The Influence of Deposition Methods of Support Layer on Cordierite Substrate on the Characteristics of a MnO2–NiO–Co3O4/Ce0.2Zr0.8O2/Cordierite Three Way Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Preparation of Cordierite Pellets
2.1.2. Preparation of Ce0.2Zr0.8O2 Powder
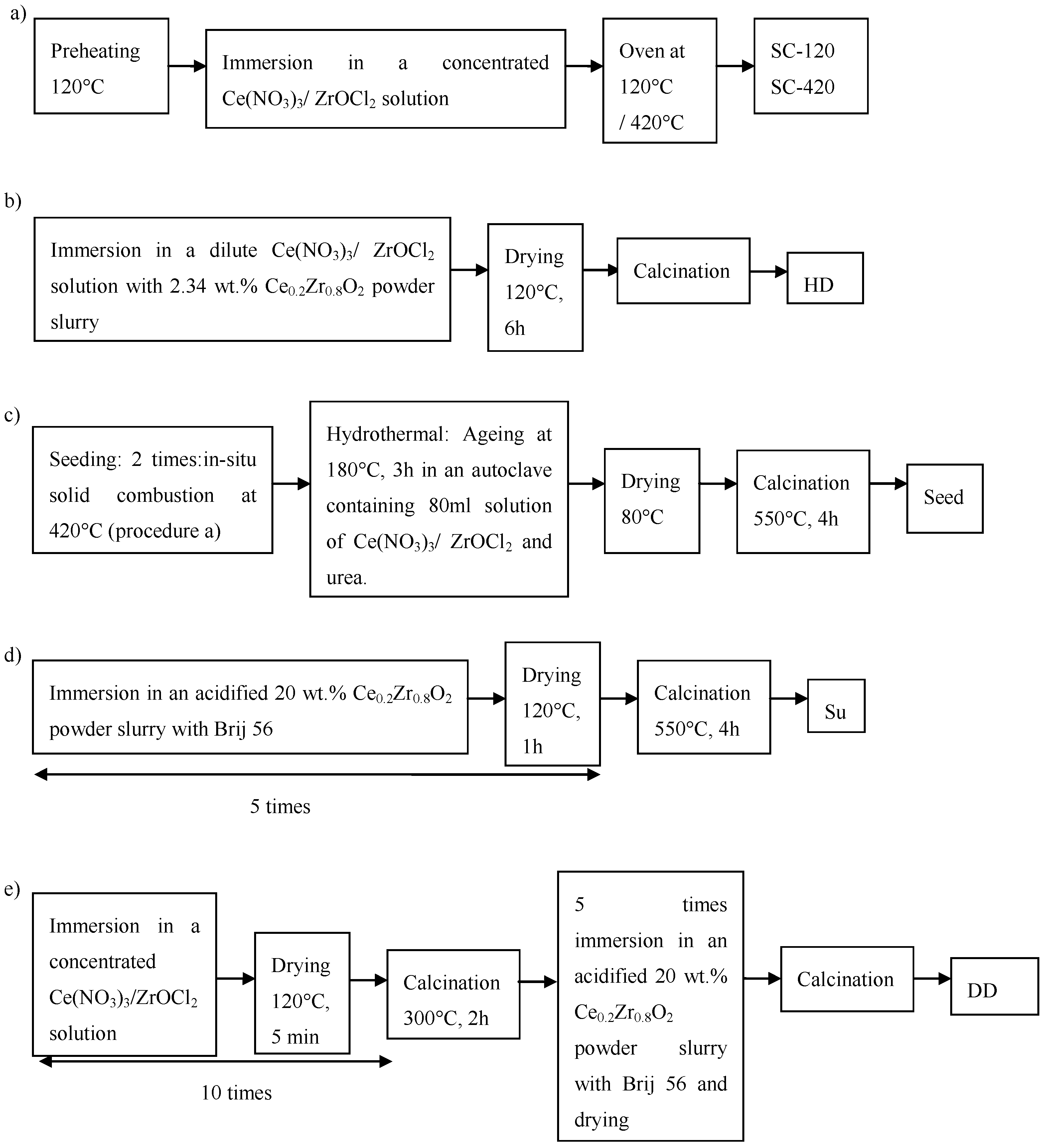
2.2. Deposition Methods of Ce0.2Zr0.8O2 Support on Cordierite Substrate
2.2.1. In Situ Solid Combustion (SC-120 and SC-420)
2.2.2. Hybrid Deposition (HD)
2.2.3. Secondary Growth on Ce0.2Zr0.8O2 Seeds (Seed)
2.2.4. Suspension (Su)
2.2.5. Double Deposition: A Combination of Wet Impregnation and Suspension (DD)
2.3. Preparation of the Complete Catalyst
2.4. Material Characterization
3. Results and Discussion
3.1. Influence of Deposition Methods on Surface Areas of Ce0.2Zr0.8O2/Cordierite Samples
| Samples | Deposition method | BET surface area (m2/g) | wt.% loading of Ce0.2Zr0.8O2 on cordierite | |
|---|---|---|---|---|
| Coated pellets | Ce0.2Zr0.8O2 powder | |||
| CordxH8 | No deposition | 20 | - | - |
| SC-120 | In situ solid combustion, oven temperature 120 °C | 18 | 50 | 3.84 |
