Effects of Solvent Diols on the Synthesis of ZnFe2O4 Particles and Their Use as Heterogeneous Photo-Fenton Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Powders
2.2. Characterization of Powders
2.3. Experimental Essays and Reaction Apparatus
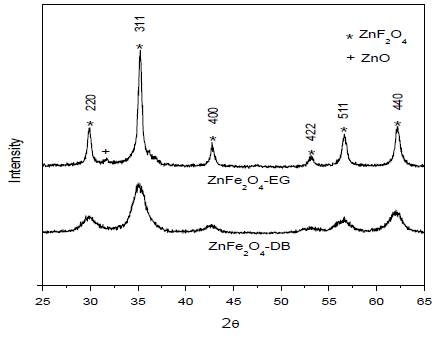
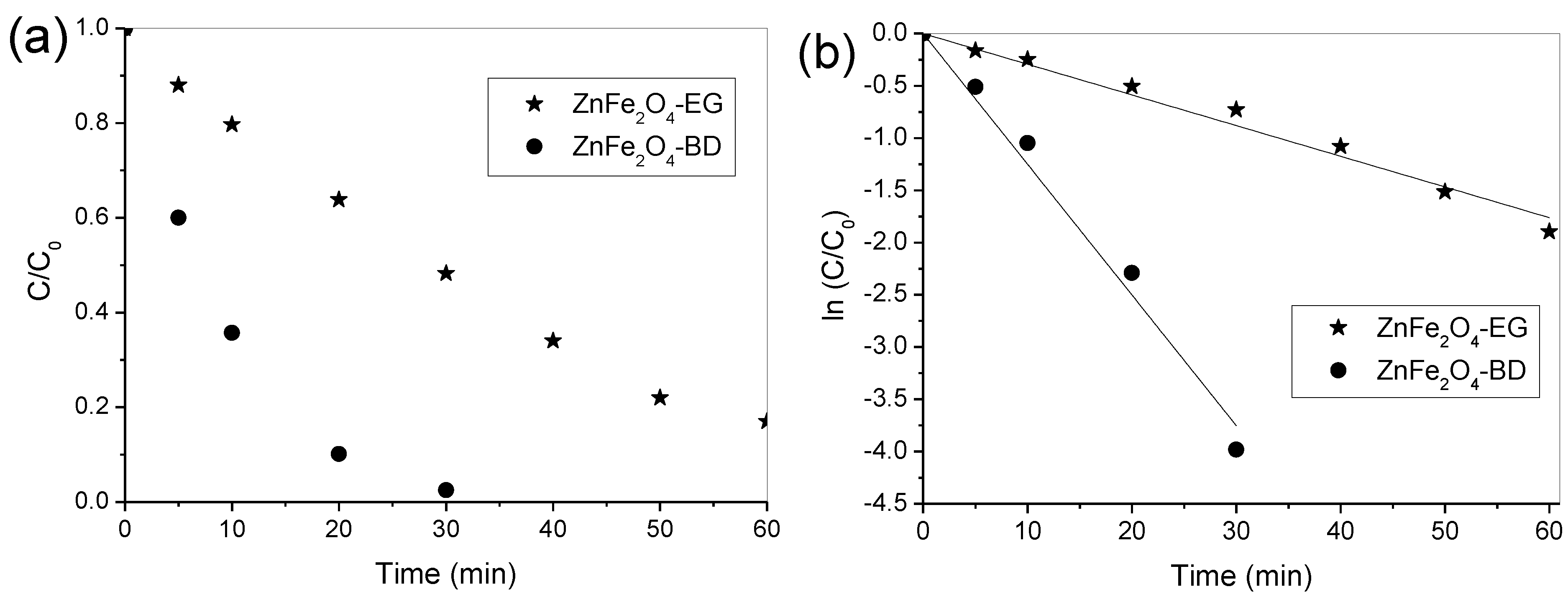
3. Results and Discussion

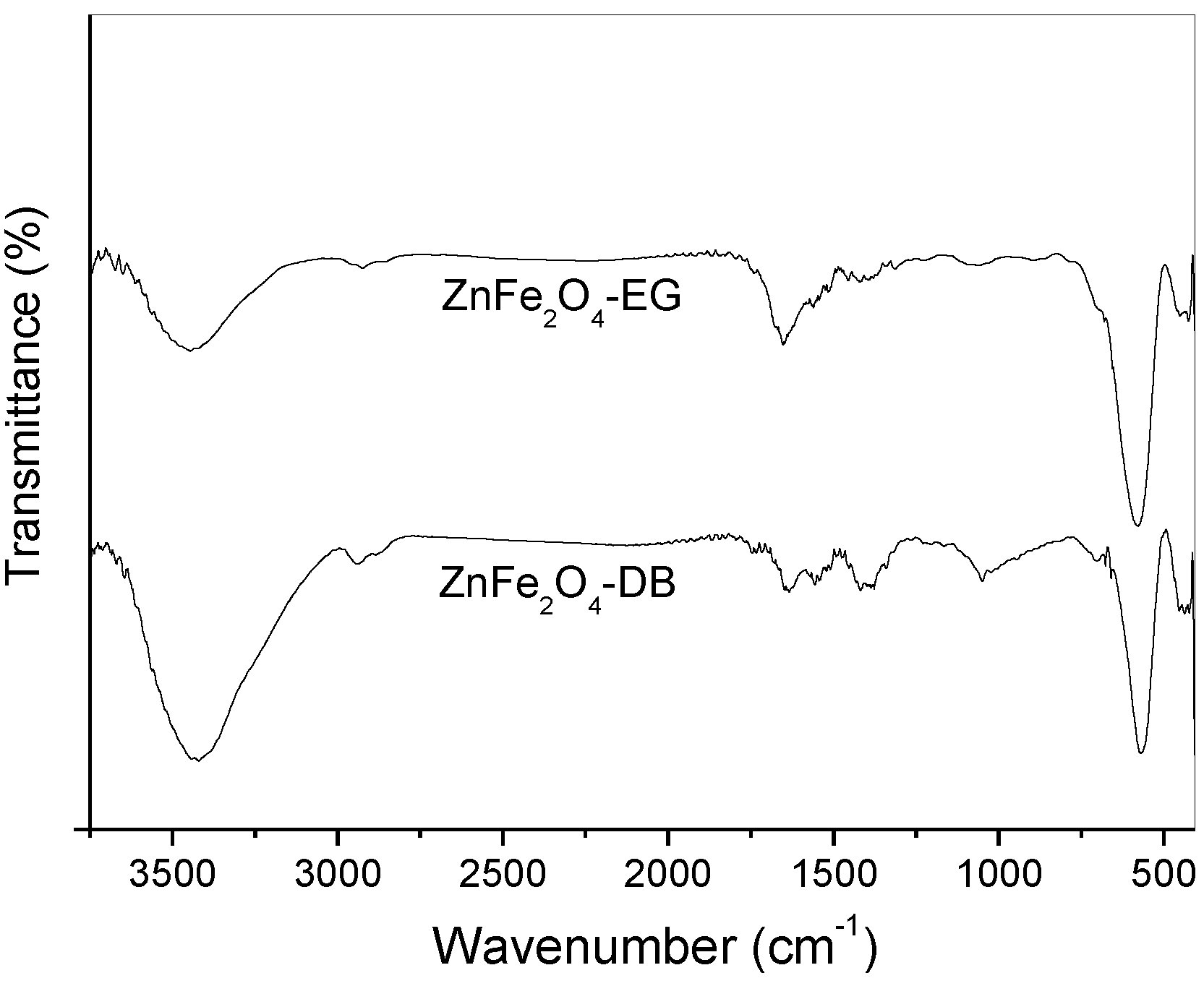
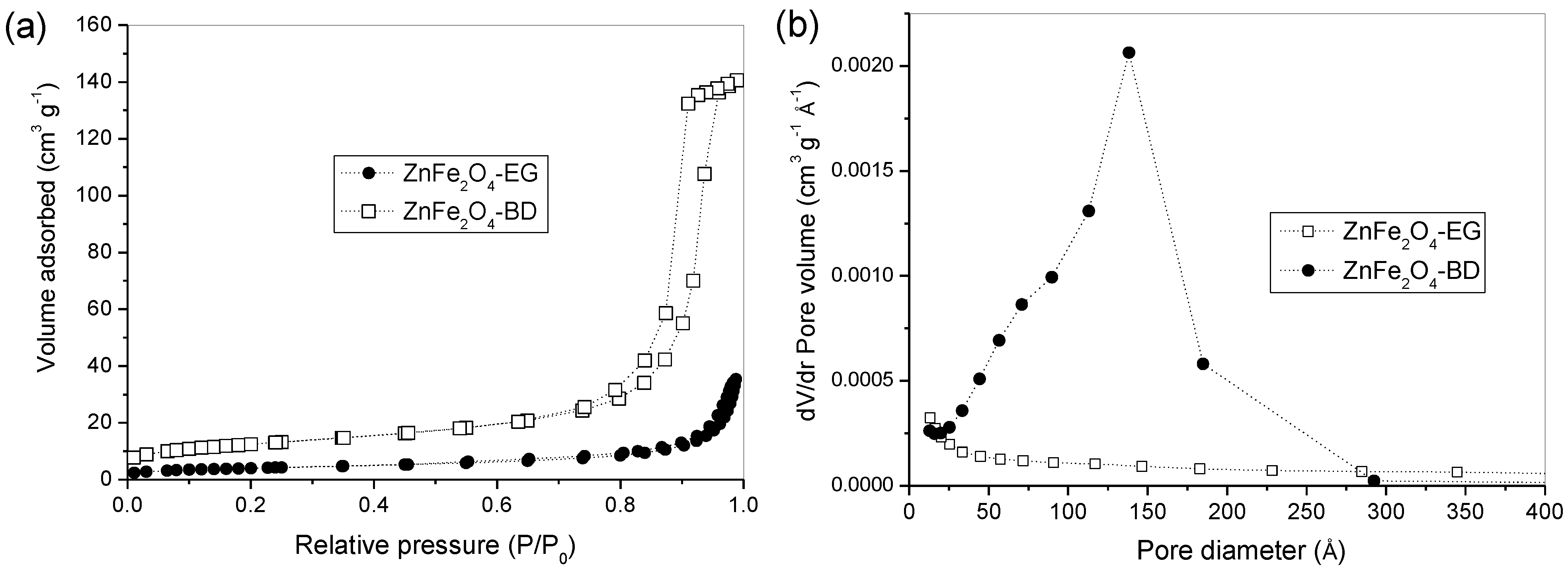
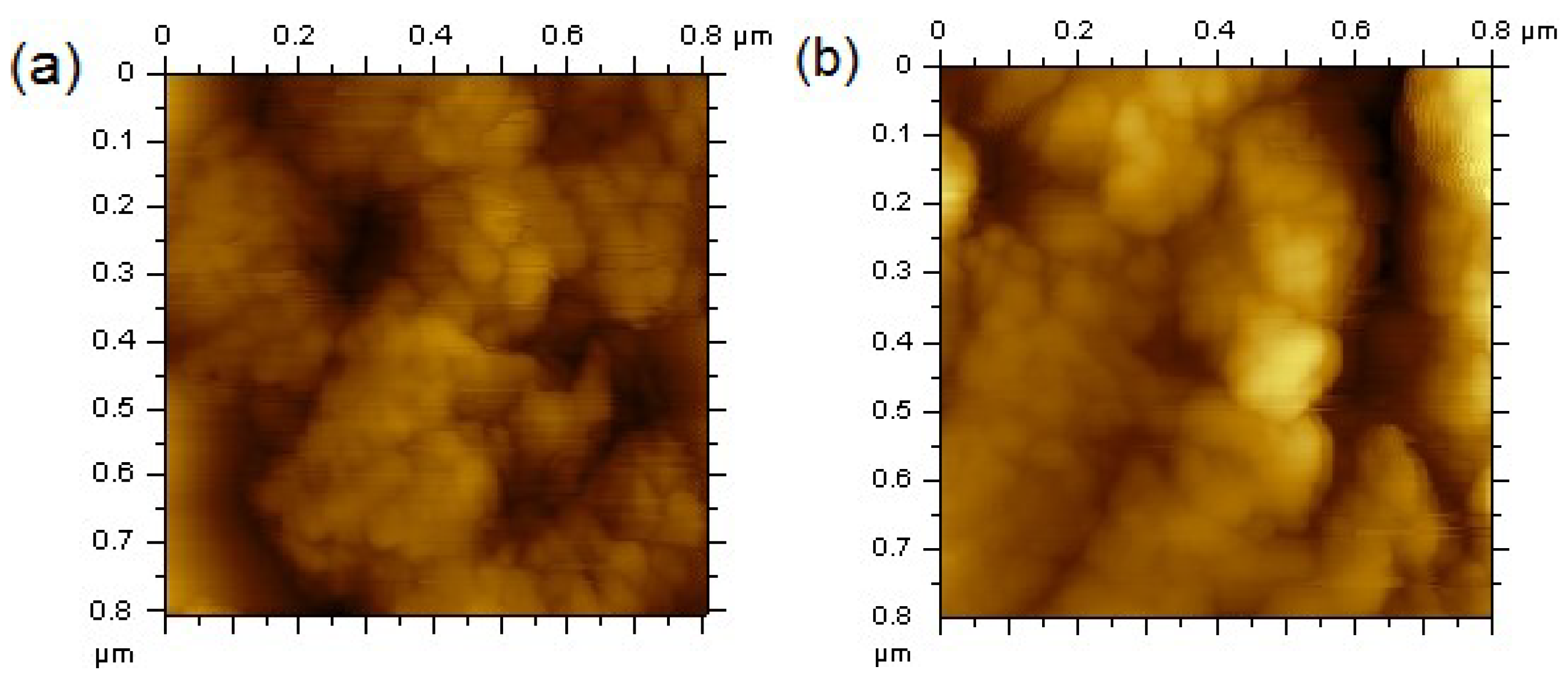
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mathew, D.S.; Juang, R.S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar]
- Pradeep, A.; Priyadharsini, P.; Chandrasekaran, G. Structural, magnetic and electrical properties of nanocrystalline zinc ferrite. J. Alloy. Compd. 2011, 509, 3917–3923. [Google Scholar]
- Fan, G.; Gu, Z.; Yang, L.; Li, F. Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 2009, 155, 534–541. [Google Scholar]
- Chu, X.; Liu, X.; Meng, G. Preparation and gas sensitivity properties of ZnFe2O4 semiconductors. Sens. Actuators B Chem. 1999, 55, 19–22. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Alonso, L.; Palacios, J.M.; Cilleruelo, C.; Abanades, J.C. Structural changes in zinc ferrites as regenerable sorbents for hot coal gas desulfurization. Solid State Ion. 2000, 138, 51–62. [Google Scholar]
- Wan, J.; Jiang, X.; Li, H.; Chen, K. Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J. Mater. Chem. 2012, 22, 13500–13505. [Google Scholar]
- Albuquerque, A.S.; Ardisson, J.D.; Bittencourt, E.; Macedo, W.A.A. Structure and magnetic properties of granular NiZn-ferrite-SiO2. Mater. Res. 1999, 2, 235–238. [Google Scholar]
