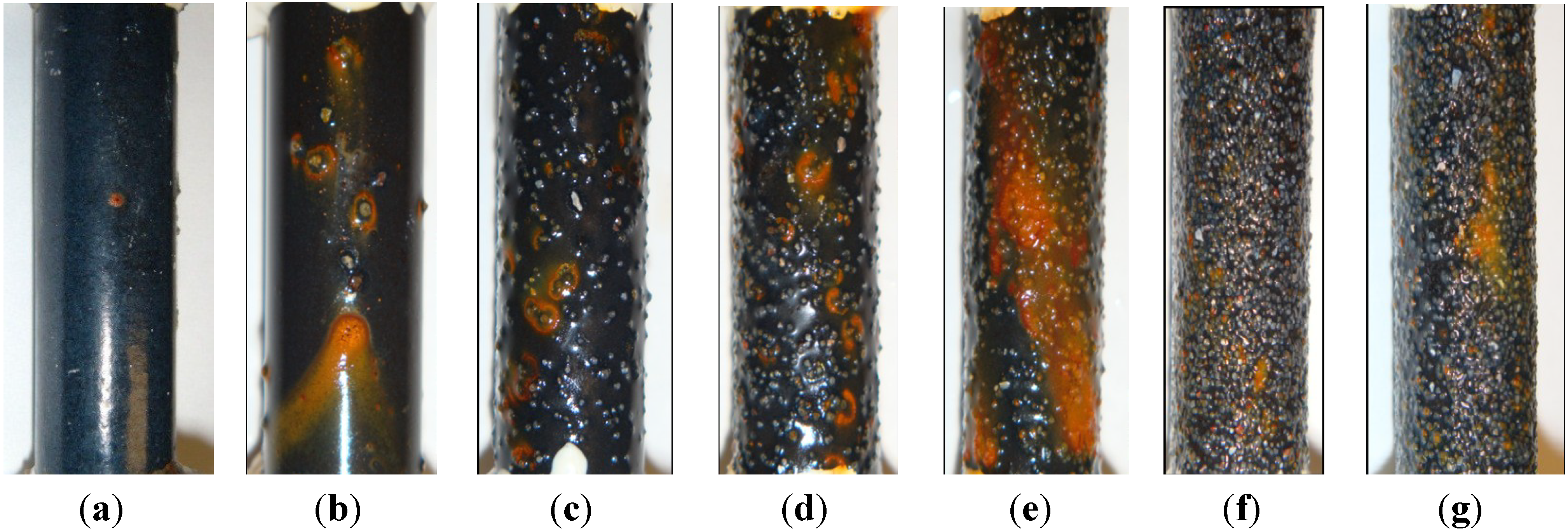
3. Experimental Section
3.1. Preparation of Enamel Coating and Samples

3.2. Characterization of Enamel Coating with Sand Particles
3.3. Electrochemical Tests
4. Conclusions
- (1)
- Enamel coating wetted well with sand particles and steel substrates. However, a weak zone was formed around the sand particles due to concentrated air bubbles.
- (2)
- The presence of micro-cracks around some sand particles was mainly because the internal pressure of released hydrogen gas exceeded the tensile strength of the enamel coating in the weak zone.
- (3)
- The addition of sand particles reduced both the coating resistance and the corrosion resistance of enamel-coated steel bars. The corrosion resistance of enamel-coated steel bars first decreased rapidly with the increasing percentage of sand particles and then remained stable after 50% sand particles by weight have been added.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobayashi, K.; Takewaka, K. Experimental studies on epoxy coated reinforcing steel for corrosion protections. Int. J. Cem. Compos. Lightweight Concr. 1984, 6, 99–116. [Google Scholar] [CrossRef]
- Darwin, A.B.; Scantlebury, J.D. Retarding of corrosion processes on reinforcing bar in concrete with an FBE coating. Cem. Concr. Compos. 2002, 24, 73–78. [Google Scholar] [CrossRef]
- Sistonen, E.; Cwirzen, A.; Puttonen, J. Corrosion mechanism of hot-dip galvanized reinforcement bar in cracked concrete. Corros. Sci. 2008, 50, 3416–3428. [Google Scholar] [CrossRef]
- Treece, R.A.; Jirsa, J.O. Bond strength of epoxy-coated reinforcing bars. ACI Mater. J. 1989, 86, 167–174. [Google Scholar]
- Miller, G.G.; Kepler, J.L.; Darwin, D. Effect of epoxy coating thickness on bond strength of reinforcing bars. ACI Struct. J. 2003, 100, 314–320. [Google Scholar]
- Manning, D.G. Corrosion performance of epoxy-coated reinforcing steel: North American experience. Constr. Build. Mater. 1996, 10, 349–365. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Hansson, C.M. Effect of surface condition on the initial corrosion of galvanized reinforcing steel embedded in concrete. Corros. Sci. 2008, 50, 2512–2522. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Volz, J.S.; Brow, R.K.; Koenigstein, M.L. Cement-modified enamel coating for enhanced corrosion resistance of steel reinforcing bars. Cem. Concr. Compos. 2013, 35, 171–180. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Brow, R.K.; Volz, J.S.; Koenigstein, M.L. Corrosion resistance and mechanism of steel rebar coated with three types of enamel. Corros. Sci. 2012, 59, 157–168. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Volz, J.S.; Brow, R.K.; Koenigstein, M.L. Microstructure and corrosion resistance of enamel coatings applied to smooth reinforcing steel. Constr. Build. Mater. 2012, 35, 376–384. [Google Scholar] [CrossRef]
- Yan, D.; Reis, S.; Tao, X.; Chen, G.; Brow, R.K.; Koenigstein, M.L. Effect of chemically reactive enamel coating on bonding strength that steel/mortar interface. Constr. Build. Mater. 2012, 28, 512–518. [Google Scholar] [CrossRef]
- Wu, C.; Chen, G.; Volz, J.S.; Brow, R.K.; Koenigstein, M.L. Local bond strength of vitreous enamel coated rebar to concrete. Constr. Build. Mater. 2012, 35, 428–439. [Google Scholar] [CrossRef]
- Moser, R.D.; Allison, P.G.; Williams, B.A.; Weiss, C.A., Jr.; Diaz, A.J.; Gore, E.R.; Malone, P.G. Improvement in the geopolymer-to-steel bond using a reactive vitreous enamel coating. Constr. Build. Mater. 2013, 49, 62–69. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W.; Tsai, C.L. Enhancement of bond strength for epoxy-coated rebar using river sand. Constr. Build. Mater. 2002, 16, 465–472. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W. The effect of particle shape on bond strength improvement of epoxy-particle coating composites. J. Mater. Sci. Technol. 2001, 9, 153–160. [Google Scholar]