| SC-420 | In situ solid combustion, oven temperature 420 °C | 14 | 46 | 10.09 |
| HD | Hybrid deposition | 18 | 33 | 0.98 |
| Seed | Secondary growth on Ce0.2Zr0.8O2 seeds | 8 | 46 (seeding) 117 (hydrothermal) | - |
| Su | Suspension | 24 | 74 | 3.5 |
| DD | Double deposition method | 25 | 56 | 4.39 |
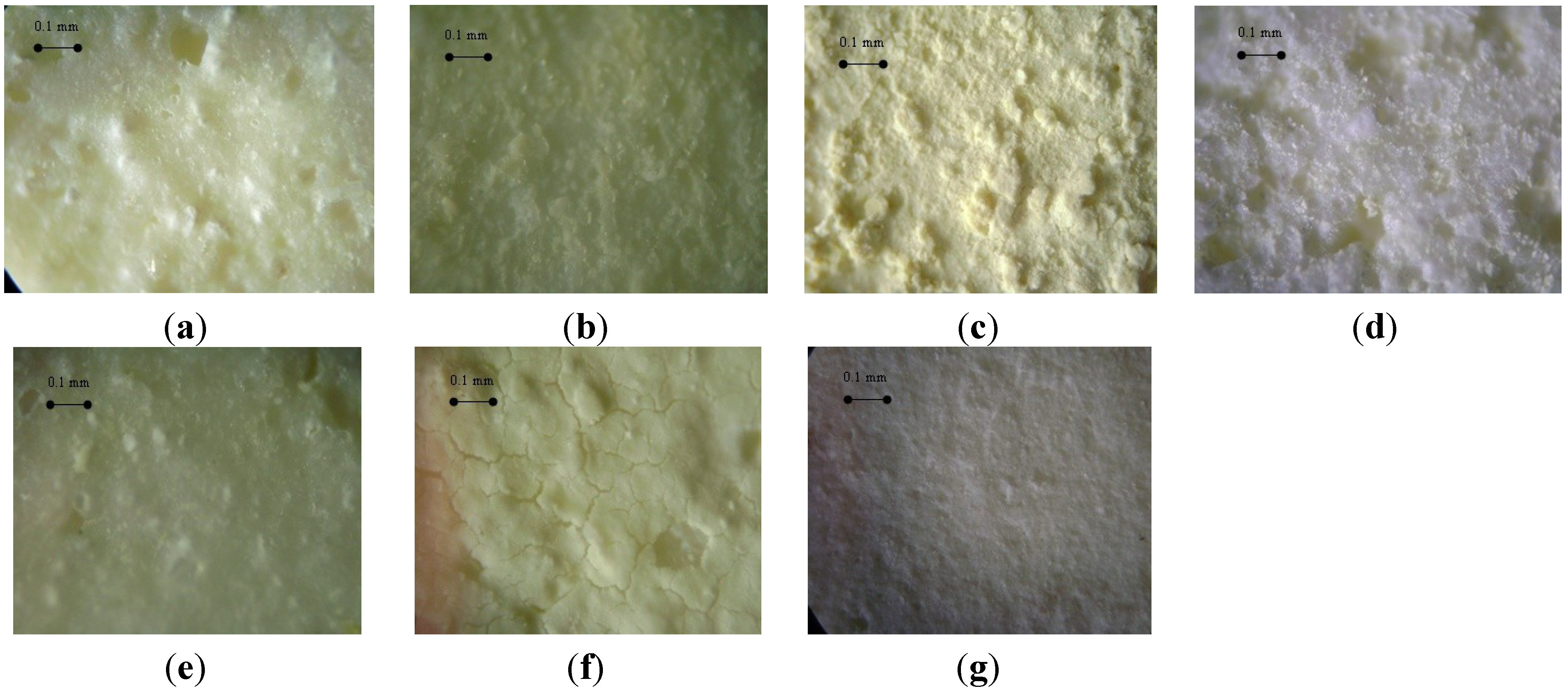
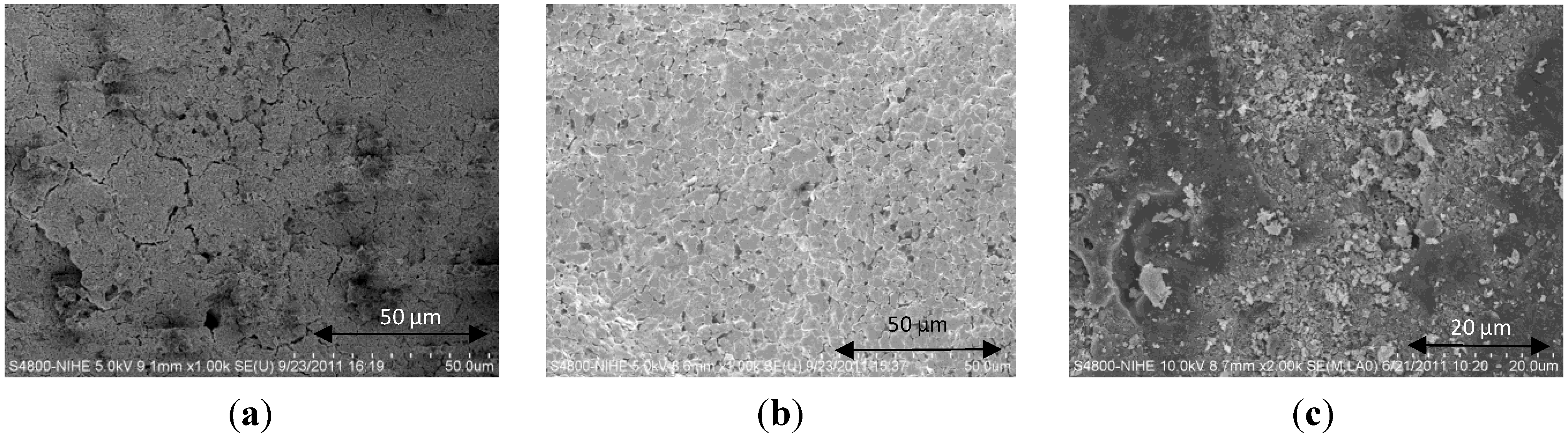
3.2. Influence of the Deposition Methods on the Morphology of Ce0.2Zr0.8O2/Cordierite Samples
3.3. Characterization of the Complete Catalyst
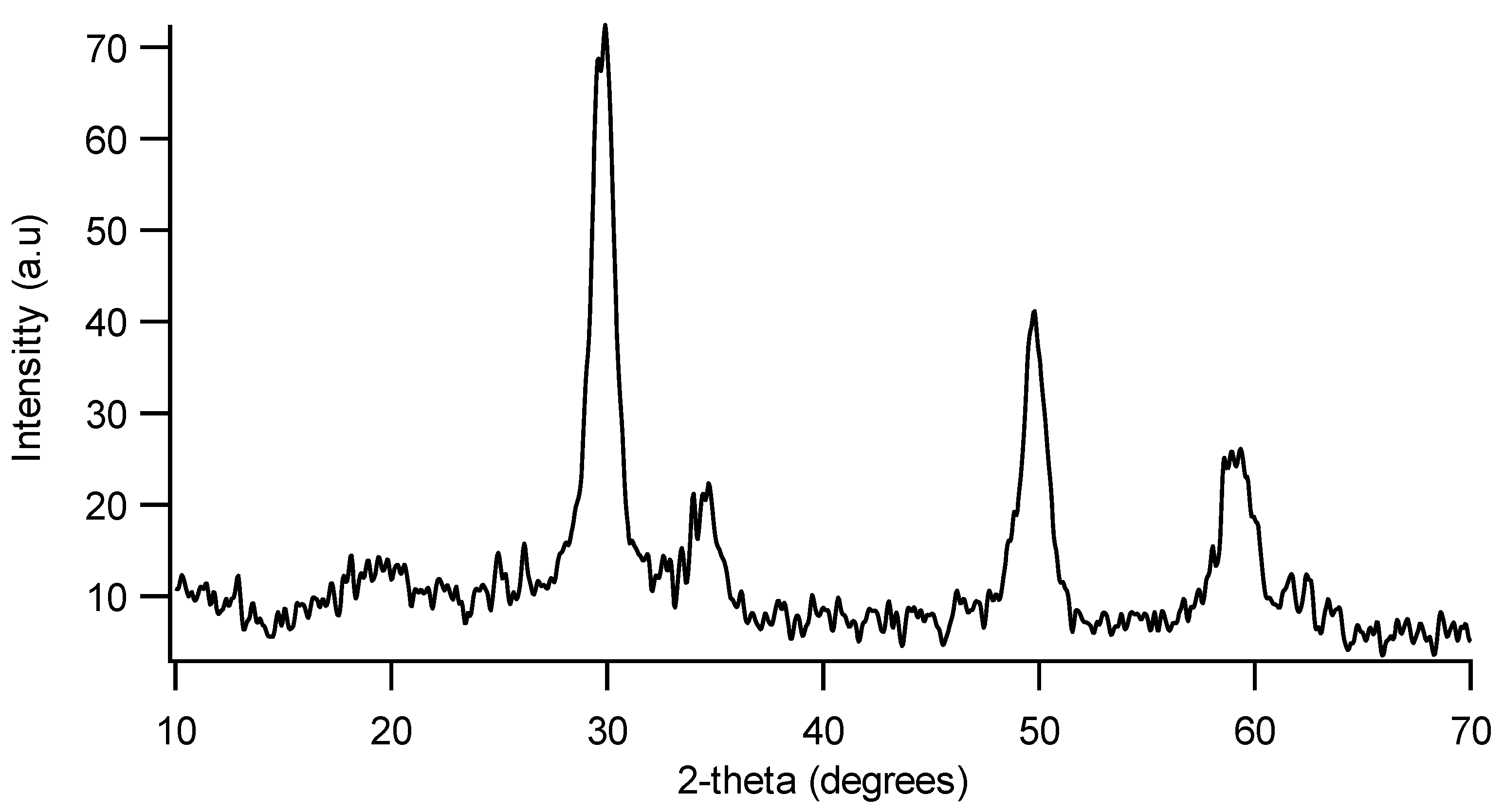

| Elements | Ca.2 MnO2–NiO–Co3O4/cordierite | Ca.3 MnO2–NiO–Co3O4/Ce0.2Zr0.8O2/cordierite |
|---|---|---|
| Mn | 14.6 | 21.4 |
| Co | 42.5 | 29.8 |
| Ni | 42.9 | 22.0 |
| Ce | 0 | 8 |
| Zr | 0 | 18.8 |
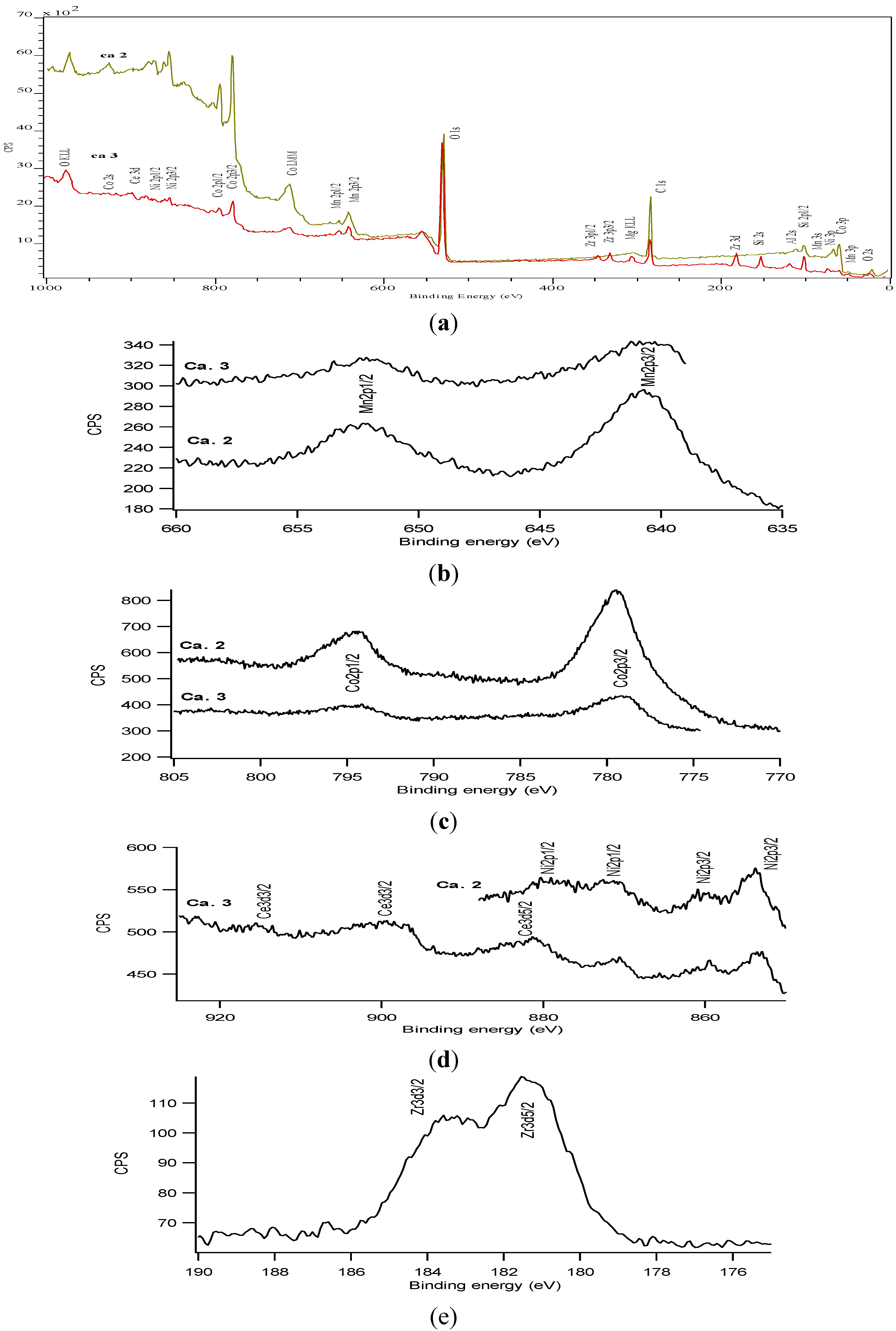

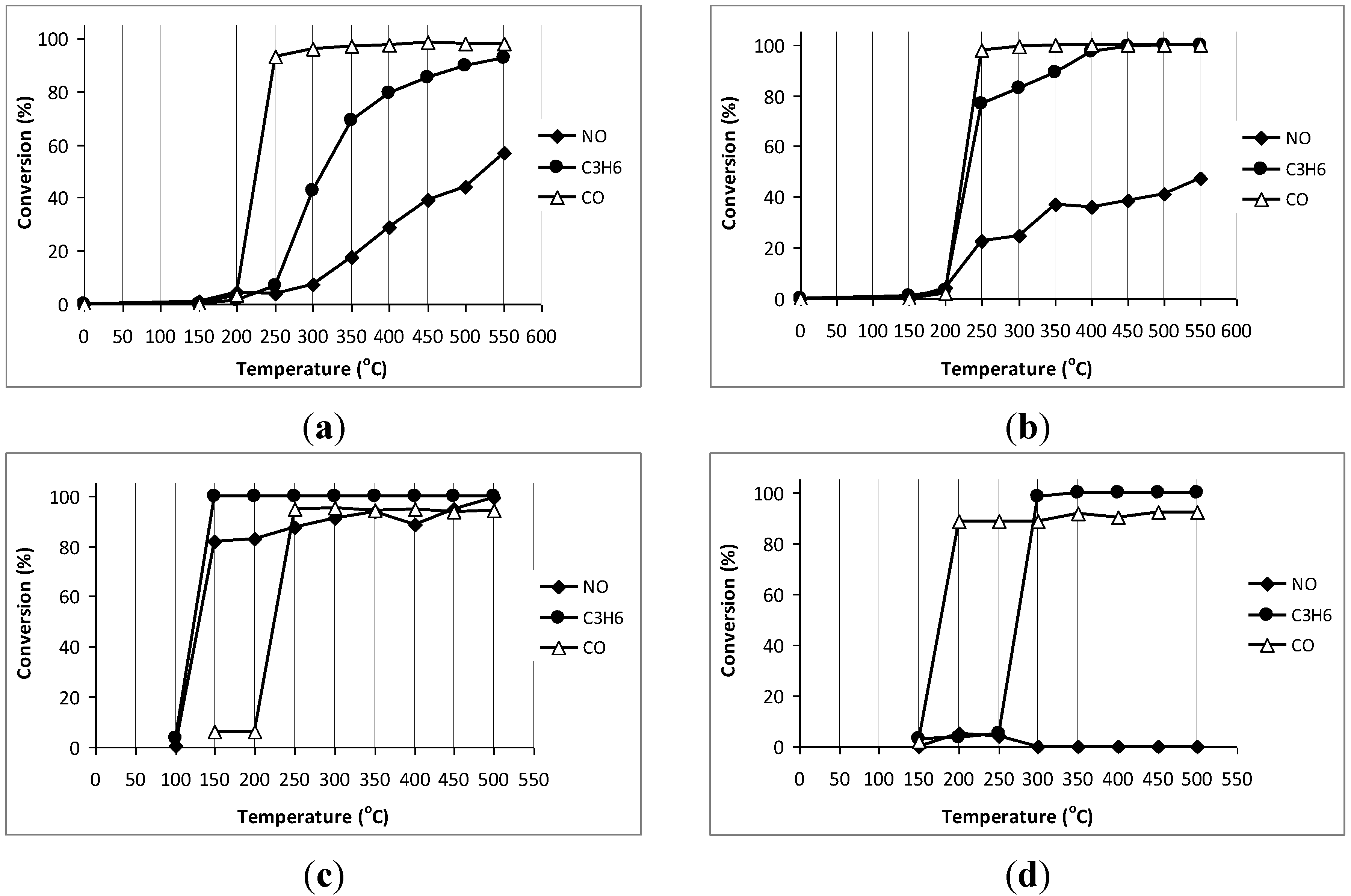
3.4. Catalytic Activities of the Final Catalysts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, B.; Wang, Q.; Li, G.; Zhou, R. Effect of synthesis condition on properties of Ce0.67Zr0.33O2 mixed oxides and its application in Pd-only three-way catalysts. J. Alloy. Compd. 