- Kumar, G.S.Y.; Naik, H.S.B.; Roy, A.S.; Harish, K.N.; Viswanath, R. Synthesis, optical and electrical properties of ZnFe2O4 nanocomposites. Nanotechnol. Nanomater. 2012, 2. [Google Scholar] [CrossRef]
- Mekap, A.; Das, P.R.; Choudhary, R.N.P. Dielectric, magnetic and electrical properties of ZnFe2O4 ceramics. J. Mater. Sci. Mater. Electron. 2013, 24, 4757–4763. [Google Scholar]
- Gama, A.M.; Rezende, M.C. Complex permeability and permittivity variation of radar absorbing materials based on MnZn ferrite in microwave frequencies. Mater. Res. 2013, 16, 997–1001. [Google Scholar] [CrossRef]
- Yelenich, O.V.; Solopan, S.O.; Kolodiazhnyi, T.V.; Dzyublyuk, V.V.; Tovstolytkin, A.I.; Belous, A.G. Magnetic properties and high heating efficiency of ZnFe2O4 nanoparticles. Mater. Chem. Phys. 2014, 146, 129–135. [Google Scholar] [CrossRef]
- Toledo-Antonio, J.A.; Nava, N.; Martinez, M.; Bokhimi, X. Correlation between the magnetism of non-stoichiometric zinc ferrites and their catalytic activity for oxidative dehydrogenation of 1-butene. Appl. Catal. A Gen. 2002, 234, 137–144. [Google Scholar]
- Casbeer, E.; Sharma, V.K.; Li, X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, G.; Wang, L.; Huang, T.; Qin, L. Highly active S-modified ZnFe2O4 heterogeneous catalyst and its photo-Fenton behavior under UV–visible irradiation. Ind. Eng. Chem. Res. 2011, 50, 7219–7227. [Google Scholar]
- Li, P.; Xu, H.Y.; Li, X.; Liu, W.C.; Li, Y. Preparation and evaluation of a photo-Fenton heterogeneous catalyst: Spinel-typed ZnFe2O4. Adv. Mater. Res. 2012, 550–553, 329–335. [Google Scholar] [CrossRef]