- Yang, X.; Jha, A.; Brydon, R.; Cochrane, R.C. An analysis of the microstructure and interfacial chemistry of steel-enamel interface. Thin Solid Films 2003, 443, 33–45. [Google Scholar] [CrossRef]
- Samiee, L.; Sarpoolaky, H.; Mirhabibi, A. Microstructure and adherence of cobalt containing and cobalt free enamels to low carbon steel. Mater. Sci. Eng. A 2007, 458, 88–95. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review-calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Garcia-Labiano, F.; Abad, A.; de Diego, L.F.; Gayan, P.; Adanez, J. Calcination of calcium-based sorbent at pressure in a broad range of CO2 concentrations. Chem. Eng. Sci. 2002, 57, 2381–2393. [Google Scholar] [CrossRef]
- Jamil, H.E.; Shriri, A.; Boulif, R.; Montemor, M.F.; Ferreira, M.G.S. Corrosion behavior of reinforcing steel exposed to an amino alcohol based corrosion inhibitors. Cem. Concr. Compos. 2005, 27, 671–678. [Google Scholar] [CrossRef]
- Behzadnascab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Jegdic, B.V.; Bajat, J.B.; Popic, J.P.; Stevanovic, S.J.; Miskovic-Stankovic, V.B. The EIS investigation of powder polymer coatings on phosphate low carbon steel: The effect of NaNO2 in the phosphating bath. Corros. Sci. 2011, 53, 2872–2880. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The corrosion protection afforded by rare earth converstion coatings applied to magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. High corrosion protection of a polyaniline/organophilic montmorillonite coating for magnesium alloy. Prog. Org. Coat. 2013, 76, 804–811. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, L.; Wang, F. Study of the failure mechanism of an epoxy coating system under high hydrostatic pressure. Corros. Sci. 2013, 74, 59–70. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Chakraborty Banerjee, P.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by grapheme coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness form constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. The effect of epoxy coating containing emeraldine base and hydrofluoric acid doped polyaniline on the corrosion protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 3747–3755. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Singh Raman, R.K. Electrochemical impedance spectroscopic investigation of the role of alkaline pre-treatment in corrosion resistance of a silane coating on magnesium alloy ZE41. Electrochim. Acta 2011, 56, 3790–3798. [Google Scholar] [CrossRef]
- National Research Council (NRC). International Critical Tables; McGraw-Hill: Washington, DC, USA, 1927; Volume 2, p. 116. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, F.; Chen, G.; Brow, R.K.; Koenigstein, M.L. Corrosion Resistance of a Sand Particle-Modified Enamel Coating Applied to Smooth Steel Bars. Materials 2014, 7, 6632-6645. https://doi.org/10.3390/ma7096632
Tang F, Chen G, Brow RK, Koenigstein ML. Corrosion Resistance of a Sand Particle-Modified Enamel Coating Applied to Smooth Steel Bars. Materials. 2014; 7(9):6632-6645. https://doi.org/10.3390/ma7096632
Chicago/Turabian StyleTang, Fujian, Genda Chen, Richard K. Brow, and Michael L. Koenigstein. 2014. "Corrosion Resistance of a Sand Particle-Modified Enamel Coating Applied to Smooth Steel Bars" Materials 7, no. 9: 6632-6645. https://doi.org/10.3390/ma7096632