2010, 508, 500–506. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Kikot, A.; Bengoa, J.F.; Tara, J.C. ZSM-5 zeolite films on cordierite modules. Effect of dilution on the synthesis medium. Mater. Lett. 2002, 52, 350–354. [Google Scholar]
- Zamaro, J.M.; Ulla, M.A.; Miro, E.E. Growth of mordenite on monoliths by secondary synthesis: Effects of the substrate on the coating structure and catalytic activity. Appl. Catal. A Gen. 2006, 314, 101–113. [Google Scholar] [CrossRef]
- Chung, K.S.; Jiang, Z.; Gill, B.S.; Chung, J.S. Oxidative decomposition of o-dichlorobenzene over V2O5/TiO2 catalyst washcoated onto wire-mesh honeycombs. Appl. Catal. A Gen. 2002, 237, 81–89. [Google Scholar] [CrossRef]
- Lisi, L.; Pirone, R.; Russo, G.; Stanzione, V. Cu-ZSM5 based monolith reactors for NO decomposition. Chem. Eng. J. 2009, 154, 341–347. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Cheng, J.; Hao, Z. Preparation of binary washcoat deposited on cordierite substrate for catalytic applications. Ceram. Int. 2010, 36, 529–534. [Google Scholar]
- Agrafiotis, C.; Tsetsekou, A. Deposition of meso-porous γ-alumina coatings on ceramic honeycombs by sol-gel methods. J. Eur. Ceram. Soc. 2002, 22, 423–434. [Google Scholar] [CrossRef]
- Biamino, S.; Fino, P.; Fino, D.; Russo, N.; Badini, C. Catalyzed traps for diesel soot abatement: In situ processing and deposition of perovskite catalyst. Appl. Catal. B Environ. 2005, 61, 297–305. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A.; Ekonomakou, A. The effect of particle size on the adhesion properties of oxide washcoats on cordierite honeycombs. J. Mater. Sci. Lett. 1999, 18, 1421–1424. [Google Scholar] [CrossRef]
- Villegas, L.; Masset, F.; Guilhaume, N. Wet impregnation of alumina-washcoated monoliths: Effect of the drying procedure on Ni distribution and on autothermal reforming activity. Appl. Catal. A Gen. 2007, 320, 43–55. [Google Scholar] [CrossRef]
- Bruneel, E.; van Brabant, J.; Le, M.T.; van Driessche, I. Deposition of a Cu/Mo/Ce catalyst for diesel soot oxidation on a sintered metal fiber filter with a CeO2 anti corrosion coating. Catal. Commun. 2012, 25, 111–117. [Google Scholar] [CrossRef]