- Wu, J.; Pu, W.; Yang, C.; Zhang, M.; Zhang, J. Removal of benzotriazole by heterogeneous photoelectro-Fenton like process using ZnFe2O4 nanoparticles as catalyst. J. Environ. Sci. 2013, 25, 801–807. [Google Scholar]
- Sun, Y.; Wang, W.; Zhang, L.; Sun, S.; Gao, E. Magnetic ZnFe2O4 octahedra: Synthesis and visible light induced photocatalytic activities. Mater. Lett. 2013, 98, 124–127. [Google Scholar] [CrossRef]
- Nan, C.; Fan, G.; Fan, J.; Li, F. Template-assisted route to porous zinc ferrite film with enhanced visible-light induced photocatalytic performance. Mater. Lett. 2013, 106, 5–7. [Google Scholar] [CrossRef]
- Dom, R.; Subasri, R.; Radha, K.; Borse, P.H. Synthesis of solar active nanocrystalline ferrite, MFe2O4 (M: Ca, Zn, Mg) photocatalyst by microwave irradiation. Solid State Commun. 2011, 151, 470–473. [Google Scholar]
- Teixeira, A.M.R.F.; Ogasawara, T.; Nóbrega, M.C.S. Investigation of sintered cobalt-zinc ferrite synthesized by coprecipitation at different temperatures: A relation between microstructure and hysteresis curves. Mater. Res. 2006, 9, 257–262. [Google Scholar]
- Li, Q.; Bo, C.; Wang, W. Preparation and magnetic properties of ZnFe2O4 nanofibers by coprecipitation—Air oxidation method. Mater. Chem. Phys. 2010, 124, 891–893. [Google Scholar]
- Liu, H.; Guo, Y.; Zhang, Y.; Wu, F.; Liu, Y.; Zhang, D. Synthesis and properties of ZnFe2O4 replica with biological hierarchical structure. Mater. Sci. Eng. B 2013, 178, 1057–1061. [Google Scholar]
- Jesus, C.B.R.; Mendonça, E.C.; Silva, L.S.; Folly, W.S.D.; Meneses, C.T.; Duque, J.G.S. Weak ferromagnetic component on the bulk ZnFe2O4 compound. J. Magn. Magn. Mater. 2014, 350, 47–49. [Google Scholar] [CrossRef]