- Liotta, L.F.; di Carlo, G.; Longo, A.; Pantaleo, G.; Venezia, A.M. Support effect on the catalytic performance of Au/Co3O4–CeO2 catalysts for CO and CH4 oxidation. Catal. Today 2008, 139, 174–179. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Zhao, B.; Shenb, M.; Zhou, R. Effect of iron doping into CeO2–ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control. J. Hazard. Mater. 2011, 186, 911–920. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Zhao, T.; Chen, D.; Holmen, A. Effect of the preparation methods and alumina nanoparticles on the catalytic performance of Rh/ZrxCe1−xO2–Al2O3 in methane partial oxidation. Catal. Today 2011, 171, 104–115. [Google Scholar]
- Gennari, F.C.; Ramos, A.C.; Condó, A.; Montinid, T.; Bengió, S.; Cortesi, A.; Andrade Gamboa, J.J.; Fornasiero, P. Hydrogen interaction with Pd/Ce0.8Zr0.2O2 nanocomposites prepared by microemulsion, coprecipitation and supercritical CO2 treatment. Appl. Catal. A Gen. 2011, 398, 123–133. [Google Scholar] [CrossRef]
- Ko, J.; Park, S.H.; Jeon, J.K.; Kim, S.S.; Kim, S.C.; Kim, J.M.; Chang, D.; Park, Y.K. Low temperature selective catalytic reduction of NO with NH3 over Mn supported on Ce0.65Zr0.35O2 prepared by supercritical method: Effect of Mn precursors on NO reduction. Catal. Today 2011, 185, 290–295. [Google Scholar]
- Liu, Q.; Liu, Z.; Huang, Z. CuO SUPPORTED on Al2O3-Coated cordierite-honeycomb for SO2 and NO removal from flue gas: Effect of acid treatment of the cordierite. Ind. Eng. Chem. Res. 2005, 44, 3497–3502. [Google Scholar] [CrossRef]
- Monte, R.D.; Fornasiero, P.; Kašpar, J.; Graziani, M.; Gatica, J.M.; Bernal, S.; Herrero, A.G. Stabilisation of nanostructured Ce0.2Zr0.8O2 solid solution by impregnation on Al2O3: A suitable method for the production of thermally stable oxygen storage/release promoters for three-way catalysts. Chem. Commun. 2000, 21, 2167–2168. [Google Scholar]