- Patil, J.Y.; Nadargi, D.Y.; Gurav, J.L.; Mulla, I.S.; Suryavanshi, S.S. Glycine combusted ZnFe2O4 gas sensor: Evaluation of structural, morphological and gas response properties. Ceram. Int. 2014, 40, 10607–10613. [Google Scholar]
- Costa, A.C.F.M.; Tortella, E.; Neto, E.F.; Morelli, M.R.; Kiminami, R.H.G.A. Sintering of Ni-Zn ferrite nanopowders by the constant heating rate (CHR) method. Mater. Res. 2004, 7, 523–528. [Google Scholar]
- Dantas, J.; Santos, J.R.D.; Cunha, R.B.L.; Kiminami, R.H.G.A.; Costa, A.C.F.M. Use of Ni-Zn ferrites doped with Cu as catalyst in the transesterification of soybean oil to methyl esters. Mater. Res. 2013, 16, 625–627. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Wan, L.; Deng, Y.; Zhan, S. Synthesis of ZnFe2O4 nanoplates by succinic acid-assisted hydrothermal route and their photocatalytic degradation of rhodamine B under visible light. J. Environ. Chem. Eng. 2014, 2, 123–130. [Google Scholar]
- Blanco-Gutierrez, V.; Climent-Pascual, E.; Torralvo-Fernandez, M.J.; Saez-Puche, R.; Fernandez-Diaz, M.T. Neutron diffraction study and superparamagnetic behavior of ZnFe2O4 nanoparticles obtained with different conditions. J. Solid State Chem. 2011, 184, 1608–1613. [Google Scholar]
- Banerjee, A.; Bid, S.; Dutta, H.; Chaudhuri, S.; Das, D.; Pradhan, S.K. Microstructural changes and effect of variation of lattice strain on positron annihilation lifetime parameters of zinc ferrite nanocomposites prepared by high enegy ball-milling. Mater. Res. 2012, 15, 1022–1028. [Google Scholar]
- Mohai, I.; Szépvölgyi, J.; Bertóti, I.; Mohai, M.; Gubicza, J.; Ungár, T. Thermal plasma synthesis of zinc ferrite nanopowders. Solid State Ion. 2001, 141–142, 163–168. [Google Scholar]
- Cao, Y.; Jia, D.; Hu, P.; Wang, R. One-step room-temperature solid-phase synthesis of ZnFe2O4 nanomaterials and its excellent gas-sensing property. Ceram. Int. 2013, 39, 2989–2994. [Google Scholar] [CrossRef]
- Manikandan, A.; Kennedy, L.J.; Bououdina, M.; Vijaya, J.J. Synthesis, optical and magnetic properties of pure and Co-doped ZnFe2O4 nanoparticles by microwave combustion method. J. Magn. Magn. Mater. 2014, 349, 249–258. [Google Scholar] [CrossRef]
- Köseoğlu, Y.; Baykal, A.; Toprak, M.S.; Gözüak, F.; Başaran, A.C.; Aktaş, B. Synthesis and characterization of ZnFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Alloy. Compnd. 2008, 462, 209–213. [Google Scholar] [CrossRef]
- Oliver, S.A. Localized spin canting in partially inverted ZnFe2O4 fine powders. Phys. Rev. B 1999, 60, 3400–3405. [Google Scholar] [CrossRef]
- Cabañas, A.; Poliakoff, M. The continuous hydrothermal synthesis of nano-particulate ferrites in near critical and supercritical water. J. Mater. Chem. 2001, 11, 1408–1416. [Google Scholar]
- Shen, Y.; Li, X.; Zhao, Q.; Hou, Y.; Tade, M.; Liu, S. Facile synthesis and characterization of ZnFe2O4/α-Fe2O3 composite hollow nanospheres. Mater. Res. Bull. 2011, 46, 2235–2239. [Google Scholar]
- Li, X.; Hou, Y.; Zhao, Q.; Wang, L. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102–108. [Google Scholar]