- Si, R.; Zhang, Y.-W.; Xiao, C.-X.; Li, S.-J.; Lin, B.-X.; Kou, Y.; Yan, C.-H. Non-template hydrothermal route derived mesoporous Ce0.2Zr0.8O2 nanosized powders with blue-shifted UV absorption and high CO conversion activity. Phys. Chem. Chem. Phys. 2004, 6, 1056–1063. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Zhao, B.; Zhou, R. The effect of La doping on the structure of Ce0.2Zr0.8O2 and the catalytic performance of its supported Pd-only three-way catalyst. Appl. Catal. B Environ. 2010, 101, 150–159. [Google Scholar] [CrossRef]
- Hori, C.E.; Permana, H.; Ng, K.Y.S.; Brenner, A.; More, K.; Rahmoeller, K.M.; Belton, D. Thermal stability of oxygen storage properties in a mixed CeO2-ZrO2 system. Appl. Catal. B Environ. 1998, 16, 105–117. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin Elmer: Waltham, MA, USA, 1978. [Google Scholar]
- Monte, R.D.; Kašpar, J. Heterogeneous environmental catalysis—A gentle art: CeO2–ZrO2 mixed oxides as a case history. Catal. Today 2005, 100, 27–35. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Suhonen, S.; Valden, M.; Hietikko, M.; Laitinen, R.; Savimäki, A.; Härkönen, M. Effect of Ce–Zr mixed oxides on the chemical state of Rh in alumina supported automotive exhaust catalysts studied by XPS and XRD. Appl. Catal. A Gen. 2001, 218, 151–160. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pham, P.T.M.; Le, M.T.; Nguyen, T.T.; Bruneel, E.; Van Driessche, I. The Influence of Deposition Methods of Support Layer on Cordierite Substrate on the Characteristics of a MnO2–NiO–Co3O4/Ce0.2Zr0.8O2/Cordierite Three Way Catalyst. Materials 2014, 7, 6237-6253. https://doi.org/10.3390/ma7096237
Pham PTM, Le MT, Nguyen TT, Bruneel E, Van Driessche I. The Influence of Deposition Methods of Support Layer on Cordierite Substrate on the Characteristics of a MnO2–NiO–Co3O4/Ce0.2Zr0.8O2/Cordierite Three Way Catalyst. Materials. 2014; 7(9):6237-6253. https://doi.org/10.3390/ma7096237
Chicago/Turabian StylePham, Phuong Thi Mai, Minh Thang Le, Tien The Nguyen, Els Bruneel, and Isabel Van Driessche. 2014. "The Influence of Deposition Methods of Support Layer on Cordierite Substrate on the Characteristics of a MnO2–NiO–Co3O4/Ce0.2Zr0.8O2/Cordierite Three Way Catalyst" Materials 7, no. 9: 6237-6253. https://doi.org/10.3390/ma7096237