- Kuai, S.; Zhang, Z.; Nan, Z. Synthesis of Ce3+ doped ZnFe2O4 self- assembled clusters and adsorption of chromium(VI). J. Hazard. Mater. 2013, 250–251, 229–237. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Liu, X.; Zheng, H.; Yong, L.; Zhang, W. Factors on the separation of photogenerated charges and the charge dynamics in oxide/ZnFe2O4 composites. J. Mater. Chem. C 2013, 1, 329–337. [Google Scholar] [CrossRef]
- Soon, A.N.; Hameed, B.H. Degradation of Acid Blue 29 in visible light radiation using iron modified mesoporous silica as heterogeneous Photo-Fenton catalyst. Appl. Catal. A Gen. 2013, 450, 96–105. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Dai, X.-H. Facile synthesis of sewage sludge-derived mesoporous material as anefficient and stable heterogeneous catalyst for photo-Fenton reaction. Appl. Catal. B Environ. 2014, 154–155, 252–258. [Google Scholar]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar]
- Köseoğlu, Y.; Baykal, A.; Gözüak, F.; Kavas, H. Structural and magnetic properties of CoxZn1−xFe2O4 nanocrystals synthesized by microwave method. Polyhedron 2009, 28, 2887–2892. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zhao, Q.; Chen, G. ZnFe2O4 multi-porous microbricks/graphene hybrid photocatalyst: Facile synthesis, improved activity and photocatalytic mechanism. Appl. Catal. B Environ. 2013, 142–143, 80–88. [Google Scholar] [CrossRef]
- Mizukami, F.; Kiyozumi, Y.; Sano, T.; Niwa, S.I.; Toba, M.; Shin, S. Effect of solvent diols and ligands on the properties of sol-gel alumina-silicas. J. Sol-Gel Sci. Technol. 1998, 13, 1027–1031. [Google Scholar] [CrossRef]
- Borhan, A.I.; Samoila, P.; Hulea, V.; Iordan, A.R.; Palamaru, M.N. Effect of Al3+ substituted zinc ferrite on photocatalytic degradation of orange I azo dye. J. Photochem. Photobiol. A Chem. 2014, 279, 17–23. [Google Scholar]
- Anchieta, C.G.; Sallet, D.; Foletto, E.L.; Silva, S.S.; Chiavone-Filho, O.; Nascimento, C.A.O. Synthesis of ternary zinc spinel oxides and their application in the photodegradation of organic pollutant. Ceram. Inter. 2014, 40, 4173–4178. [Google Scholar]
- Blanco-Gutierrez, V.; Saez-Puche, R.; Torralvo-Fernandez, M.J. Superparamagnetism and interparticle interactions in ZnFe2O4 nanocrystals. J. Mater. Chem. 2012, 22, 2992–3003. [Google Scholar]
- National Council on Environmental (Brazil). Available online: http://www.mma.gov.br/port/conama (accessed on 6 July 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anchieta, C.G.; Cancelier, A.; Mazutti, M.A.; Jahn, S.L.; Kuhn, R.C.; Gündel, A.; Chiavone-Filho, O.; Foletto, E.L. Effects of Solvent Diols on the Synthesis of ZnFe2O4 Particles and Their Use as Heterogeneous Photo-Fenton Catalysts. Materials 2014, 7, 6281-6290. https://doi.org/10.3390/ma7096281
Anchieta CG, Cancelier A, Mazutti MA, Jahn SL, Kuhn RC, Gündel A, Chiavone-Filho O, Foletto EL. Effects of Solvent Diols on the Synthesis of ZnFe2O4 Particles and Their Use as Heterogeneous Photo-Fenton Catalysts. Materials. 2014; 7(9):6281-6290. https://doi.org/10.3390/ma7096281
Chicago/Turabian StyleAnchieta, Chayene Gonçalves, Adriano Cancelier, Marcio Antonio Mazutti, Sérgio Luiz Jahn, Raquel Cristine Kuhn, Andre Gündel, Osvaldo Chiavone-Filho, and Edson Luiz Foletto. 2014. "Effects of Solvent Diols on the Synthesis of ZnFe2O4 Particles and Their Use as Heterogeneous Photo-Fenton Catalysts" Materials 7, no. 9: 6281-6290. https://doi.org/10.3390/ma7096281